Abstract
Stingless bees are the largest group of eusocial pollinators with diverse natural histories, including obligate cleptobionts (genus Lestrimelitta) that completely abandoned flower visitation to rely on other stingless bees for food and nest materials. Species of Lestrimeliita are thought to specialize upon different host species, and deception through chemical similarity has been proposed as a mechanism to explain this phenomenon. In the Yucatan Peninsula of Mexico, Scaptotrigona pectoralis is a species chemically distinct from, and not preferred as a host by, locally widespread Lestrimeliita niitkib; witnessing attacks on S. pectoralis colonies offered the opportunity to test the sensory deception hypothesis to cletoparasitism. Analysis of cuticular profiles revealed that the Lestrimelitta attacking S. pectoralis differed significantly in odour bouquet to L. niitkib and, in contrast, it resembled that of S. pectoralis. Further analyses, including morphometrics, mtDNA barcoding, and the examination of taxonomic features, confirmed the existence of two sympatric Lestrimelitta species. The results give support to the hypothesis of chemical deception as a cleptobiotic strategy in Lestrimelitta sp. This is the first evidence that sympatric cleptobionts of the same genus select hosts in accordance with species-specific cuticular profiles, with possible consequences for ecological adaptation and the evolution of these remarkable organisms and the community of stingless bee hosts.
Similar content being viewed by others
Introduction
Cleptobiosis is the behavior of stealing food, or sometimes nesting materials or other items of value, either from members of the same or a different species1. Given its obvious advantages, facultative cleptobiosis is widespread in the animal kingdom with most animal species occassionaly robbing food from conspecifics and heterospecifics. In contrast, obligate cleptobiosis is very rare, perhaps because it entails extreme specialization in a narrow niche and can only evolve under very specific circumstances1. Obligate highly eusocial cleptobionts have been found only in the Tribe Meliponini or stingless bees2.
The stingless bees are the most diversified group of highly eusocial bees encompassing over 500 species that are the predominant pollinators in the tropics worldwide3. One contrasting trait of stingless bees compared with the honey bee (Tribe Apini), the other group of highly eusocial bees, is its wide variation in morphology and lifestyles, sometimes with extreme adaptations4, among these the evolution of obligate cleptobiosis. Obligate cleptobiotic stingless bees are presently classified in two genera, the Paleotropical Cleptotrigona Moure, 1961, with one known species and the Neotropical Lestrimelitta Friese, 1903 with over two dozen species5. Cleptobiotic stingless bees represent a rare example of a highly specialized adaptation (including changes in morphology) as a consequence of having completely abandoned the collection of pollen, nectar and resins from plants to thrive exclusively on the robbing of resources and nest materials from other non-cleptiobiotic species of stingless bees2.
An interesting feature of cleptobiotic stingless bees is their preference for certain host stingless bee species to rob and, likewise, there are many potential host stingless bee species that are seldom or never raided. Moreover, different species seem to prey upon different hosts2,6,7,8,9. The mechanisms that Lestrimelitta use to overcome colony defenses and to select certain host species over others are poorly understood, although there are two basic strategies that cleptobionts can use to enter a target colony: force and chemical deception2,10. Because of its readily noticeable raids, it has been generally accepted that force and the use of citral (and other mandibular propaganda pheromones) may be the predominant mechanisms of host exploitation by Lestrimelitta2,9. However, recently, evidence for the possible role of chemical deception was obtained for L. niitkib of the Yucatan Peninsula11. Comparing the cuticular profiles of L. niitkib with its hosts has revealed similarity in the alkene fragment with some of L. niitkib’s preferred species (namely, Nannotrigona perilampoides Cresson, 1878 and Plebeia spp.) but a significantly distant profile with non-preferred ones like Melipona (Melikerria) beecheii Bennett, 1831 and Scaptotrigona pectoralis (Dalla Torre, 1896)11. Furthermore, potential host species with chemically distant profiles reacted more rapidly to the presence of Lestrimelitta workers in their colony (but not so chemically similar hosts), presumably because their different profiles allowed a fast detection of the intruder. This evidence suggested that sensory deception may be relevant in the selection and invasion of hosts by cleptobiotic stingless bees11.
Cuticular hydrocarbons (CHCs) are well known to play an important role in social insects12. Insect CHCs represent the dominant fraction of the waxy lipid layer located on insect epicuticle. The original role of CHCs is thought to be protection of the insect against desiccation13. However, CHCs secondarily evolved as important cues in chemical communication, most prominently in intra and inter specific recognition14. In stingless bees, unsaturated cuticular hydrocarbons, alkenes and alkadienes seem to be the main compounds responsible for nestmate recognition15. Because of their different ecological properties and specificity, CHCs have been proposed as candidate traits through which ecological adaptation could lead to selective mating and reproductive isolation16,17,18.
Long-term records of the incursion of L. niitkib on stingless bee yards have indicated that S. pectoralis is never attacked and that, on the contrary, cleptobiont colonies can be killed by the latter7,19. Given this background information, witnessing successful attacks of Lestrimelitta on S. colonies in the Eastern part of the Yucatan Peninsula was, thus, highly unusual and offered us the opportunity to test predictions of the hypothesis of sensory deception though chemical similarity. In addition, it allowed for a better understanding of the relative importance of chemical deception in Lestrimelitta ecology and, ultimately, to ask whether cuticular cues matter in the evolution of eusocial stingless bee cleptobionts and their hosts.
Materials and methods
Collection of biological material
Two separate attacks of Lestrimelitta sp. were witnessed on two different colonies of S. pectoralis kept in a stingless bee yard in the locality of Felipe Carrillo Puerto, Quintana Roo (Q Roo), Yucatan Península (19°35′02″ N 88°02′13″ W) (Supplementary files, Fig. S1 online). At each attack, two samples of arriving Lestrimelitta sp. workers were collected. One sample was used for chemical Gas Chromatography and Mass Spectrometry (GCMS) and consisted of the extracts from three individual workers that had their legs previously removed to avoid contamination with resin/pollen products, and that were sequentially submerged for 1 min in glass vials (Agilent Tech.) containing 500 µl of hexane. Another sample of at least ten workers was collected in Eppendorf tubes containing ethanol for morphometric analyses and DNA barcoding. In addition to the samples of attacking Lestrimelitta, we also collected the same type of samples and number of workers from five S. pectoralis colonies (including the two raided colonies) and from five N. perilampoides colonies (the preferred host species of L. niitkib) from QRoo. Host species and identity are well documented for L. niitkib in the state of Yucatan where attacks on S. pectoralis have not been witnessed7,11. As controls, we collected samples from Mérida in the neighboring state of Yucatan (Supplementary files, Fig. S1 online), from five colonies of the two same putative host species using similar procedures and number of individuals. Samples from five colonies of L. niitkib were also collected in Yucatan as well as from QRoo using the same methods. All samples were kept at -20 °C until further analyses.
Comparison of cuticular profiles
To compare the cuticular profiles of the different species and localities we used GC–MS. For this, one microliter of each extract in hexane was placed in the inlet port of an Agilent Technologies gas chromatograph (7890) coupled with a mass spectrometer (5975C). The inlet in splitless mode was set at 300 °C (splitless time, 1.5 min). A fused-silica column HP-5MS (5% phenyl-95% polydimethylsiloxane; 30 m, 0.25 mm ID, 0.25 µm) was employed with helium (purity 99.99) carrier gas at 1.0 mL/min. The oven program started at 40 °C and reached 300 °C at 10 °C/min with a holding of 13 min. The eluent was transferred into the MS detector via a transfer line held at 300 °C with 3 min of solvent delay. Typical conditions of MS detector were optimized through the autotune software option. The electron impact mode (70 eV) was used as an ionization source (230 °C) and masses were monitored between 25 and 525 m/z. The temperature of the quadrupole was 150 °C. The total analysis time was 39 min.
The relative contribution of each cuticular hydrocarbon (alkanes and alkenes) was calculated based on the average ion current peak areas obtained for colonies of each species. The assignment of chromatographic peaks was accomplished by comparison of the experimental mass spectra with the spectra in the NIST data base20. Also, retention indices were obtained for each peak using reference samples containing n-alkanes (C10 to C30).
We focused further analyses on unsaturated cuticular hydrocarbons, which are known to serve as recognition cues in stingless bees15.
To test for chemical similarity between Lestrimelitta sp. with L. niitkib and potential hosts, we submitted the data of peak areas of each unsaturated hydrocarbon (all 14 alkene isomers detected) to a principal component analysis (PCA). We then used these new PCs as variables in separate analyses to establish relationships between groups21. PCA scores were calculated for the first three components that included the largest amount of variation in the data. We also compared the scores among species and localities using a GLM analysis (with Bonferroni correction)22. The scores for the first two components were also used to produce plots of the corresponding values for each colony and species onto a bidimensional scale.
Morphometric comparisons
For the comparison of size and shape of the samples collected from different species and localities, we analyzed the morphometrics of meristic characters and the geometry of the forewing.
For comparison of meristic characters, the head, thorax, right forewing and right hind leg of ten workers from each sample were dissected and mounted on slides using routine procedures. The structures were photographed using a Leica S8 APO microscope and four characters related to bee size were measured using ImageJ23 on each worker: head width, intertegular distance, forewing length, and femur length24. A General Linear Model (GLM) in the statistical software SAS22 was used to compare the size of the different structures between species and localities. Additionally, a PCA was used to obtain parameters of overall body size combining the four meristic traits measured on individual bees. The resulting coefficients for the first three PCs were used to calculate scores as individual measures of body size and were compared using a GLM in SAS22.
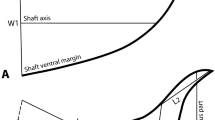
The shape and size of the forewing was also compared between Lestrimelitta sp. and L. niitkib using geometric morphometrics. Twelve intersections of the forewing veins (Supplementary files, Fig. S2 online), were established as homologous landmarks using tpsDig2 software version 2.1225. The coordinates of the landmarks were Procrustes fitted to evaluate existing shape variation using the software MorphoJ version 1.07a26. Within the MorphoJ software, further statistical computations including Procrustes ANOVA, Discriminant Function Analysis (DFA), Principal Component Analysis (PCA) and multivariate regression analyses were conducted to discriminate between bee types27.
DNA barcoding
Genetic differences among Lestrimelitta sp. and L. niitkib were analysed by comparing the DNA barcode region of the mitochondrial gene cytochrome oxidase I or Cox1. Barcoding is an accepted technique at discriminating species in almost all groups of animals28 and has been successfully applied for such purposes in stingless bees29. DNA was extracted from one individual per colony using a Chelex protocol and amplified with universal PCR primers for animal barcoding30 using standard methods as part of the CBol initiative to barcode the bees of the world31. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 1132. In short, sequences were aligned, the best model for sequence evolution tested, and then sequences were compared using neighbor joining (NJ) and maximum likelihood (ML) after correction for the best model: the Tamura 3-parameter model with rate variation among sites modelled using a discrete gamma distribution (T92 + G). Two sequences of Lestrimelitta danuncia from Costa Rica (BOLD database, kindly provided by L. Packer) were included as representative congeners as was one of the stingless bee Plebeia frontalis (downloaded from the NCBI database), as an outgroup.
Taxonomic identification
As a final step, specimens of Lestrimelitta sp. and L. niitkib, were compared using traits that are discriminatory in the taxonomy of bees of this genus, namely, the shape of the propodeal spiracle, length of the mesotibial spur, presence or absence of hairs on the body, and the length, density, and type of pubescence33,34. To date, two large species groups can be recognized within Lestrimelitta based on the shape of the propodeal spiracle: the exclusively South American L. limao (Smith, 1863) species group, which consists of species with an ovoid propodeal spiracle, 2–3 times longer than broad35,36, and the L. ehrhardti (Friese, 1931) species group found in both Central and South America, which are distinguished by an elongate propodeal spiracle, at least 4.6 times longer than broad35.
Results
Comparison of cuticular profiles
A variety of linear alkanes and alkenes were found in the studied species in the range of carbon lengths of C19 to C33 (Figs. 1 and 2). Alkadienes and branched alkanes were not detected in any species (Fig. 1 and Supplementary files Table S1 online). If more than one alkene isomer was present, as indicated by differences in the retention time, they were numbered accordingly (Supplementary files, Table S1 online). The comparison of whole chromatograms (Fig. 1) and the graphs of relative alkene proportion (Fig. 2) showed obvious differences between Lestrimelitta sp. and L. niitkib from both Yucatan and Q Roo.
Typical GC/MS chromatograms of cuticular extracts of stingless bee species and localities from the Yucatan Peninsula. (1) benzaldehyde, (2) Z-Citral, (3) E-Citral, (4) 2-tridecanone, (5) not identified, (6) 2-pentadecanone, (7) not identified, (8) methyl octadecanoate, (9) not identified, (10) tricosene, (11) tricosane, (12) pentacosene, (13) pentacosane, (14) heptacosene, (15) heptacosane, (16) nonacosene.
PCA further confirmed these differences. The first three components for the alkene based PCA encompassed 76% of the variance in the data, with PC1 alone explaining 42% of the variation (Supplementary files, Table S2 online). The compounds with highest correlation coefficients with PC1 were the three isomers of C25:1 followed by the three isomers of C23:1. For PC2, the compounds with highest correlations were the third isomer of C25:1 and the first isomer of C29:1 (Supplementary files, Table S3 online).
Colony scores were obtained by means of the corresponding coefficients for each PC and further compared by means of a GLM analysis (Supplementary files, Table S4 online). The results of both the GLM analysis and the plot of scores against PC1 and PC2 (Fig. 3) revealed significant statistical differences between Lestrimelitta sp. and L. niitkib from Yucatan and QRoo and also with N. perilampoides from both states. On the other hand, scores for PC1 of S. pectoralis from both Yucatan and QRoo were not different to Lestrimelitta sp. (Fig. 3, Supplementary files Table S4 online), confirming that their similarity was mainly due to C25:1 and C23:1. Lestrimelitta sp. and S. pectoralis did differ in PC2 scores, suggesting differentiation in other components of their alkene bouquet, namely the third isomer of C25:1 and the first isomer of C29:1 (Fig. 3) Chemical distances were also compared among the bee species and populations, which provided further support for the differences found in the PCA (Supplementary files, Fig. S3 online).
Morphometric comparisons
Given the chemical similarity between populations of L. niitkib from Yucatan and QRoo, individuals of this species from both localities were pooled in one group for morphometric comparison with Lestrimelitta sp. Comparison of the four individual meristic characters revealed two that showed significant differences in the length of the femur and forewing (Supplementary files, Table S5 online). For this PCA, the first three Components encompassed 95% of the variation in the data, with PC1 explaining 59% of the total variance (Supplementary files, Table S6 online). Head width had the highest correlation with PC1, while forewing length had the highest correlation with PC2 (Supplementary files, Table S7 online). Highly significant differences resulted when the scores of both Lestrimelitta species were compared for all three Components (Supplementary files, Table S5 online). The plot of individual scores against PC1 and PC2 confirmed the morphometric separation of Lestrimelitta sp. and L. niitkib along PC2, indicating that differences were due to the length of the forewing (Fig. 4).
Geometric morphometrics of the forewings confirmed these PCA-based results. The Procrustes ANOVA (Supplementary Files, Table S3) revealed significant differences in size; the forewings of Lestrimelitta sp. were significantly larger (centroid size: X̄ ± DS = 594.59 ± 8.09) compared with those of L. niitkib (centroid size: X̄ ± DS = 523.51 ± 16.03). The DFA also showed significant differences in the shape of the forewing (Procrustes distance = 0.034, T2 = 1086.87, P ≤ 0.001, permutation at 1000 iterations); mean shapes of forewings of both species are each displayed by a wireframe (Supplementary Fig. S3 online). Notably, the PCA showed that the majority of the shape variation in the forewings was explained in the first three dimensions, accounting for 71% of the total variance (variance explained: PC1 = 53%; PC2 = 12%; PC3 = 6%) (Fig. 5A). After allometric correction through the covariance matrix of the residuals of the regression, the shape variation decreased considerably, accounting for 51% of the total variance (variance explained: PC1 = 25%; PC2 = 17%; PC3 = 9%) (Fig. 5B). This multivariate regression showed high allometric influence of 39.18% (p ≤ 0.0001), meaning that most of the difference in shape between the forewings of both species is due to their size.
DNA barcoding
The sequencing of the Cox 1 region of mtDNA generated a final dataset of 603 bases, and allowed assignment of the samples of Lestrimelitta from the Yucatán Peninsula according to their genetic similarity to species already in the BOLD database37. The resulting NJ tree assigned the specimen of L. niitkib as expected, together (Fig. 6). However, the specimen of Lestrimelitta sp. was not assigned to the latter branch, but to L. danuncia, a species not yet reported in Mexico38, though we note weak bootstrap support for the branch (see also ML tree in supplementary file Fig. S4 online).
NJ tree obtained from the analysis of the fragment Cox 1 of the mt DNA of Lestrimelitta specimens from the Yucatán Peninsula (Yuc Yucatan State; QRoo Quintana Roo State) and from Costa Rica (Lestrimelitta danuncia), with Plebeia frontalis as outgroup. Values show bootstrap branch support (500 replicates). The bar represents nucleotide sequence divergence.
The two Lestrimelitta sp. from QRoo were identical, 0 bp difference. The two L. danuncia samples had 10 pb differences (variability within the species = 10/603 or 0.017). The two Lestrimelitta sp. from QRoo differed from the two L. danuncia samples by on average 5 bp (divergence to danuncia = 5/603 or 0.008). In detail, the two Lestrimelitta sp. QRoo differed from the L. danuncia samples by 2 bp and 8 bp (i.e. (2 + 8)/2 = average 5 bp).
Taxonomic identification
The species performing the attacks on S. pectoralis in QRoo belongs to the Lestrimelitta species group with an elongated spiracle of the propodeum, the ehrhardti group of species33 (Supplementary files, Fig. S5 online). Currently 13 species are recognized within this group. Noteworthy, the two Lestrimelitta reported from Mexico belong to this group: L. chamelensis and L. niitkib38. However, both species have a greatly reduced mesotibial spur, in strong contrast with Lestrimelitta sp. which has an elongated one (Fig. 7). This bee has a long mesotibial spur, similar to L. danuncia (currently not reported from Mexico). In addition, there are subtle differences in the setae on the anterior edge of the mesoscutum between Lestrimelitta sp. and L. danuncia (data not shown). Such differences could represent regional variation or a new species, sister to L. danuncia, as also the barcoding suggests. Presently, Lestrimelitta sp. has only been found in QRoo, but it represents a new report for Mexico.
Discussion
In this study, the unusual attacks of Lestrimelitta to a seemingly non-preferred host provided the opportunity to test the relative importance of chemical deception in these obligate cleptobionts. GCMS analyses, complemented with genetic and taxonomic tools, led to the identification of an unreported species for México. Importantly, this is the first record of a chemical comparison between sympatric cleptobionts or cleptoparasites species within the same genus. The results, a) reveal that different species of Lestrimelitta resemble the alkene profiles of their preferred hosts, which implies that chemical deception seems to be common in these organisms, b) that the cleptobiont chemical profiles are species-specific and, c) that, in sympatric species, chemical profiles may relate to the differential selection of hosts, thus possibly serving to avoid resource competition and support niche separation. These results have several implications.
Our study extends the findings of chemical cleptobiont-host similarity found in L. niitkib to a possible new species of Lestrimelitta, adding support to the notion of chemical deception as a mechanism used by obligate stingless bee cleptobionts to select and invade their hosts. Chemical similarity between Lestrimelitta and its preferred hosts could help scouts to avoid nest guards without eliciting an aggressive response, a common strategy also used by other social parasites39,40. In contrast, species with chemically distant profiles react rapidly to Lestrimelitta intruders, which invariably leads to their elimination11, thus preventing attacks to their colonies. However, in chemically similar species, Lestrimelitta scouts may enter the host colony and collect information for the recruitment of nestmates.
Noteworthy, Lestrimelitta does not show an exact cuticular profile (chemical crypsis) with that of their host, as occurs in cleptoparasites that live inside their host’s nest41,42,43. Thus, this may be better classified as a case of chemical masquerade43. The relationship of Lestrimelitta with its hosts is temporary, and chemical deception probably becomes only useful to avoid initial detection during the first stages of their raids. Under this scenario, evolving towards exact mimicry of a single host (crypsis) would probably be too costly to the already specialized narrow niche of obligate cleptobiosis in a highly eusocial organism1. Instead, it would be more advantageous to own a profile resembling several hosts, which could help to better deal in case of changes in host diversity and abundance11.
One key aspect in the dynamics of this system is understanding how Lestrimelitta comes to chemically resemble their hosts. It has been suggested that chemical similarities found between L. niitkib and its preferred hosts, N. perilampoides and Plebeia, may derive from phylogenetic relatedness11. Alternatively, like other cleptoparasites, Lestrimelitta could produce cues that match the host's chemical profile44, or acquire them by exposure to the nest or host environment45.
Presently, it is difficult to propose a clear origin of the cuticular compounds used in host deception, but since Lestrimelitta uses the larval food and nest materials of their hosts, it is likely that both could serve as sources of chemical cues. Larval food shows contrasting variation among and within species of stingless bees, presumably derived from the different floral sources exploited46. However, when pollen of different sources was fed to adult Frieseomelitta stingless bees, no change of their cuticular profile occurred47. Likewise, larval food from different colonies used to rear gynes of S. pectoralis did not produce significant differences in the cuticular profiles of the emerging adults48. Although these experiments suggest that food may not be the origin of intraspecific CHC chemical differences in stingless bees, its effect in interspecific cross-fostering experiments (reproducing the model of Lestrimelitta-hosts) has not been analyzed.
The cerumen (a mix of beewax and plant resins) robbed by Lestrimelitta from host colonies to build their nest structures could also be a source of cuticular odours. Cerumen has been acknowledged as an important source of chemicals used in recognition by Frieseomelitta stingless bees. Individuals confined in contact with the cerumen of a foreign nest were quickly rejected when placed back in their own nest47. However, in Tetragonisca angustula, no significant rates of rejection occurred when individuals were put in contact with cerumen from their own or a foreign nest49. It is known that stingless bee species incorporate resin compounds in their own cuticular profile, thereby enriching their chemical diversity50. However, the bioassays performed so far are not conclusive and more work is needed so as to assess the role of cerumen and resin in stingless bee recognition.
Another way by which Lestrimelitta may acquire the host’s odours is by physical contact. Such a mechanism has been assumed in myrmecophilous insects, which, similarly to Lestrimelitta, only partially mimic their hosts40,51. In the course of nest attacks, Lestrimelitta comes into contact with hosts and host nest materials, making it possible to acquire odours of the raided species in the process. However, although the exchange of surface compounds through physical contact has been previously demonstrated among social insect nestmates52, evidence for its occurrence among parasites and hosts is limited53.
One important finding was that the cuticular profile of the recently identified Lestrimelitta sp. is qualitatively and quantitatively different to that of sympatric L. niitkib (in particular in the proportions of alkanes and alkenes). Distinct, species-specific chemotypes could prove useful in the chemotaxonomy of cleptobiotic bees54. Notably, the profiles of the two Lestrimelitta in this study closely resembled their specific hosts. The profile of L. niitkib resembled that of N. perilampoides, its preferred host11, while that of Lestrimelitta sp., resembled the profile of S. pectoralis, but it was different to N. perilampoides. This apparent selection of host species in relation to differences in the chemical profile of the cleptobionts is remarkable because, from a common strategy to overcome their host chemical detection systems, modifications could have evolved so that different species of Lestrimelitta chemically resemble their respective hosts. Thus, each sympatric species of cleptobiont might be chemically coevolving with a group of hosts. If so, cuticular cues involved in host selection could be key traits in the differentiation of Lestrimelitta species or chemotypes. In insects, adaptation to novel environments can involve changes in cuticular hydrocarbons that could lead to environmentally based divergent selection16,55. Mechanisms of sympatric speciation through intraspecific social parasitism have been proposed for the evolution of Hymenopteran workerless parasites56 and cryptic species divergence in ants57. Our model system could be driven by competition for resources among cleptobionts, i.e. host shifts. A parasite-host race emergence, as in other cases of sympatric speciation57,58,59, could have profound effects in the evolution of stingless bees.
In conclusion, by chemically exploiting species-specific hosts, sympatric cleptobionts may be under a type of ecological adaptation. If such adaptation has a genetic basis, it may eventually lead to their reproductive isolation60. In stingless bees, gene flow between populations could be further reduced because of their philopatric mode of reproduction and short colony dispersal, reinforcing genetic differentiation61.
A note should be made on the high abundance of citral in both species of Lestimelitta in this study. Propaganda substances produced in the mandibular and labial glands of the cleptobiont are known to have different effects on hosts, from disruption to retreat, and thus seems to play a key role in the process of host raiding2,62. Similarly, some host species seem to be raided by the use of sheer force2. Therefore, it is likely that adaptation to evade detection by chemical means could be one of various strategies which Lestrimelitta may use to exploit different species of stingless bee or at different stages during raids11.
Our study leaves many open questions which we hope will encourage investigation of the scarcely studied relationship between stingless bees and cleptobionts. Regions with a diversity of sympatric Lestrimelitta species may be ideal to test the chemical deception hypothesis. Empiric evidence is required on how chemical convergence between cleptobiont and host arises, as well as the role of different compounds, propaganda substances and their mixtures in deception. It is key to determine how chemical resemblance is acquired, and thus if there is an arms race between host and cleptobionts, which may differ under sympatry versus allopatry. Answers to these questions will help to deepen our knowledge of mutualistic interactions and species divergence, and to understand the importance of cleptobionts in the evolution and health of rich and varied stingless bee communities. This could improve their image as pests of stingless beekeeping and stop the destruction of their colonies61.
Data availability
JJGQE is the corresponding author from whom materials can be requested.
References
Breed, M.D., Cook, C. & Krasnec, M.O. Cleptobiosis in social insects. Psyche 484765 (2012).
Sakagami, S., Roubik, D. & Zucchi, R. Ethology of the robber stingless bee, Lestrimelitta limao (Hymenoptera: Apidae). Sociobiology 21, 237–277 (1993).
Rasmussen, C. & Cameron, S. A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Lin. Soc. 99, 206–232 (2010).
Roubik, D. W. Ecology and Natural History of Tropical Bees (Cambridge University Press, 1989).
Camargo, J. M. F. & Pedro, S. R. M. Meliponini Lepeletier, 1836. in Catalogue of the Bees (Hymenoptera, Apoidea) in the Neotropical Region (ed Moure, J. S.). 272–578. (Sociedade Brasileira de Entomologia, 2007).
Nogueira-Neto. P. Behavior problems related to the pillages made by some parasitic stingless bees (Meliponinae, Apidae). in Development and Evolution of Behavior: Essays in Memory of T.C. Schneirla (ed. Aronson, L.R.). 416–434. (W. H. Freeman, 1970).
Quezada-Euán, J. J. G. & González-Acereto, J. Notes on the nest habits and host range of cleptobiotic Lestrimelitta niitkib (Ayala 1999) (Hymenoptera: Meliponini) from the Yucatan Peninsula, Mexico. Acta Zool. Mexicana 86, 245–249 (2002).
Rech, A. R., Schwade, M. A. & Schwade, M. R. M. Abelhas-sem-ferrão amazônicas defendem meliponarios contra saques de outras abelhas. Acta Amazon. 43, 389–394 (2013).
Grüter, C., von Zuben, L. G., Segers, F. H. I. D. & Cunningham, J. P. Warfare in stingless bees. Insect. Soc. 63, 223–236 (2016).
Cini, A., Bruschini, C., Poggi, L. & Cervo, R. Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim. Behav. 81, 1139–1145 (2011).
Quezada-Euán, J. J. G. et al. Does sensory deception matter in eusocial obligate food robber systems? A study of Lestrimelitta and stingless bee hosts. Anim. Behav. 85, 817–823 (2013).
van Zweden, J. S. & D’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist, G. J. & Bagnères, A. G.) 222–243 (Cambridge University Press, 2010).
Blomquist, G. J. & Bagnères, A. G. Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology (Cambridge University Press, 2010).
Nash, D. R. & Boomsma, J. J. Communication between hosts and social parasites. In Sociobiology of Communication: An Interdisciplinary Perspective (eds d’Etorre, P. & Hughes, D. P.) 55–79 (Oxford University Press, 2008).
Martin, S. J., Shemilt, S., da S Lima, C. B. & de Carvalho, C. A. L. Are isomeric alkenes used in species recognition among neo-tropical stingless bees (Melipona spp). J. Chem. Ecol. 43, 1066–1072 (2017).
Chung, H. & Carroll, S. B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37, 822–830 (2015).
Finck, J., Berdan, E. L., Mayer, F., Ronacher, B. & Geiselhardt, S. Divergence of cuticular hydrocarbons in two sympatric grasshopper species and the evolution of fatty acid synthases and elongases across insects. Nat. Sci. Rep. 6, 33695 (2016).
Buellesback, J., Vetter, S. G. & Schmitt, T. Differences in the reliance on cuticular hydrocarbons as sexual signaling and species discrimination cues in parasitoid wasps. Front. Zool. 15, 22 (2018).
Medina-Medina, L.A. & Gonzalez-Acereto, J.A. La respuesta defensiva de Scaptotrigona pectoralis como un contundente escudo de protección contra las incursiones de Lestrimelitta niitkib dirigidas a otras especies de abejas sin aguijón. in VI Congreso Iberoamericano de Apicultura. 171–173. (1998).
National Institute of Standards and Technology. Mass Spectral Library. (NIST/EPA/NIH, 2011).
Tabachnick, B. G. & Fidell, L. S. Using Multivariate Statistics (Harper Collins College, 1996).
SAS Institute. SAS/STAT 9.2 User’s Guide. (SAS Institute Cary, 2008).
Rasband, W.S. ImageJ. (U.S. National Institutes of Health, 1997–2012).
Quezada-Euán, J.J.G., Paxton, R.J., Palmer, K.A., May-Itzá, W.D.J., Tay, W.T. & Oldroyd, B.P. Morphological and molecular characters reveal differentiation in a Neotropical social bee, Melipona beecheii (Apidae: Meliponini). Apidologie 38, 247–258 (2007).
Rohlf, F. J. TPSDIG: Version 2.12. (New York State University, 2008).
Klingenberg, C. P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357 (2011).
Francoy, T.M., Grassi, M.L., Imperatriz-Fonseca, V.L., May-Itzá, W.D.J. & Quezada-Euán, J.J.G. Geometric morphometrics of the wing as a tool for assigning genetic lineages and geographic origin to Melipona beecheii (Hymenoptera: Meliponini). Apidologie 42, 499–507 (2011).
Ratnasingham, S. & Hebert, P. D. N. A DNA-based registry for all animal species: the Barcode Index Number (BIN) system. PLoS ONE 8, e66213 (2013).
Hurtado-Burillo, M., Ruiz, C., May-Itzá, W.D.J., Quezada-Eúan, J.J.G., & De la Rúa, P. Barcoding stingless bees: Genetic diversity of the economically important genus Scaptotrigona in Mesoamerica. Apidologie 44, 1–10 (2013).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrigenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 3, 294–299 (1994).
Packer, L., Sheffield, C.S., Gibbs, J., de Silva, N., Best, L.R. et al. The campaign to barcode the bees of the world: progress, problems and prognosis. in Memorias VI Congreso Mesoamericano Sobre Abejas Nativas, Guatemala. 178–180. (2009).
Tamura, K., Stecher, G., & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evolut. https://doi.org/10.1093/molbev/msab120. (2021)
Oliveira, F.F.d. & Marchi, P. Três espécies novas de Lestrimelitta Friese (Hymenoptera, Apidae) da Costa Rica, Panamá e Guiana Francesa. Rev. Bras. Entomol. 49, 1–6 (2005).
González, V. H. & Griswold, T. L. New species and previously unknown males of neotropical cleptobiotic stingless bees (Hymenoptera, Apidae, Lestrimelitta). Caldasia 34, 227–245 (2012).
Marchi, P. & Melo, G.A.R. Revisão taxonômica das espêcies brasileiras do gênero Lestrimelitta Friese (Hymenoptera, Apidae, Meliponina). Rev. Bras. Entomol. 50, 6–30 (2006).
Gonzalez, V., Rasmussen, C. & Velasquez, A. Una especie nueva de Lestrimelitta y un cambio de nombre en Lasioglossum (Hymenoptera: Apidae, Halictidae). Rev. Colomb. Entomol. 36, 319–324 (2010).
Ratnasingham, S. & Hebert, P. D. N. BOLD: The barcode of life data system (https://www.barcodinglife.org). Mol. Ecol. Notes 7, 355–364 (2007).
Ayala, R. Revisión de las abejas sin aguijón de México (Hymenoptera: Apidae: Meliponini). Folia Entomol. Mexicana 106, 1–123 (1999).
Ruano, F. & Tinaut, A. The assault process of the slave-making ant Rossomyrmex minuchae (Hymenoptera: Formicidae). Sociobiology 43, 201–209 (2004).
Errard, C. et al. Coevolution-driven cuticular hydrocarbon variation between the slave-making ant Rossomyrmex minuchae and its host Proformica longiseta (Hymenoptera: Formicidae). Chemoecology 16, 235–240 (2006).
Dettner, K. & Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 39, 129–154 (1994).
Lenoir, A., d’Ettorre, P. & Errard, C. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599 (2001).
Von Beeren, C., Pohl, S. & Witte, V. On the use of adaptive resemblance terms in chemical ecology. Psyche 2012, 635761 (2012).
Lambardi, D., Dani, F. R., Turillazzi, S. & Boomsma, J. J. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 61, 843–851 (2007).
Uboni, A., Bagnères, A. G., Christidès, J. P. & Lorenzi, M. C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 58, 1259–1264 (2012).
Quezada-Euán, J. J. G. et al. Body size differs in workers produced across time and is associated with variation in the quantity and composition of larval food in Nannotrigona perilampoides (Hymenoptera, Meliponini). Insect. Soc. 58, 31–38 (2011).
Nunes, T. M., Mateus, S., Turatti, I. C., Morgan, E. & Zucchi, R. Nestmate recognition in the stingless bee Frieseomelitta varia (Hymenoptera, Apidae, Meliponini): Sources of chemical signals. Anim. Behav. 81, 463–467 (2011).
Gutiérrez, E., Ruiz, D., Solís, T., May-Itzá, W.d.J., Moo-Valle, H. & Quezada-Euán, J.J.G. Does larval food affect cuticular profiles and recognition in eusocial bees? A test on Scaptotrigona gynes (Hymenoptera: Meliponini). Behav. Ecol. Sociobiol. 70, 871–879 (2016).
Jones, S. M. et al. The role of wax and resin in the nestmate recognition system of a stingless bee, Tetragonisca angustula. Behav. Ecol. Sociobiol. 66, 1–12 (2012).
Leonhardt, S.D. Chemical ecology of stingless bees. J. Chem. Ecol. 43, 385–402 (2021).
Akino, T. Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News 11, 173–181 (2008).
Lenoir, A., Hefetz, A., Simon, T. & Soroker, V. Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol. Entomol. 26, 275–283 (2001).
Von Beeren, C. et al. Chemical and behavioral integration of army ant-associated rove beetles—A comparison between specialists and generalists. Front. Zool. 15, 8 (2018).
Kather, R. & Martin, S. J. Cuticular hydrocarbon profiles as a taxonomic tool: Advantages, limitations and technical aspects. Physiol. Entomol. 37, 25–32 (2012).
Menzel, F., Blaimer, B. B. & Schmitt, T. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B-Biol. Sci. 284, 20161727 (2017).
Savolainen, R. & Vepsäläinen, K. Sympatric speciation through intraspecific social parasitism. Proc. Nat. Acad. Sci. 100, 7169–7174 (2003).
Hartke, J., Sprenger, P.P., Sahm, J, Winterberg, H., Orivel, J. et al. Cuticular hydrocarbons as potential mediators of cryptic species divergence in a mutualistic ant association. Ecol. Evolut. 9, 9160–9176 (2019).
Doebeli, M. & Dieckmann, U. Evolutionary branching and sympatric speciation caused by different types of ecological interactions. Am. Nat. 156, S77–S101 (2000).
Thibert-Plante, X. & Gavrilets, S. Evolution of mate choice and the so-called magic traits in ecological speciation. Ecol. Lett. 16, 1004–1013 (2013).
Cabej, N. R. Epigenetic Principles of Evolution (Elsevier, 2012).
Quezada-Euán, J. J. G. Stingless Bees of Mexico: The Biology, Management and Conservation of an Ancient Heritage (Springer, 2018).
Von Zuben, L. G. et al. Interspecific chemical communication in raids of the robber bee Lestrimelitta limao. Insect. Soc. 63, 339–347 (2016).
Acknowledgements
MV received support from CONACYT for his postgraduate studies. The study was performed with support from projects CONACYT CAR-21861 and SAGARPA-CONCAYT 291333 to JJGQE. We are very thankful to Liam Graham and Laurence Packer (University of York, Canada) for their collaboration in DNA barcoding and providing sequences. We thank Biol. Teresita Solís for her help during sample collection and processing.
Author information
Authors and Affiliations
Contributions
M.V. and J.J.G.Q.E. outlined the study and organized the different approaches and sections in the ms, DM performed GC–MS analyses and interpretation, RM performed geometric morphometrics analysis and interpretation, FFO revised specimens for key taxonomic differences, RJP performed Cox1 sequencing and molecular analyses. All authors wrote and revised the ms.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vázquez, M., Muñoz, D., Medina, R. et al. Sympatric cleptobiotic stingless bees have species-specific cuticular profiles that resemble their hosts. Sci Rep 12, 2621 (2022). https://doi.org/10.1038/s41598-022-06683-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06683-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.