Abstract
The association between phthalate exposure and breast cancer remains controversial. We performed a prospective patient cohort design to explore the interaction between creatinine-corrected urinary phthalate metabolites and hormone receptors as well as body mass index (BMI) on recurrent breast cancer. In this follow-up study, 636 female breast cancer patients and 45 new recurrent cases diagnosed for a total of 1576.68 person-years of follow-up were recruited. Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) was negatively associated with breast cancer recurrence, with adjusted hazard ratio (aHR) 3rd vs. 1st quartile of 0.15 (95% CI 0.04–0.51). The MEOHP presented as a non-monotonic dose–response (NMDR) curve, being U-shaped. In the stratification of hormone receptors, MEOHP still exhibited a U-shaped dose–response curve. The third quartile of MEOHP showed significant lowest recurrent risk in the status of ER-positive (aHR 0.18, 95% CI 0.05–0.66), PR-negative (aHR 0.14, 95% CI 0.03–0.63), and HER2-negative (aHR 0.24, 95% CI 0.08–0.76). Whether in BMI < 25 or in BMI ≥ 25, the third quartile of MEOHP was negatively associated with recurrent breast cancer, and there was a negative interaction on an additive scale between MEOHP and BMI (pinteraction = 0.042). The association between MEOHP and recurrent breast cancer was modified by hormone receptors and BMI.
Similar content being viewed by others
Introduction
About 2.3 million breast cancer cases were newly diagnosed in 2020 worldwide. Breast cancer has surpassed lung cancer as the most common cancer, and the number of deaths was about 685,000. Breast cancer is the most commonly diagnosed cancer (24.5% of female cancers) and the leading cause of cancer death (15.5% of female cancer deaths) in women1,2. The highest estimated age-standardized incidence of breast cancer in most areas was between the ages of 60 and 74 years, which is available online at the International Agency for Research on Cancer (IARC) (https://gco.iarc.fr). In Taiwan, data from the Ministry of Health and Welfare (MOHW) showed that the age-adjusted incidence rates of breast cancer rise sharply to 45–49 years and are increasing over time; the age-adjusted incidence rates of 45–49 years in females were 64.96 per 100,000 in 2010 to 74.57 per 100,000 in 2015, and in 2018, it was 79.40 per 100,000. Furthermore, the 5-year survival rate of breast cancer between 2014 and 2019 was 88.6% in Taiwan. Studies have indicated that women with local recurrence have a higher risk of metastasis and death than women without local recurrence3,4.
Phthalates are man-made chemicals used in many products, including personal care products and consumer products5. Phthalates do not form a covalent bond with the plastic matrix and leak from containers to the surroundings, causing human exposure6, and as they are ubiquitous in air, dust, water, and food, humans could be exposed through inhalation, ingestion, and skin absorption7,8. Some artificial chemicals have similar structure to endogenous estrogen and can bind to estrogen receptors (ER)9. Phthalates are known environmental endocrine-disrupting chemicals (EDCs) that could bind to and interact with ER and progesterone receptor (PR)10,11; however, the association of phthalates with hormone-dependent cancer, such as breast cancer, remains conflicting.
A very recent prospective study indicated that phthalate metabolites in urine were not associated with breast cancer in postmenopausal women12. Three other retrospective case–control studies had inconsistent findings; one study suggested that most urinary phthalate metabolites were negatively associated with breast cancer and breast cancer-specific mortality among predominantly white women13; another Mexican study reported that mono-ethyl phthalate (MEP) was associated with breast cancer while mono-benzyl phthalate (MBzP) and mono-(3-carboxypropyl) phthalate (MCPP) were negatively related to breast cancer14, although the last study in Native Alaskans showed that mono-2-ethylhexyl phthalate (MEHP) was associated with breast cancer15. Other cancer epidemiological studies still had different findings. Thyroid cancer and benign nodule were associated with mono-methyl phthalate (MMP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and MEHP, but mono-n-butyl phthalate (MnBP) was observed to have a negative correlation16. A population-based nested case–control study in Taiwan found that MBzP, MnBP, and mono-isobutyl phthalate (MiBP) were associated with prostate cancer in obese men17. So far, few epidemiological studies have addressed the relationship between phthalate exposure and recurrent breast cancer.
Phthalates are rapidly metabolized to the corresponding metabolites through hydrolysis and oxidation, making phthalates more soluble and easily excreted into the urine18. Urine is a non-invasive sample and easier to obtain than blood, and most phthalate metabolites are easily detectable in urine than in other body fluids19; furthermore, the concentration of phthalate metabolites in urine is about 50 times higher than in blood, so quantifying urinary phthalate metabolite concentrations is the best way to assess phthalate exposure in epidemiological studies20,21.
To our best knowledge, this is the first study using a prospective patient cohort design to explore the interaction between phthalate exposure and hormone receptors as well as body mass index (BMI) on breast cancer recurrence.
Methods
Study population
Seven hundred and twenty-six female patients diagnosed with breast cancer by breast surgeon specialists and histopathological sections were recruited from Kaohsiung Medical University (KMU) Chung-Ho Memorial Hospital (a medical center in southern Taiwan) from September 2013 to June 2018 and follow-up of these patients were continued until July 2019. Patients under 20 years of age, being non-citizens (n = 1), having benign breast disease (n = 11), suffering from any other cancer (n = 1), and with incomplete data (n = 11) were excluded. Due to 66 patients not having urine samples, only 636 breast cancer patients completed phthalate metabolite examinations in urine.
In this patient cohort study, 636 breast cancer patients were recruited in this study and 47 patients already with recurrence were excluded in this study design and the patients were followed up every 6 months until the end of this study. For recurrence-free survival analyses, the breast cancer recurrence and metastases were study outcomes (recurrent patients including patients who died of breast cancer), while non-recurrent breast cancer patients were censored at the time of lost follow-up, withdrawal, and last follow-up date. Finally, a total of 589 non-recurrent breast cancer patients with 1576.68 person-years were followed up, and 45 new recurrent breast cancer cases were diagnosed. The prospective follow-up was performed to explore the association between phthalate exposure and the risk of breast cancer recurrence with new recurrent patients (n = 45) vs. non-recurrent patients (n = 544) (Fig. 1). Researchers regularly followed patients for breast cancer recurrence, metastasis, and survival every 6 months by medical record, and extracted the patient's medical history, including breast density, treatment, TNM stage (TNM classification of malignant tumors), ER, PR, human epidermal growth factor receptor 2 (HER2), etc. Immunohistochemistry (IHC) was used to test hormone receptors.
All these patients provided blood and spot morning urine samples and completed questionnaires with trained interviewers to obtain information concerning sociodemographic characteristics, alcohol use, smoking status, dietary habits, reproductive history, family history of breast cancer, etc. According to the standard classification of the World Health Organization (WHO), BMI was categorized as non-obesity (BMI < 25 kg/m2) and overweight or obesity (BMI ≥ 25 kg/m2). Written informed consents were obtained from all participants before data and biological specimen collection. The study protocol was approved by the Institutional Review Board (IRB) of Kaohsiung Medical University (KMU) Chung-Ho Memorial Hospital (IRB no. KMUHIRB-20120104, KMUHIRB-20140055, and KMUHIRB-G(I)-20150026). The study was performed in accordance with the Declaration of Helsinki.
Phthalate reagents
Nine phthalate analytical standards were purchased from Cambridge Isotope Laboratories (CIL) (Andover, Massachusetts, USA); namely, MEP (Product Code: ULM-4585-MT-1.2), MnBP (ULM-6148-MT-1.2), MiBP (ULM-7919-MT-1.2), MEHP (ULM-4583-MT-1.2), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) (ULM-4662-MT-1.2), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) (ULM-8149-MT-1.2), MEOHP (ULM-4663-MT-1.2), MMP (ULM-6697-MT-1.2), and MBzP (ULM-6149-MT-1.2). Nine phthalate internal standards were purchased from Toronto Research Chemicals (TRC) (North York, Ontario, Canada) and Cambridge Isotope Laboratories (CIL) respectively; namely, MEP-d4 (Cat. No.: M542582), MnBP-d4 (M525102), MiBP-d4 (M547702), MEHP-d4 (M542492), MEHHP-d4 (M542512), MECPP-13C4 (Product Code: CLM-8148-MT-1.2), MEOHP-13C4 (CLM-6640-MT-1.2), MMP-d4 (M566542), and MBzP-d4 (M524902).
Sample preparation
The first spot morning urine samples were collected in a sterile brown glass bottle from all breast cancer participants and then frozen at − 20 °C until analysis. For analysis, urine samples were thawed at 4 °C, and then 1 mL urine sample was transferred to a glass tube, followed by the addition of 10 μL mixed isotope internal standards solution and 250 μL ammonium acetate (1 M, pH 6.5). After adding 3 μL beta-glucuronidase from E. coli K12 (Roche Diagnostics GmbH, Mannheim, Germany), the mixture was shaken and incubated for 90 min at 37 °C in a water bath, and then placed for 10 min at room temperature. The mixture was mixed with 2 mL phosphate buffer (pH 2) for 30 s and centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was purified with solid-phase extraction cartridge (Agilent ABS Elut-NEXUS) (Agilent Technologies, Palo Alto, CA, USA). After sample loading, 2 mL formic acid and 1 mL water were added to the cartridge, and then eluted with 2 mL acetonitrile and 2 mL ethyl acetate and dried by nitrogen gas at 55 ℃. The residue was reconstituted by adding 200 μL water and taken for liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis.
Urinary phthalate metabolites assessment by LC–MS/MS
The concentrations of nine urinary phthalate metabolites: MEP, MnBP, MiBP, MEHP, MEHHP, MECPP, MEOHP, MMP, and MBzP, were measured by the same technician in double-blinded fashion using LC–MS/MS, which was a Waters ACQUITY UPLC system (Waters Corporation, Milford, MA, USA) coupled with a Finnigan TSQ Quantum Ultra triple-quadrupole MS (Thermo Fisher Scientific, San Jose, CA, USA) equipped with an electrospray ionization (ESI) source in a negative ion mode and combined with Xcalibur software (Thermo Fisher Scientific).
A 10 μL quantity of each sample was injected into a ACQUITY UPLC CSH Phenyl-Hexyl Column (2.1 × 100 mm, 1.7 μm) (Waters Corporation, Milford, MA, USA) equipped with a BEH C18 column VanGuard Pre-column (2.1 × 5 mm, 1.7 μm) (Waters Corporation) with a flow rate of 250 μL/min at 40 ℃ in the gradient from 30% solvent B (0.1% acetic acid in acetonitrile) for 0.0–5.0 min, then increased to 40% B for 10.0 min, 50% B for 12.0 min, and 100% B for 13.0 min, then reduced back down to 10% B in 13.1–15.0 min (solvent A: 0.1% acetic acid in water). The negative ion mode was set as follows: ion spray voltage, 3000 V; sheath gas (N2), 28 psi; auxiliary gas (N2), 10 psi; and collision gas (Ar), 1.0 mTorr. The detection rates, precursor ion (m/z), product ion (m/z), retention times (min), and collision energy (V) were shown in Supplementary Table S1.
Method validation and sample determination
All experiments were done in the laboratory at the Center for Research Resources and Development in KMU, having an external quality assurance by German External Quality Assessment Scheme for toxicological analyses. Blank sample and quality control were simultaneously performed in each experiment along with the analytical protocol. The urinary concentrations of phthalate metabolites were adjusted by creatinine for urine dilution. Creatinine-adjusted units (μg/g) were calculated by dividing the phthalate metabolite concentrations (μg/L) by creatinine concentrations (mg/dl) and multiplying by 10022.
The Σ4DEHP was the sum of urinary MEHP, MEHHP, MECPP, and MEOHP concentrations to represent similar source exposure23. If the values of concentrations were less than the limit of detection (LOD), the values were replaced with LOD/\(\sqrt 2\). The detection rates of MMP and MBzP were 0% and 68.9% separately; thus, the two metabolites were not considered in further analysis. The detection rates of the other seven urinary phthalate metabolite concentrations were 98.4% to 100%. We calculated the intra- and inter-batch coefficient of variation (CV) for individual metabolites. Intra-batch CV: MnBP (12.63%), MiBP (13.56%), MEHP (12.50%), MEHHP (7.89%), MECPP (13.85%), and MEOHP (9.97%). Inter-batch CV: MnBP (8.18%), MiBP (6.34%), MEHP (7.83%), MEHHP (11.19%), MECPP (11.76%), and MEOHP (4.47%).
Statistical analysis
The continuous and categorical variables of sociodemographic and clinical characteristics for new recurrence and non-recurrence groups were compared using Independent sample t-test and Chi-square test or Fisher’s exact test. If the sociodemographic and clinical characteristics as risk factors associated with breast cancer were significantly different between the two groups, they were selected as potential confounding factors in the adjusted model. The values of creatinine-corrected urinary phthalate metabolite concentrations were non-normal distributions, so the Mann–Whitney U test or ln-transformed for Independent sample t-test were used to analyze the differences between two groups. Spearman correlation coefficients were used to evaluate correlations between individual phthalate metabolite concentrations. The concentrations were categorized into quartiles according to the distribution of phthalate metabolite concentrations in all participants with the first quartile being defined as the reference group.
Multivariable Cox proportional hazards model was performed for recurrence-free survival analysis with new recurrent breast cancer patients as related to phthalate exposure. The hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the risk of recurrent breast cancer in relation to phthalate exposure. The study patients were also stratified by the status of ER (ER-negative vs. ER-positive), PR (PR-negative vs. PR-positive), and HER2 (HER2-negative vs. HER2-positive) as well as BMI (BMI < 25 kg/m2 vs. BMI ≥ 25 kg/m2) to estimate the interaction effect of phthalate exposure and hormone receptors as well as BMI on the risk of breast cancer recurrence. The interaction was estimated for survival analysis based on additive scale in this prospective follow-up design24,25. The measurement for interaction effect as additive scale was relative excess risk due to interaction (RERIHR = eβ1+β2+β3 − eβ1 − eβ2 + 1)24,26.
Because the associations between recurrent breast cancer and eight phthalate metabolites were evaluated at the same time, we used Bonferroni correction (p-value multiplied by eight phthalate comparisons) to reduce the potential problems (Type I error) raised from multiple comparisons. The analyses were performed using SPSS 22.0 and all tests were two-sided. p-values < 0.05 were considered statistically significant.
Ethics declarations
Written informed consents were obtained from all participants before data and biological specimen collection. The study protocol was approved by the Institutional Review Board (IRB) of Kaohsiung Medical University (KMU) Chung-Ho Memorial Hospital (IRB no. KMUHIRB-20120104, KMUHIRB-20140055, and KMUHIRB-G(I)-20150026). The study was performed in accordance with the Declaration of Helsinki.
Consent to participate/consent to publish
Written informed consents were obtained from all participants before data and biological specimen collection. All the authors have read and approved the paper for publication.
Results
Patient characteristics in patient cohort
In Table 1, as of June 2018, 726 breast cancer patients were enrolled in this study, of which 636 patients underwent urinary phthalate metabolite examination. In this patient cohort study, 589 non-recurrent breast cancer were followed up with 1576.68 person-years, and 45 new recurrent breast cancer cases were diagnosed. Compared to non-recurrent patients, new recurrent patients were less married (70.5%), smoked more (15.9%), had higher breast cancer stages (59.1%), poorer cell differentiation (47.7%), larger tumor size (61.9%), less ER-positive status (66.7%), less PR-positive status (44.4%), more chemotherapy (88.9%), and more radiotherapy (80.0%).
Urinary phthalate metabolite profiles
Urinary phthalate metabolite concentrations were significantly positively related to each other as shown in Supplementary Table S2. The geometric mean concentrations of metabolites were not significant between the two groups (Supplementary Table S3). In the concentration of metabolites, the concentration of MEP was highest, followed by MECPP, and then MnBP.
The results of prospective follow-up
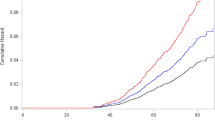
The recurrence-free survival analysis of patient cohort was shown in Table 2. MEOHP were significantly negatively associated with breast cancer recurrence with adjusted HRs 3rd vs. 1st quartile of 0.15 (95% CI 0.04–0.51, p-value = 0.003). After performing Bonferroni correction for eight phthalate comparisons, only MEOHP was still significant (p-value = 0.024; 0.003 multiplied by eight phthalate comparisons). Furthermore, the dose–response curve of MEOHP presented as non-monotonic dose–response (NMDR), which was U-shaped.
The interaction effect on breast cancer recurrence
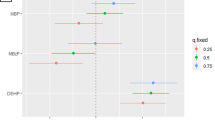
The effect of MEOHP on the risk of breast cancer recurrence stratified by the status of ER, PR, and HER2 as well as BMI were illustrated as in Table 3. Compared to the first quartile, the third quartile of MEOHP presented the lowest breast cancer recurrence risk in ER-positive status (aHR 0.18, 95% CI 0.05–0.66, p-value = 0.009), PR-negative status (aHR 0.14, 95% CI 0.03–0.63, p-value = 0.011), and HER2-negative status (aHR 0.24, 95% CI 0.08–0.76, p-value = 0.015). The dose–response curves of MEOHP still presented as U-shaped (Supplementary Fig. S1). The association between MEOHP and recurrent breast cancer was significantly modified by BMI (pinteraction = 0.042). Compared to the first quartile, the third quartile of MEOHP presented the lowest breast cancer recurrence risk in BMI < 25 (aHR 0.25, 95% CI 0.07–0.93, p-value = 0.039) and MEOHP was still negatively associated with recurrent breast cancer in BMI ≥ 25 (aHR 0.11, 95% CI 0.01–0.92, p-value = 0.041). There was a negative interaction effect on an additive scale between MEOHP and BMI on recurrent breast cancer risk (RERIHR = − 1.97).
Discussion
This study is the first patient cohort design to explore the interaction between phthalate exposure and hormone receptors as well as BMI on recurrent breast cancer. It is suggested that MEOHP were negatively associated with recurrent breast cancer and these effects were modified by hormone receptors and BMI. MEOHP was associated with the lowest breast cancer recurrence risk in the status of ER-positive, PR-negative, and HER2-negative (molecular subtype of breast cancer: luminal A) and the dose–response was U-shaped. The effects of EDCs have often been observed as an NMDR curve in animal, cell culture, and epidemiological studies27. EDCs have different mechanisms due to different doses, including cell and tissue specificity, receptor selectivity, receptor competition, and negative feedback27,28. An animal study found that adult mice exposed to BPA resulted in mammary cancer and displayed an NMDR curve29. An epidemiological study also found an inverted U-shaped association between diabetes and persistent organic pollutants30.
Breast cancer patients with hormone receptor positive (HR+)/HER2− subtype (approximating luminal A subtype) had the best survival pattern of 4-year breast cancer-specific survival31. Luminal B, HER2‐enriched, and triple‐negative tumors had higher recurrence risk and bad prognosis compared with luminal A tumors32,33. A Poland population-based case–control study reported that exposure to organic solvents was positively associated with breast cancer and might be limited to ER−/PR− cases34, but little heterogeneous effect by ER status between most phthalate metabolites and breast cancer was observed13, which was similar to our study. Few epidemiological studies are available to explore the association between recurrent breast cancer and molecular subtypes in relation to phthalate exposure. The present study suggests that exposure to MEOHP might be associated with decreasing breast cancer recurrence risk, especially in the status of ER-positive, PR-negative, and HER2-negative.
Phthalates can be added in personal care products to hold color and fix fragrance, and women are exposed to phthalates through usage of such products35. DEHP, as a plasticizer to increase flexibility, is present in medical devices and construction material36,37. Compared to nonusers, the concentration of MiBP is higher in users of perfume35,38 and MEOHP is higher in bottled water38. Our previous study found that MEHHP, MECPP, MEOHP, and Σ4DEHP were positively associated with increased intron 1 methylation level and gene expression in ADAM33 gene and the phenomenon may be related to decreased breast cancer risk39. Two studies and a multiethnic cohort study in whites observed an non-significant negative association between MEOHP and breast cancer13,14,40; a prospective study and an Alaska study showed that MEOHP were not associated with breast cancer12,15. A cross-sectional study in the National Health and Nutrition Examination Survey (NHANES) also found that phthalate metabolites were not significantly associated with breast cancer41, but MEOHP was related to male-specific thyroid cancer16, and MEHHP, MEOHP, MECPP, MCMHP, MBzP, MiBP, and MnBP were also associated with prostate cancer in a waist ≥ 90 cm subgroup17; however, four studies observed that MBzP was negatively associated with breast cancer risk12,13,14,40. So far, the inconsistent results of these studies were still unclear, and this could explain the different results from study designs, races, populations, cultures, environmental, dietary habits, and even different diseases.
The mechanisms of phthalates are nothing more than peroxisome proliferator-activated receptors (PPARs), DNA methylation, and DMA damage. PPARs have three isoforms identified: PPARα, PPARβ/δ, and PPARγ. PPARs play an important role in lipid metabolism and are involved in cellular differentiation and carcinogenesis42. PPARγ has significant expression in human mammary adenocarcinomas and reduces the tumor growth of malignant breast epithelial cells43. It is associated with longer breast cancer-specific survival and expresses more in luminal ER-positive tumors, suggesting that PPARγ is a better marker for prognosis in luminal breast cancer patients44. MEHP and MEOHP bonded to the ligand binding pocket of PPARγ and produced interaction as confirmed by reporter gene assay45; therefore, the negative correlation between MEOHP and breast cancer recurrence might be caused by the combination of MEOHP and PPARγ, resulting in reduced risk of breast cancer recurrence in our study. PPARα and PPARγ have opposite effects on human breast cells. PPARα causes the proliferation of breast cells; however, PPARγ inhibits cell proliferation. MEHP activates both human PPARα and PPARγ, but not PPARβ. However, MnBP cannot activate any PPAR isoform, but MnBP is an antagonist against both human PPARβ and PPARγ46. Because different phthalates have different mechanisms on different PPARs, this leads to different growth effects on breast cells.
Studies have suggested that the additive scale could better reflect biologic interaction and is important for public health interventions in a prospective study24,25. In our study, there was a negative interaction on an additive scale between MEOHP and BMI on recurrent breast cancer. The prevalence of obesity is a global problem and the phthalate exposure, a EDCs, might be one of the reasons for obesity47,48. A meta-analysis study indicated that high heterogeneity between studies for MEOHP and obesity was insufficient to perform meta-analysis; however, the association between MEOHP and obesity was positive in most adult and child studies, but there was little statistical significance49. The study found adipocyte in culture medium accumulated MEHP50 and individuals undergoing weight loss had higher levels of MEP51. The negative association between some phthalates and obesity could be explained by the cumulative capacity and lipophilic capacity of phthalates in adipose tissue52. A large study also showed that the association between breast cancer-specific mortality and MEOHP was modified by BMI13. In our study, whether in BMI < 25 or in BMI ≥ 25, MEOHP was negatively associated with recurrent breast cancer. However, few studies have explored the association between recurrent breast cancer, phthalate exposure, and obesity. For further analysis in the future, the obesity indicators, including BMI, waist circumference, waist-hip ratio, and body fat ratio, should be considered while sample size should be expanded for exploring the association between phthalate exposure, obesity, and recurrent breast cancer.
In sociodemographic characteristics, we found a higher frequency of being single/unmarried in recurrent breast cancer patients. Some studies have found that married breast cancer patients have lower mortality rate than unmarried patients53,54. Compared to married women, unmarried women were 1.18-times more likely to have later stage breast cancer at breast cancer diagnosis55. Furthermore, married patients were more likely to receive chemotherapy and radiotherapy than those unmarried53,54. Two epidemiological studies on pregnant women also found that the concentrations of certain phthalates were higher in unmarried Caucasian women relative to those married56. Being unmarried might also be a risk factor for recurrent breast cancer due to lack of care and companionship, and having some undesirable living habits; therefore, further study is needed to explore the relationship between recurrent breast cancer and the sources of phthalate exposure, such as environmental exposure and the use of phthalate products.
Our study has some limitations. Firstly, only a single-spot morning urine was used to evaluate phthalate exposures; however, there were no differences between two consecutive first-morning urine tests, suggesting that the phthalate exposure patterns of women were relatively stable57. In addition, phthalates have short biological half-lives and metabolize quickly within 24 h58. Previous studies have indicated that the collection of multiple spot urine samples was more conducive to assess most complete phthalate exposures compared with a single urine measurement59. The within-person reproducibility of most phthalate metabolites was modest, so the results would tend to be attenuated60. In our prospective patient cohort design, due to the non-differential misclassification of phthalate exposure, the association between phthalate exposure and recurrent breast cancer may be underestimated. Secondly, the questionnaire did not include environmental exposure issues, including the use of plastic containers and personal care products. However, the concentrations of phthalate metabolites in urine already represented the phthalate exposures of the body from various sources. Thirdly, since only one-time measurement of phthalate exposures and disease outcome was performed, it was difficult to explore the association between phthalate exposure and recurrent breast cancer; however, in our patient cohort study, we excluded patients who already had recurrence and followed up new patients to recurrence status. Fourthly, although the number of new recurrent samples was small, it could explore the temporary association between the risk of breast cancer recurrence and phthalate exposure in this patient cohort design.
Our study is the first pioneer patient cohort study to assess the interaction between phthalate exposure and hormone receptors as well as BMI on recurrent breast cancer. We found that the negative association between MEOHP and breast cancer recurrence was modified by the hormone receptors and BMI. There was also a negative interaction between MEOHP and BMI on recurrent breast cancer. From a future perspective, it is necessary to recruit large sample sizes and new recurrent breast cancer patients to understand how phthalate exposure could affect the recurrence and prognosis, while the biological mechanism between phthalate exposure and recurrent breast cancer also warrants exploration.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Abbreviations
- IARC:
-
International Agency for Research on Cancer
- MOHW:
-
Ministry of Health and Welfare
- EDCs:
-
Endocrine-disrupting chemicals
- BMI:
-
Body mass index
- KMU:
-
Kaohsiung Medical University
- IRB:
-
Institutional Review Board
- WHO:
-
World Health Organization
- IHC:
-
Immunohistochemistry
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- TNM stage:
-
TNM classification of malignant tumors
- MEP:
-
Mono-ethyl phthalate
- MnBP:
-
Mono-n-butyl phthalate
- MiBP:
-
Mono-isobutyl phthalate
- MEHP:
-
Mono-2-ethylhexyl phthalate
- MEHHP:
-
Mono-(2-ethyl-5-hydroxyhexyl) phthalate
- MECPP:
-
Mono-(2-ethyl-5-carboxypentyl) phthalate
- MEOHP:
-
Mono-(2-ethyl-5-oxohexyl) phthalate
- MMP:
-
Mono-methyl phthalate
- MBzP:
-
Mono-benzyl phthalate
- MCPP:
-
Mono-(3-carboxypropyl) phthalate
- ESI:
-
Electrospray ionization
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- LOD:
-
Limit of detection
- CV:
-
Coefficient of variation
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- RERI:
-
Relative excess risk due to interaction
- NMDR:
-
Non-monotonic dose–response
- NHANES:
-
National Health and Nutrition Examination Survey
- PPARs:
-
Peroxisome proliferator-activated receptors
References
Wild, C. P., Weiderpass, E. & Stewart, B. W. World Cancer Report: Cancer Research for Cancer Prevention (International Agency for Research on Cancer, 2020).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Dent, R. et al. Factors associated with breast cancer mortality after local recurrence. Curr. Oncol. 21, e418–e425. https://doi.org/10.3747/co.21.1563 (2014).
Sirohi, B., Leary, A. & Johnston, S. R. Ipsilateral breast tumor recurrence: Is there any evidence for benefit of further systemic therapy? Breast J. 15, 268–278. https://doi.org/10.1111/j.1524-4741.2009.00716.x (2009).
Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019 (Centers for Disease Control and Prevention, 2019).
Sicinska, P. Di-n-butyl phthalate, butylbenzyl phthalate and their metabolites induce haemolysis and eryptosis in human erythrocytes. Chemosphere 203, 44–53. https://doi.org/10.1016/j.chemosphere.2018.03.161 (2018).
Wallner, P., Kundi, M., Hohenblum, P., Scharf, S. & Hutter, H. P. Phthalate metabolites, consumer habits and health effects. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph13070717 (2016).
Benjamin, S. et al. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard Mater. 340, 360–383. https://doi.org/10.1016/j.jhazmat.2017.06.036 (2017).
Key, T. J., Verkasalo, P. K. & Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2, 133–140. https://doi.org/10.1016/s1470-2045(00)00254-0 (2001).
Zuccarello, P. et al. Implication of dietary phthalates in breast cancer. A systematic review. Food Chem. Toxicol. 118, 667–674. https://doi.org/10.1016/j.fct.2018.06.011 (2018).
Sheikh, I. A. et al. Endocrine disruption: In silico perspectives of interactions of di-(2-ethylhexyl)phthalate and its five major metabolites with progesterone receptor. BMC Struct. Biol. 16, 16. https://doi.org/10.1186/s12900-016-0066-4 (2016).
Reeves, K. W. et al. Urinary phthalate biomarker concentrations and postmenopausal breast cancer risk. J. Natl. Cancer Inst. 111, 1059–1067. https://doi.org/10.1093/jnci/djz002 (2019).
Parada, H. Jr. et al. Urinary phthalate metabolite concentrations and breast cancer incidence and survival following breast cancer: The Long Island Breast Cancer Study Project. Environ. Health Perspect. 126, 047013. https://doi.org/10.1289/ehp2083 (2018).
Lopez-Carrillo, L. et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 118, 539–544. https://doi.org/10.1289/ehp.0901091 (2010).
Holmes, A. K. et al. Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska Native women. Int. J. Circumpolar Health 73, 25760. https://doi.org/10.3402/ijch.v73.25760 (2014).
Liu, C. et al. Urinary biomarkers of phthalates exposure and risks of thyroid cancer and benign nodule. J. Hazard Mater 383, 121189. https://doi.org/10.1016/j.jhazmat.2019.121189 (2020).
Chuang, S. C. et al. Phthalate exposure and prostate cancer in a population-based nested case-control study. Environ. Res. 181, 108902. https://doi.org/10.1016/j.envres.2019.108902 (2020).
Silva, M. J. et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 77, 561–567. https://doi.org/10.1007/s00204-003-0486-3 (2003).
Hines, E. P., Calafat, A. M., Silva, M. J., Mendola, P. & Fenton, S. E. Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ. Health Perspect. 117, 86–92. https://doi.org/10.1289/ehp.11610 (2009).
Calafat, A. M. et al. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ. Health Perspect. 123, A166-168. https://doi.org/10.1289/ehp.1510041 (2015).
Engel, S. M. & Wolff, M. S. Causal inference considerations for endocrine disruptor research in children’s health. Annu. Rev. Public Health 34, 139–158. https://doi.org/10.1146/annurev-publhealth-031811-124556 (2013).
Yaghjyan, L., Sites, S., Ruan, Y. & Chang, S. H. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int. J. Obes. (Lond.) 39, 994–1000. https://doi.org/10.1038/ijo.2015.8 (2015).
Starling, A. P. et al. Predictors and long-term reproducibility of urinary phthalate metabolites in middle-aged men and women living in urban Shanghai. Environ. Int. 84, 94–106. https://doi.org/10.1016/j.envint.2015.07.003 (2015).
Knol, M. J., van der Tweel, I., Grobbee, D. E., Numans, M. E. & Geerlings, M. I. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 36, 1111–1118. https://doi.org/10.1093/ije/dym157 (2007).
Rod, N. H., Lange, T., Andersen, I., Marott, J. L. & Diderichsen, F. Additive interaction in survival analysis: Use of the additive hazards model. Epidemiology 23, 733–737. https://doi.org/10.1097/EDE.0b013e31825fa218 (2012).
VanderWeele, T. J. Causal interactions in the proportional hazards model. Epidemiology 22, 713–717. https://doi.org/10.1097/EDE.0b013e31821db503 (2011).
Vandenberg, L. N. et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. https://doi.org/10.1210/er.2011-1050 (2012).
Dutta, S., Haggerty, D. K., Rappolee, D. A. & Ruden, D. M. Phthalate exposure and long-term epigenomic consequences: A review. Front. Genet. 11, 405. https://doi.org/10.3389/fgene.2020.00405 (2020).
Jenkins, S., Wang, J., Eltoum, I., Desmond, R. & Lamartiniere, C. A. Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ. Health Perspect. 119, 1604–1609. https://doi.org/10.1289/ehp.1103850 (2011).
Lee, D. H. et al. Low dose of some persistent organic pollutants predicts type 2 diabetes: A nested case-control study. Environ. Health Perspect. 118, 1235–1242. https://doi.org/10.1289/ehp.0901480 (2010).
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 27, 619–626. https://doi.org/10.1158/1055-9965.Epi-17-0627 (2018).
Gao, J. J. & Swain, S. M. Luminal A breast cancer and molecular assays: A review. Oncologist 23, 556–565. https://doi.org/10.1634/theoncologist.2017-0535 (2018).
Chen, J. et al. The efficacy of molecular subtyping in predicting postoperative recurrence in breast-conserving therapy: A 15-study meta-analysis. World J. Surg. Oncol. 12, 212. https://doi.org/10.1186/1477-7819-12-212 (2014).
Peplonska, B. et al. Occupational exposure to organic solvents and breast cancer in women. Occup. Environ. Med. 67, 722–729. https://doi.org/10.1136/oem.2009.046557 (2010).
Parlett, L. E., Calafat, A. M. & Swan, S. H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo Sci. Environ. Epidemiol. 23, 197–206. https://doi.org/10.1038/jes.2012.105 (2013).
Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 29, 134–139. https://doi.org/10.1111/j.1365-2605.2005.00567.x (2006).
Sampson, J. & De Korte, D. DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 21, 73–83. https://doi.org/10.1111/j.1365-3148.2010.01056.x (2011).
Romero-Franco, M. et al. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ. Int. 37, 867–871. https://doi.org/10.1016/j.envint.2011.02.014 (2011).
Yang, P. J. et al. Breast cancer is associated with methylation and expression of the a disintegrin and metalloproteinase domain 33 (ADAM33) gene affected by endocrinedisrupting chemicals. Oncol. Rep. 40, 2766–2777. https://doi.org/10.3892/or.2018.6675 (2018).
Wu, A. H. et al. Urinary phthalate exposures and risk of breast cancer: The Multiethnic Cohort study. Breast Cancer Res. 23, 44. https://doi.org/10.1186/s13058-021-01419-6 (2021).
Morgan, M., Deoraj, A., Felty, Q. & Roy, D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol. Cell Endocrinol. 457, 89–102. https://doi.org/10.1016/j.mce.2016.10.003 (2017).
Berger, J. & Moller, D. E. The mechanisms of action of PPARs. Annu. Rev. Med. 53, 409–435. https://doi.org/10.1146/annurev.med.53.082901.104018 (2002).
Mueller, E. et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol. Cell 1, 465–470. https://doi.org/10.1016/s1097-2765(00)80047-7 (1998).
Abduljabbar, R. et al. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res. Treat. 150, 511–522. https://doi.org/10.1007/s10549-015-3348-9 (2015).
Kratochvil, I. et al. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor gamma. Rapid Commun. Mass Spectrom. 33(Suppl 1), 75–85. https://doi.org/10.1002/rcm.8258 (2019).
Venkata, N. G. et al. Mono(2-ethylhexyl)phthalate and mono-n-butyl phthalate activation of peroxisome proliferator activated-receptors alpha and gamma in breast. Toxicol. Lett. 163, 224–234. https://doi.org/10.1016/j.toxlet.2005.11.001 (2006).
Hruby, A. & Hu, F. B. The epidemiology of obesity: A big picture. Pharmacoeconomics 33, 673–689. https://doi.org/10.1007/s40273-014-0243-x (2015).
Baillie-Hamilton, P. F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement Med. 8, 185–192. https://doi.org/10.1089/107555302317371479 (2002).
Ribeiro, C. et al. Association between the exposure to phthalates and adiposity: A meta-analysis in children and adults. Environ. Res. 179, 108780. https://doi.org/10.1016/j.envres.2019.108780 (2019).
Chiang, H. C. et al. Mono(2-ethylhexyl)phthalate accumulation disturbs energy metabolism of fat cells. Arch. Toxicol. 90, 589–601. https://doi.org/10.1007/s00204-014-1446-9 (2016).
Dirtu, A. C. et al. Phthalate metabolites in obese individuals undergoing weight loss: Urinary levels and estimation of the phthalates daily intake. Environ. Int. 59, 344–353. https://doi.org/10.1016/j.envint.2013.06.023 (2013).
Jackson, E., Shoemaker, R., Larian, N. & Cassis, L. Adipose tissue as a site of toxin accumulation. Compr. Physiol. 7, 1085–1135. https://doi.org/10.1002/cphy.c160038 (2017).
Liu, Y. L. et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: An analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res. Treat. 178, 379–388. https://doi.org/10.1007/s10549-019-05385-8 (2019).
Martinez, M. E. et al. Prognostic significance of marital status in breast cancer survival: A population-based study. PLoS ONE 12, e0175515. https://doi.org/10.1371/journal.pone.0175515 (2017).
Hinyard, L., Wirth, L. S., Clancy, J. M. & Schwartz, T. The effect of marital status on breast cancer-related outcomes in women under 65: A SEER database analysis. Breast 32, 13–17. https://doi.org/10.1016/j.breast.2016.12.008 (2017).
Wenzel, A. G. et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193, 394–402. https://doi.org/10.1016/j.chemosphere.2017.11.019 (2018).
Hoppin, J. A., Brock, J. W., Davis, B. J. & Baird, D. D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect. 110, 515–518. https://doi.org/10.1289/ehp.02110515 (2002).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP) (Agency for Toxic Substances and Disease Registry, 2019).
Preau, J. L. Jr., Wong, L. Y., Silva, M. J., Needham, L. L. & Calafat, A. M. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: An observational study. Environ. Health Perspect. 118, 1748–1754. https://doi.org/10.1289/ehp.1002231 (2010).
Townsend, M. K., Franke, A. A., Li, X., Hu, F. B. & Eliassen, A. H. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: A prospective cohort study. Environ. Health 12, 80. https://doi.org/10.1186/1476-069x-12-80 (2013).
Acknowledgements
We would like to thank all the subjects who participated in this study and the staffs in the Center for Research Resources and Development in KMU for helping us perform the experiments of phthalate metabolites examination.
Funding
The work was supported by the National Science Council of Taiwan (NSC 102-2632-B-037-001-MY3), and partially from the Kaohsiung Medical University ‘Aim for the Top Universities Grant’ (KMU-TP103A16, KMU-TP104A01, and KMU-TP105A05). The funding sources had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
P.J.Y.: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. M.F.H.: Investigation, Resources. F.O.Y.: Investigation, Resources. T.H.H.: Project administration. Y.J.L.: Investigation. E.M.T.: Funding acquisition, Resources, Supervision. T.N.W.: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, PJ., Hou, MF., Ou-Yang, F. et al. Association between recurrent breast cancer and phthalate exposure modified by hormone receptors and body mass index. Sci Rep 12, 2858 (2022). https://doi.org/10.1038/s41598-022-06709-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06709-3
This article is cited by
-
Prospective association between phthalate exposure in childhood and liver function in adolescence: the Ewha Birth and Growth Cohort Study
Environmental Health (2023)
-
Association between phthalate metabolites in human amniotic fluid and offspring birth size: a sub-study of the PERSIAN birth cohort
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.