Abstract
The association between type 1 diabetes and mental health disorders could be exacerbated in a stressful environment. This study aimed to evaluate the effectiveness of a teleguided intervention on emotional disorders in patients with type 1 diabetes during the COVID-19 outbreak. An open-label clinical trial was performed during the social distancing period in the COVID-19 outbreak in Brazil. Individuals with type 1 diabetes aged ≥ 18 years were randomized to receive a teleguided multidisciplinary intervention or the usual care plus an educational website access. The primary outcome was a positive screening for emotional disorders (Self Report Questionnaire 20) after a 16-week intervention. Secondary outcomes included evaluation of patients’ perceptions of pandemic-related changes, diabetes-related emotional distress, eating disorders, and sleep disorders. Data were analyzed with the intent‐to‐treat principle. Fifty-eight individuals (mean age, 43.8 ± 13.6 years) were included (intervention group, n = 29; control group, n = 29). The primary outcome was not different between the groups. The intervention group felt more supported in their diabetes care during the social distancing period (82.8% vs. 48.3% in the control group, P < 0.01). Both groups reported a similar self-perceived worsening of physical activity habits and mental health during the outbreak. There was no benefit to using the telehealth strategy proposed for emotional disorders in patients with type 1 diabetes during the COVID-19 outbreak. Further studies are needed to determine the impact on metabolic parameters and to understand why it is so difficult to emotionally support these patients.
Trail Registration: ClinicalTrials.gov (NCT04344210), 14/04/2020.
Similar content being viewed by others
Introduction
Type 1 diabetes mellitus is a chronic disease that is increasing in both incidence and prevalence1. In 2019, there were approximately 1.1 million individuals under the age of 20 years with this diagnosis2. This reflects an increase in the annual incidence of the disease of approximately 2–3%. Brazil has the third highest incidence of type 1 diabetes, with approximately 7.3 new cases per thousand inhabitants per year2. The challenges of living with diabetes are reflected in different spheres of life for those who receive this diagnosis. There is often a compromise in interpersonal relationships, financial demands and emotional overload related to dependence on continuous health care3. The emotional response of living with the disease is commonly manifest as depression and anxiety, which are two- to four-times more prevalent in those living with diabetes compared to those without diabetes4. In Southern Brazil, about 20% of patients with type 1 diabetes are diagnosed with depression and 40% with anxiety. Those patients who had concurrent diabetes and psychiatric illnesses also had worse glycemic control5.
The prevalence of emotional disorders in patients with type 1 diabetes could be even more expressive in a stressful environment, such as the COVID-19 outbreak. Several measures have been taken to prevent the spread of COVID-19, including isolation of suspected cases, tracking and monitoring of contacts, and the recommendation of social distancing, especially for high-risk groups such as patients with diabetes6,7,8. The COVID-19 preventive measures have the potential to affect the mental well-being of these patients. A previous study that was performed by our group showed that up to 94% of patients with type 1 diabetes have positive screening results for a mental health disorder during the pandemic6. These data highlight the need for mental health access and support for patients with type 1 diabetes during and after this outbreak.
Teleinterventions could be used as a strategy to reduce the impact of the COVID-19 outbreak on the mental health of patients with type 1 diabetes. Previous studies have shown that telehealth strategies can result in improvements in patient satisfaction with the care and quality of life. Moreover, telemedicine has the potential to increase access to healthcare, which may improve diabetes management and reduce severe hypoglycemic episodes9,10,11. The use of a multidisciplinary teleintervention has been shown to be effective in reducing mental health disorders in patients with type 2 diabetes during periods of crisis, such as the COVID-19 outbreak12. However, there are no studies to date that assessed the effectiveness of this type of intervention in patients with type 1 diabetes. This study is part of a protocol that assessed the use of teleguided interventions on emotional disorders in patients with diabetes during the COVID-19 outbreak, and presents the evaluation performed in patients with type 1 diabetes.
Methods
Study design
A randomized clinical trial was performed to assess the impact of a teleintervention in type 1 diabetes during an outbreak. Previous databases were used to identify potential participants for the study, which refer to records of main institutions where patients with type 1 diabetes undergo outpatient follow-up and contained information on telephone number and recent glycated hemoglobin assessment. A medical record review was later performed to identify those who met the inclusion criteria for the study. Potential participants were contacted by telephone and invited to participate in the study, and an inclusion in the protocol was performed at that time to respect social distancing measures.
Participants
Individuals with a previous diagnosis of type 1 diabetes with regular follow-up in two public care centers in Southern Brazil were selected. Patients aged ≥ 18 years and with a measurement of glycated hemoglobin (HbA1c) between January and March 2020 were included. The exclusion criteria was patients who had a medical history of any condition that prevented their understanding of the questionnaires (such as dementia) and interaction with researchers by telephone (such as deafness). Institutionalized and hospitalized patients at the time of inclusion were also not included.
Enrollment and study procedures
Enrollment began on April 14, 2020 and ended on April 29, 2020. The first confirmed case of COVID-19 in Brazil was on February 26, 2020, and the formal recommendation for social distancing for risk groups in Southern Brazil (Porto Alegre city) started on March 22, 2020. Thus, patients were included in the study approximately 2 months after the first case of COVID-19 in the country and 1 month after the beginning of the contact restriction measures. Potential participants were randomly invited to participate in the study. An inclusion questionnaire was applied when the participant was enrolled into the study. Randomization was performed in enrollment into the study, in a 1:1 ratio that was provided by the Randomization.com website. The electronic system generated randomization patterns for the sequence of inclusion of participants with type 1 diabetes in the study. The main researcher was responsible for generating the randomization patterns, which were performed before inclusion of participants in the study. Participants were randomly contacted, without any prior knowledge of them by the research team, and then allocated to each group based on their inclusion number and the pre-established allocation pattern. Participants who were enrolled received a second call to start the intervention procedures or to receive guidance on an educational website that was available to the control group.
Teleintervention characteristics
A multidisciplinary team composed of 6 members (2 general practitioners, 1 endocrinologist, 1 nutritionist, 1 physical educator, and 1 psychologist) was responsible for preparing protocols for appointments that were performed remotely. The original clinical trial design envisaged a similar intervention for patients with type 1 diabetes or type 2 diabetes12. However, all protocols were customized for the particularities of type 1 diabetes. The objective of this strategy was to provide guidance tools and to represent a support channel for the needs of patients with type 1 diabetes during the COVID-19 outbreak. The interface protocols used are available as supplementary material S1.
For the maintenance of remote connections, a group of moderators, which corresponded to 3 postgraduate students and 5 undergraduate medical students, was responsible for mediating contact between the patients and the multidisciplinary team. The moderators went through a training process to qualify them to make the proposed remote appointments. Then, these moderators were responsible for performing the weekly teleinterventions and discussing potential questions regarding the participants with the multidisciplinary team. An online instant messaging group was created so that the multidisciplinary team could instantly respond to the moderators' demands. The moderators were responsible for transmitting the information to participants, and there was no direct contact between the multidisciplinary team and the patients included. The participants were assigned to a specific moderator, who accompanied the same participant throughout the intervention. The assignment was performed based on the participant inclusion number, matching with a list of moderators in alphabetical order.
The duration of the proposed intervention was 16 weeks. The main pillar of this intervention was the provision of weekly telephone contacts between patients and health professionals. Each remote appointment was scheduled to last about 10 min and aimed to address different topics related to the control of diabetes, the presence of emotional overload, and the maintenance of healthy habits during the outbreak.
In addition to developing the protocols, the multidisciplinary team was also responsible for addressing diabetes care demands during the study period. Moderators could access the multidisciplinary team at any time during the follow-up period to address specific patient demands. During the remote contacts, patients were routinely asked for reports on glycemic controls, and were encouraged to maintain good adherence to treatment during each call. Prescription adjustments were discussed with an endocrinologist if recurrent hypoglycemia was reported.
Participants who were randomized to the control group received the usual care during the outbreak, in accordance with the pandemic-related restrictions. For this group, a website was made available with recommendations about maintaining healthy habits during crisis situations. This proposal aimed to offer a reliable source of information during the outbreak for these participants without interacting directly with them.
Outcome measures
Emotional disorder outcomes and changes that occurred during the pandemic were assessed using specific questionnaires, which were applied via telephone calls. All participants were evaluated when they were enrolled into the study (baseline) and after 16 weeks of intervention (follow-up).
Primary outcome
The primary outcome was the presence of a positive screening for emotional disorders at the 16-week follow-up. The Brazilian version of the Self Report Questionnaire-20 (SRQ-20) was used for this evaluation, and a positive screening result was considered if the score was ≥ 713. The choice of this questionnaire was based especially on the wide range of psychiatric disorders that it assesses (anxiety disorders, depression, and somatoform disorders) compared to other mental health scores.
Secondary outcomes
An evaluation of patients’ perceptions (subjective assessment of changes that occurred with the pandemic in relation to eating habits, physical activity, glycemic control and mental health) was performed as pre-planned in the protocol. For this assessment, participants’ were asked to give a score (0–10) for adherence to diet, maintenance of physical activity, glycemic control, and mental health according to their impression before and during the pandemic (follow-up period). Moreover, psychosocial aspects and perceptions about diabetes care during the pandemic were assessed by asking the participants about the presence of respiratory symptoms. Finally, social distancing measures, financial and medical assistance difficulties that may have occurred during the outbreak period were asked with yes/no answer options.
In addition, an assessment of differences between the groups for diabetes-related emotional distress, eating disorders, and sleep disorders was performed using screening tools. The Brazilian version of the Problem Areas in Diabetes Scale (B-PAID) was used to evaluate diabetes-related emotional distress (considered positive if the score was ≥ 40)14. The Brazilian version of the Eating Attitudes Test (EAT-26) was used to assess eating disorders (considered positive if the score was ≥ 20)15. The Brazilian version of the Mini Sleep Questionnaire (MSQ) was used to evaluate sleep disorders screening (considered positive if the score was ≥ 31)16.
Demographics and clinical data
Personal information, such as age, marital status, race/ethnicity, diabetes duration, disease complications, current medications, and psychiatric history were obtained from each patient’s medical records and then verified by the participant. The HbA1c (high-performance liquid chromatography method) results were obtained from records and collected between January and March 2020. Diabetes complications were evaluated using the presence of retinopathy, which was considered based on the last fundus examination. For neuropathy, the presence of a previous diagnosis or an altered monofilament 10-g test result at a medical appointment was considered. For diabetic kidney disease, the presence of macro/microalbuminuria or chronic kidney disease attributed to diabetes in medical records was considered. A history of coronary heart disease, stroke, heart failure, or peripheral arterial disease that was recorded in the patients’ medical records indicated cardiovascular disease.
Power estimations for the primary outcome
The initial protocol was designed to assess mental health and metabolic outcomes in patients with type 1 or type 2 diabetes. The results that were found in patients with type 2 diabetes were described elsewhere12. The metabolic outcomes were not assessed at this time because of the second wave of the pandemic in Brazil and the requirement for exposure of the participants to collect samples for laboratory tests. Thus, in this analysis, the primary outcome was only the emotional disorder assessment in type 1 diabetes. A previous study found that, with the use of a remote intervention in patients with diabetes, changes were significantly greater in the intervention group compared to a control group, with a large between-group effect size (d = 0.83)17. Accordingly, the sample size was calculated for independent samples and dichotomous outcomes, considering the presence of the positive screening for the emotional disorder assessment. Fifty-eight participants were required to detect a difference in emotional disorders between groups considering an estimated withdrawal rate of 10%17. This final sample size ensured that a two-sided test with α = 0.05 would have 85% power to detect a mean difference between groups for the primary outcome.
Statistical analysis
We used SPSS v.22 software (IBM Corp., Armonk, NY, USA) software for the analyses. Participants’ characteristic data were reported as the mean ± standard deviation (SD) if the data were normally distributed. Differences between groups for baseline data were evaluated using an unpaired t-test and the Mann–Whitney U test for continuous variables and the Chi-square test was used for categorical variables.
Outcome data were analyzed using the intention‐to‐treat principle. We used the Markov Chain Monte Carlo multiple imputation algorithm to deal with the missing data. Clinical and psychosocial aspects and perceptions about diabetes care during the study were assessed using the Chi-square test. Data on patients’ perceptions of changes in habits that occurred during the pandemic were reported as the median ± interquartile range (IQR), and analyses were performed using the Mann–Whitney U test for the between-groups comparisons and the Wilcoxon Rank test for the within-group comparisons. Results of the questionnaires were analyzed for the presence of a positive screening result for the disorder based on previously cited cutoff values. Comparisons of positive screening between groups were performed using the Chi-square test/Fisher’s exact test and comparisons of within-group data were performed using the McNemar’s test. Comparisons within groups were performed post hoc and sought to assess changes from baseline to follow-up within each arm of the study. Two-tailed tests were used to determine significance at the 5% level.
Ethics approval and consent to participate
The informed consent form was read by the telephone contact for all participants who were included in the study. Agreement was registered using an audio recording or an electronic message. The study followed international recommendations for conducting research with humans and was approved by the institutional ethics committee (CONEP No. 4.029.368). This trial was registered at ClinicalTrials.gov (registration: NCT04344210). This reporting follows the CONSORT statement18.
Consent for publication
All authors have reviewed the final version of the manuscript and agree with the publication of the results presented.
Results
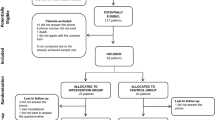
Overall, 117 potentially eligible patients were identified, and the enrollment stopped when 58 individuals with type 1 diabetes provided informed consent. Sixteen remote appointments were planned, but the median number of calls that were received by the participants in the intervention group was 13.0 (IQR 11.3–15.8). The moderators made three contact attempts weekly if the participant did not answer on the first call. Only three participants received fewer than 10 calls due to the difficulty of contacting them (one participant received nine calls, one participant received eight calls, and one participant received only two calls). Four participants needed clinical support to adjust their insulin dose due to recurrent hypoglycemia, which was discussed with an endocrinologist from the multidisciplinary team. At the end of the follow-up, six participants withdrew from the study: four participants did not answer the phone (three in the intervention group and one in the control group); one participant was hospitalized; and one participant did not respond to the final questionnaires and requested to be removed from the study (the latter two participants were both in the intervention group) (Fig. 1).
Participants had a mean age of 43.8 ± 13.6 years, 50.0% were female, and 31.0% were married. Most participants were white and had a lower-to-middle income. The mean diabetes duration was 25.2 ± 11.6 years and the HbA1c value was 8.7 ± 1.5% (72.0 ± 16.4 mmol/mol). A previous depression diagnosis was found in 25.9% and a previous anxiety diagnosis in 3.4% of participants. There were no differences between groups regarding the baseline characteristics (Table 1).
Primary outcome
Emotional disorders evaluation
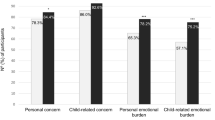
For emotional disorders in the groups (SRQ 20 questionnaire), a positive screening result was found in 51.7% and 41.4% of participants in the intervention and the control group at the baseline, respectively (P = 0.43). In the follow-up, a positive screening result was found in 48.3% and 34.5% of participants in the intervention and control groups, respectively (P = 0.29) (see Fig. 2). For within-group analyses, there was no difference in the baseline and follow-up results (P < 0.99) for within-group comparison in the intervention group and P = 0.79 for within-group comparison in the control group).
Participants with positive screening for mental health disorders based on cutoff values of the questionnaires. For the evaluation of emotional disorders, a score greater than or equal to 7 on SRQ 20 is considered positive. Diabetes-related emotional distress is considered when the B-PAID score is greater than or equal to 40. The presence of positive screening for an eating disorder is considered when the EAT 26 score is greater than or equal to 20. A positive screening for sleep disorder is considered when a score greater than or equal to 31 is present in the MSQ. *P = 0.04 for comparison between groups after the intervention.
Secondary outcomes
Psychosocial aspects and perceptions about diabetes care during the pandemic
During the pandemic, 29.3% of participants followed the guidance of complete social distancing and 58.7% of participants followed the guidance only partially (maintained basic activities). Around 70.7% had contact only with the family during the study period. For diabetes care, 38.0% received remote care from their attending physician and 22.4% considered their medical care to be worse during the outbreak. Additionally, 20.7% of the participants reported difficulties obtaining medical assistance during the period, and 19.0% reported difficulties in getting medication prescriptions. Most participants (53.5%) reported financial difficulties and 6.9% lost their jobs during the pandemic. The two groups were comparable in most of the evaluated characteristics. However, participants from the intervention group reported more frequently that they felt supported in their diabetes care during the social distancing period (82.8% vs. 48.3%, P < 0.01) (see Table 2).
Changes in habits during the COVID-19 outbreak
Participants were asked to provide a score, from zero to 10, for the quality of some aspects in their daily routine before and during the COVID-19 outbreak. Comparisons between the groups in relation to the evaluation periods showed a similarity in self-reported scores for eating habits, physical activity, glycemic control, and mental health. For within-group comparisons, both control and intervention groups showed worse physical activity and mental health parameters during the pandemic. For the self-reported score for physical activity, the intervention group had a median score of 5.0 (IQR 3.0–8.0) before the pandemic and 4.0 (IQR 1.0–6.64) during the pandemic (P = 0.02), while the control group had a median of 6.0 (IQR 1.0–9.0) and 4.0 (IQR 0.0–7.0) before and during the pandemic, respectively (P = 0.001). For mental health, the intervention group had a median score of 9.0 (IQR 8.0–10.0) and 8.0 (IQR 7.0–9.0) before and during the pandemic (P = 0.02), while the control group had a median score of 8.0 (IQR 7.0–10.0) and 7.0 (IRQ 5.0–10.0) before and during the pandemic, respectively (P = 0.01) (see Table 3).
Diabetes-related emotional distress
For diabetes-related emotional distress between groups (B-PAID questionnaire), the presence of a positive screening result was found in 27.6% and 13.8% of participants in the intervention and control groups at the baseline, respectively (P = 0.20). In the follow-up, a positive screening result was found in 27.6% and 27.6% of participants in the intervention and control groups (P < 0.99), (see Fig. 2). There was no difference in the within-group analyses for the baseline and follow-up results.
Eating disorders
When assessing eating disorders between groups (EAT-26 questionnaire), the presence of a positive screening result was equal (72.4%) in the intervention and control groups (P < 0.99) at the baseline. At the follow-up visit, a positive screening result was found in 62.1% and 75.9% of participants in the intervention and control groups, respectively (P = 0.26) (see Fig. 2). The within-group analyses showed that there were no changes in screening for the intervention and control groups between the baseline and follow-up responses.
Sleep disorders
When evaluating sleep disorders between groups (MSQ questionnaire), a positive screening result was found in 75.9% and 58.6% of participants in the intervention and control groups at the baseline, respectively (P = 0.16). At the follow-up visit, a positive screening was found in 82.8% and 58.6% of participants in the intervention and control groups (P = 0.04) (see Fig. 2). When corrected for the questionnaire scores at baseline, there was no difference between groups [OR 3.4 (95% IC, 0.9–11.8)]. There was no change in the within-group difference at baseline and follow-up in the intervention and control groups (P = 0.73 and P < 0.99, respectively).
Discussion
The psychological impact of the COVID-19 pandemic has the potential to generate lasting and persistent damage to the population. This study assessed the impact of a telehealth intervention on emotional disorders in patients with type 1 diabetes during the social distancing period. The intervention was not effective in reducing the prevalence of emotional disorders, diabetes-related emotional distress, or eating and sleep disorders at the end of the follow-up period. However, the participants in the intervention group felt almost twice as much support in their diabetes care during the outbreak. For patients’ perceptions about changes that occurred in relation to the period before the pandemic, both groups reported that there was a worsening in physical activity habits and mental health parameters, with no improvement related to the intervention that was performed.
Remote strategies are aids in the care of type 1 diabetes. Different studies have shown a reduction in episodes of hypoglycemia and an improvement in the quality of life related to this type of intervention9,10,11. However, improving mental health parameters in these patients is still a challenge. In our study, a telehealth strategy was not effective in mitigating the effects of the COVID-19 outbreak on emotional disorders in type 1 diabetes. Interestingly, a similar intervention was developed for patients with type 2 diabetes, and it was effective in reducing the prevalence of mental disorders by up to 36% during the pandemic12. The main studies on teleinterventions that showed positive results in mental health issues included patients with type 1 diabetes or type 2 diabetes17,19,20,21,22. It is possible that the positive results in these studies could be mediated mainly by patients with type 2 diabetes who seem to respond more positively to the teleinterventions. Some factors could explain this difference between the types of diabetes. First, it is possible that, because these individuals have lived with type 1 diabetes for a long time, they have a greater capacity for self-care, autonomy, and security in relation to their diabetes care 23. Thus, providing lifestyle and diabetes care strategies remotely may be insufficient to mitigate the effects of the outbreak on these patients’ mental health. Second, it is possible that these patients, who are already emotionally fragile, need a longer intervention time to show significant emotional benefits. Third, it is possible that the younger age of patients with type 1 diabetes makes them more psychologically resilient to the emotional impact of an outbreak24. In this case, the prevalence of mental health disorders could reflect an already chronic condition, requiring more complex strategies to mitigate its effects. In addition, it is possible that patients with type 1 diabetes do not perceive themselves as part of the group that is at a higher risk for the disease, and thus, they are affected less by this situation. Still, the differences in the response to teleintervention between patients with type 1 or type 2 diabetes is thought-provoking and deserves to be better understood.
Support for diabetes care during crisis situations can directly impact glycemic control in patients with type 1 diabetes. Previous studies showed that patients who receive support and maintain regular contact with health professionals through telemedicine have an improvement of up to 0.91% in HbA1c levels25,26. Other studies show that this impact can also be reflected in other areas including control of blood pressure and weight as well as dyslipidemia, and it also promotes a better quality of life27,28. In our study, patients who maintained regular contact with health professionals reported that they felt almost twice as much support in their diabetes care during the period of social distancing. The impact that this contact will have in terms of glycemic control in this context remains hypothetical, and studies evaluating metabolic outcomes are necessary to better understand this effect.
Self-reported scores for physical activity and mental health were evaluated, demonstrating that the COVID-19 outbreak had a negative impact on both the intervention and control groups. Restrictions on outdoor activities, gyms, and public swimming contributed to a less active lifestyle. Additionally, home confinement can lead to symptoms of anxiety and sadness, which may have a negative impact on the activity practice levels and mental health perceptions. It was expected that the intervention group could have had better results for physical activity and mental health scores by the end of study, which was not shown, and this raises a concern. Physical activity and emotional well-being are an important part of diabetes care, and they can lead to better glycemic control and also help to improve the ability to perform diabetes tasks29. Therefore, the reduction of physical activity levels and the worsening perception of mental health that was seen in this study can lead to serious consequences in diabetes control, which could predispose these patients to further chronic complications30,31.
Studies that were performed during the COVID-19 pandemic showed that over 20% of patients with pre-existing psychiatric disorders reported worsening of their symptoms while social distancing32. In our study, over 40% of the participants had a positive screening result for emotional disorders after 20 weeks of social isolation in Brazil, reaching almost 70% of eating and sleep disorders. There was a tendency toward worsening sleep parameters in the intervention group at the end of the follow-up. However, this result seems to be mediated by a difference that already existed at baseline. When the difference in the same group was evaluated by comparing the baseline and follow-up results, there were no significant changes in the prevalence that was present in both within-group analyses, which reinforces the impression that the difference that was found is due to chance or mediated by a small difference that already existed at baseline. Thus, the high prevalence of these disorders is an alert for health professionals, who must be attentive to signs of intense suffering and provide specialized mental health care.
This study has some limitations. Although the number of participants was in accordance with the calculated sample size, we considered that a relatively small sample was included in this study. Considering the number of dropouts in the follow-up that occurred especially in the intervention group, and less frequently in the control group, it is possible that the assessment of the primary outcome may have been compromised. In addition, the scales that were used to assess emotional disorders were designed and validated for self-application. Because the questionnaires were applied remotely to preserve patient safety during the pandemic, and mediated by a third individual, it may result in difficulty for patients to be completely honest in their responses, and it could be a potential source of bias. The scales that were used to assess psychiatric disorders are screening tools and have no diagnostic value. Finally, the inclusion of a sample from only two university hospitals in the same region of Brazil may also compromise the external validity of the study.
The COVID-19 outbreak has the potential to generate negative mental health outcomes for patients with type 1 diabetes. Although the present study has shown that there is no benefit in using a telehealth strategy for emotional disorders during the COVID-19 pandemic, patients who remained in regular contact with health professionals felt more support in their diabetes care while in social distancing. Further studies are needed to understand whether this support is reflected in improved glycemic control and an improved quality of life for these patients when in crisis situations. In addition, a better understanding of why it is so difficult to emotionally support patients with type 1 diabetes is essential for the development of effective strategies in the future.
Data availability
Deidentified participant data and informed consent form will be available for one year after publication of the article upon justified request to the e-mail address of the main researcher and with a signed data access agreement.
Change history
11 March 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-022-08539-9
References
Mayer-Davis, E. J. et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 376(15), 1419–1429. https://doi.org/10.1056/nejmoa1610187 (2017).
Saeedi, P., Petersohn, I., & Salpea, P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 157:107843 (2019). https://doi.org/10.1016/j.diabres.2019.107843
Raymond, J. Updates in behavioural and psychosocial literature in adolescents with type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 22(4), 265–269. https://doi.org/10.1097/MED.0000000000000167 (2015).
Buchberger, B. et al. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 70, 70–84. https://doi.org/10.1016/j.psyneuen.2016.04.019 (2016).
Fritzen, T. M. et al. High prevalence of psychiatric disorders and its association with worse glycemic control in patients with type 1 diabetes. Endocr. Abstr. 70, 449. https://doi.org/10.1530/endoabs.70.AEP449 (2020).
Alessi, J. et al. Mental health in the era of COVID-19: Prevalence of psychiatric disorders in a cohort of patients with type 1 and type 2 diabetes during the social distancing. Diabetol. Metab. Syndr. 12(1), 1–10. https://doi.org/10.1186/s13098-020-00584-6 (2020).
Alessi, J., De Oliveira, G. B., Schaan, B. D. & Telo, G. H. Dexamethasone in the era of COVID-19: Friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol. Metab. Syndr. 12(1), 1–11. https://doi.org/10.1186/s13098-020-00583-7 (2020).
People at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fspecific-groups%2Fhigh-risk-complications.html
Guljas, R., Ahmed, A., Chang, K. & Whitlock, A. Impact of telemedicine in managing type 1 diabetes among school-age children and adolescents: An integrative review. J. Pediatr. Nurs. 29(3), 198–204. https://doi.org/10.1016/j.pedn.2013.10.013 (2014).
Fogel, J. L. & Raymond, J. K. Implementing telehealth in pediatric type 1 diabetes mellitus. Pediatr. Clin. North Am. 67(4), 661–664. https://doi.org/10.1016/j.pcl.2020.04.009 (2020).
Bertuzzi, F. et al. Teleconsultation in type 1 diabetes mellitus (TELEDIABE). Acta Diabetol. 55(2), 185–192. https://doi.org/10.1007/s00592-017-1084-9 (2018).
Alessi, J. et al. Telehealth strategy to mitigate the negative psychological impact of the COVID-19 pandemic on type 2 diabetes: A randomized controlled trial. Acta Diabetol. 15, 1–11. https://doi.org/10.1007/s00592-021-01690-1 (2021).
Gonçalves, D. M., Stein, A. T. & Kapczinski, F. Avaliação de desempenho do self-reporting questionnaire como instrumento de rastreamento psiquiátrico: Um estudo comparativo com o Structured Clinical Interview for DSM-IV-TR. Cad Saude Publica. 24(2), 380–390. https://doi.org/10.1590/S0102-311X2008000200017 (2008).
Bighetti F. Tradução e validação do eating attitudes test (EAT-26) em adolescentes do sexo feminino na cidade de Ribeirão Preto - SP. Tese (Mestrado em Enferm em Saúde Pública). Published online 2003.
Falavigna, A. et al. Consistency and reliability of the Brazilian Portuguese version of the Mini-Sleep Questionnaire in undergraduate students. Sleep Breath. 15(3), 351–355. https://doi.org/10.1007/s11325-010-0392-x (2011).
Gross, C. C., Scain, S. F., Scheffel, R., Gross, J. L. & Hutz, C. S. Brazilian version of the Problem Areas in Diabetes Scale (B-PAID): Validation and identification of individuals at high risk for emotional distress. Diabetes Res. Clin. Pract. 76(3), 455–459. https://doi.org/10.1016/j.diabres.2006.09.022 (2007).
Ebert, D. D. et al. The 6-month effectiveness of Internet-based guided self-help for depression in adults with Type 1 and 2 diabetes mellitus. Diabet. Med. 34(1), 99–107. https://doi.org/10.1111/dme.13173 (2017).
Rennie, D. CONSORT revised—Improving the reporting of randomized trials. J. Am. Med. Assoc. 285(15), 2006–2007. https://doi.org/10.1001/jama.285.15.2006 (2001).
Nobis, S. et al. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: A randomized controlled trial. Diabetes Care 38(5), 776–783. https://doi.org/10.2337/dc14-1728 (2015).
Cohen, L. B., Taveira, T. H., Wu, W. C. & Pirraglia, P. A. Pharmacist-led telehealth disease management program for patients with diabetes and depression. J. Telemed. Telecare. 26(5), 294–302. https://doi.org/10.1177/1357633X18822575 (2020).
Nobis, S. et al. Efficacy and cost-effectiveness of a web-based intervention with mobile phone support to treat depressive symptoms in adults with diabetes mellitus type 1 and type 2: Design of a randomised controlled trial. BMC Psychiatry 13, 1–10. https://doi.org/10.1186/1471-244X-13-306 (2013).
Newby, J. et al. Web-based cognitive behavior therapy for depression in people with diabetes mellitus: A randomized controlled trial. J. Med. Internet. Res. 19(5), 1. https://doi.org/10.2196/jmir.7274 (2017).
Chiang, J. L., Kirkman, M. S., Laffel, L. M. B. & Peters, A. L. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 37(7), 2034–2054. https://doi.org/10.2337/dc14-1140 (2014).
Pearman, A., Hughes, M. L., Smith, E. L. & Neupert, S. D. Age differences in risk and resilience factors in COVID-19-related stress. J. Gerontol. Ser. B. https://doi.org/10.1093/geronb/gbaa120 (2020).
Andrès, E. et al. Telemonitoring in diabetes: evolution of concepts and technologies, with a focus on results of the more recent studies. J. Med. Life. 12(3), 203–214. https://doi.org/10.25122/jml-2019-0006 (2019).
Charpentier, G. et al. The diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: A 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 study). Diabetes Care 34(3), 233–239. https://doi.org/10.2337/dc10-1259 (2011).
Andrès, E. et al. Experimentation of 2.0 telemedicine in elderly patients with chronic heart failure: A study prospective in 175 patients. Eur. J. Intern. Med. 51, e11–e12. https://doi.org/10.1016/j.ejim.2018.02.022 (2018).
Andrès, E. et al. E-Health: A promising solution for optimizing management of chronic diseases: Example of the national e-Health project E-care based on an e-platform in the context of chronic heart failure. Eur. Res. Telemed. 4(3), 87–94. https://doi.org/10.1016/j.eurtel.2015.08.001 (2015).
Association AD. Standards of Medical Care in Diabetes. Vol 44. (2021).
Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2(2), 1143–1211. https://doi.org/10.1002/cphy.c110025 (2012).
Anderson, E. & Durstine, J. L. Physical activity, exercise, and chronic diseases: A brief review. Sport Med. Heal Sci. 1(1), 3–10. https://doi.org/10.1016/j.smhs.2019.08.006 (2019).
Zhou, J., Liu, L., Xue, P., Yang, X. & Tang, X. Mental health response to the COVID-19 outbreak in China. Am. J. Psychiatry. 177(7), 574–575. https://doi.org/10.1176/appi.ajp.2020.20030304 (2020).
Acknowledgements
This work was conducted with support from Medical Science Program: Endocrinology of Universidade Federal do Rio Grande do Sul, School of Medicine of Pontifícia Universidade Católica do Rio Grande do Sul, Hospital de Clínicas de Porto Alegre and Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul.
Funding
This study was supported by FIPE (Research and Events Incentive Fund) of Hospital de Clínicas de Porto Alegre, and Medical Science Program: Endocrinology of Universidade Federal do Rio Grande do Sul. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.A.: conceptualization, methodology, data curation, writing—original draft preparation. G.B.O., D.W.F., B.A.; G.L.K., A.S.B., C.P.K., A.B., T.C. and G.H.T.: methodology, data curation. B.D.S.: conceptualization, supervision, writing—reviewing and editing. G.H.T.: Conceptualization, writing—original draft preparation, supervision. J.A. is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-022-08539-9
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alessi, J., Becker, A.S., Amaral, B. et al. RETRACTED ARTICLE: Type 1 diabetes and the challenges of emotional support in crisis situations: results from a randomized clinical trial of a multidisciplinary teleintervention. Sci Rep 12, 3086 (2022). https://doi.org/10.1038/s41598-022-07005-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07005-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.