Abstract
Tropodiaptomus is one of the most specious genera in the family Diaptomidae, but it is often rare in terms of distribution and abundance. Moreover, Tropodiaptomus species show a noteworthy variability in some of the morphological characters considered of prime importance in diaptomid taxonomy, and the presence of cryptic or pseudocryptic species is likely. Thus, through a geographically-wide sampling in Thailand, we aimed to investigate the local diversity of the genus and to compare the morphological and molecular diversity pattern based on mitochondrial and nuclear genes sequences. DNA taxonomy was also implemented in order to check whether the Tropodiaptomus lineages were independent species according to the “evolutionary genetic species concept”. Six Tropodiaptomus morphospecies were found, three of which are putative species new to Science pending a formal description. The finding of such a high incidence of undescribed species stresses the existence of a significant “Linnean shortfall” affecting Thai diaptomids. The molecular results showed that most of the studied species could be identified consistently with their morphology-based taxonomy. However, Tropodiaptomus vicinus and T. cf. lanaonus showed a high level of genetic diversity, suggesting that traditional morphological techniques might be inadequate for correctly assessing their taxonomical status.
Similar content being viewed by others
Introduction
Taxonomic crypsis, i.e., the existence of distinct evolutionary entities which cannot be told apart based on morphology, is particularly frequent for those taxa where morphological conservatism or poorly understood morphological plasticity prevent from a morphology-based sound assessment of their actual diversity (e.g.1). Such a phenomenon is well known for copepods (e.g.2), and has been lately observed among several Holarctic diaptomid copepod genera (e.g.3,4,5,6,7). Conversely, no such evidence is to date available for diaptomids occurring in the Oriental region. Since cryptic and pseudocryptic species are assumed to be widely distributed in nature and among biogeographical regions8, and their discovery and description are pivotal to the correct assessment of actual biodiversity patterns9, we have investigated the morphological and genetic diversity patterns of the diverse copepod genus Tropodiaptomus Kiefer, 1932 in Thailand with the aim of contributing to a sounder assessment of the actual diaptomid diversity of Indo-Burma biodiversity hotspot.
With over 60 formally described species, mostly distributed around Africa and Asia10,11, Tropodiaptomus is the most speciose genus in the family Diaptomidae. To date, eight Tropodiaptomus species, i.e., Tropodiaptomus doriai (Richard, 1894), T. hebereri (Kiefer, 1930), T. lanaonus Kiefer, 1982, T. megahyaline Saetang, Sanoamuang & Maiphae, 2020, T. oryzanus Kiefer, 1937, T. ruttneri (Brehm, 1924), T. vicinus (Kiefer, 1930) and Tropodiaptomus sp., have been recorded from Thailand, where they occur in various types of habitats such as ponds, lakes, rivers, roadside canals, rice fields, and so forth (e.g.12,13,14, and references therein), being always rare both in term of distribution and abundance. Studies available to date for Asian Tropodiaptomus focused on morphological characters only. However, as already stressed by Lai & Fernando15, Lai et al.16, Defaye17 and Ambedkar18, Tropodiaptomus species show a noteworthy variation in the morphology of male fifth pair of legs and antennule, i.e., those characters which are traditionally used for species identification in diaptomid copepods (e.g.13,19). It is thus likely that the diversity of the genus is to date inadequately known, thus preventing the understanding of the distribution, ecology, and natural history of its species.
Based on an integrative approach including morphological and genetic data, we thus aim (i) to investigate the species diversity of the genus Tropodiaptomus in Thailand, (ii) to compare morphological and molecular diversity patterns based on mitochondrial and nuclear genes sequences, and (iii) to investigate the taxonomical value of the morphological characters currently used for species identification.
Materials and methods
Sample collection and morphological identification
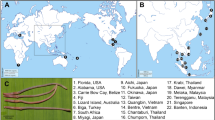
Out of 468 zooplankton samples collected from 2017 to 2019 in 196 permanent and temporary water bodies throughout Thailand, 29 samples containing Tropodiaptomus spp. were found from 23 sites (Fig. 1, Table 1). Copepods were sampled with a 60 µm mesh-sized hand net, immediately preserved in 99% ethanol and kept in a cool box. Tropodiaptomus specimens were then sorted out in the laboratory under a stereo microscope, prepared according to Dussart and Defaye20, and identified to species level according to Lai et al.16, Lai and Fernando21, Kiefer22, Sanoamuang23 and Saetang et al.13. The morphology of 108 collected specimens was carefully checked and compared with that of the known species of the genus. Particular attention was paid to the ornamentation of the basis and second exopod segment of male right fifth pair of legs (P5) and of the exopod segment of male left P5, to the length of the spinous process occurring on the antepenultimate segment of adult male right antennule, and to the number of setae occurring on the 13th segment of male left antennule. Collected samples are now stored at the Department of Zoology, Kesetsart University, Thailand.
Sampling sites of the analyzed Tropodiaptomus samples. Refer to Table 1 for locality codes. (This map was created by GEO-Informatics Research Center for Natural Resource and Environment, Prince of Songkla University and
DNA extraction, amplification, and sequencing
One to three specimens (62 individuals in total) selected from each population were soaked in distilled water for 5–10 min. DNA extraction was then performed according to Garcia-Morales and Elias-Gutierrez24 with a modified HotSHOT protocol25. The extracted DNA was amplified by polymerase chain reaction (PCR).
Fragments of the mitochondrial marker 12S rRNA and of the nuclear gene Internal Transcribed Spacer 2 (ITS2) were amplified in the 62 specimens using the primer pairs and conditions described by Vecchioni et al.26 and White et al.27, respectively. In addition, a fragment of the nuclear gene 28S rRNA was amplified following the protocol described in Vecchioni et al.26. However, due to its conservative nature, the 28S rRNA was amplified only in a subset of specimens selected from the major clades obtained based on the “12S-ITS2 dataset” (see below). Details on primers and thermal cycles are reported in Supplementary Table S1.
After PCR, 5 μl of each PCR product were used to perform electrophoresis on 2% agarose gel, with a voltage of 90 V, for 20 min. The outcome of the electrophoresis was verified using a UV transilluminator. The samples that showed a clear, single band, with the expected weight for each gene fragment was purified using the ExoSAP-IT kit (Affymetrix USB). Sequencing was operated by Macrogen Inc. (Madrid, Spain) via an ABI 3130xL sequencer (Applied Biosystems). The same primers used for the PCRs were used for the direct sequencing of the PCR products. The quality of the resulting chromatograms was verified by measuring their “Phred score” value28. Among these, only the sequences that showed continuous readings of high-quality bases (QV > 20) were kept for subsequent analyses.
Sequence alignment and phylogenetic analyses
12S and 28S chromatograms were checked and manually edited using the software Chromas v.2.6.2 (Technelysium, Pty. Ltd. 1998, Queensland, Australia). For ITS2, the forward and reverse sequences were assembled and the contig was examined through SEQUENCHER v.4.1.4 as suggested by Fontaneto et al.29. For ITS2, homozygotes consensus sequences were obtained directly from the contig. Heterozygotes sequences were carefully inspected and manually constructed when the chromatograms exhibited double peaks at a single position. Conversely, the two haplotypes were reconstructed using CHAMPURU v.1.030 (http://jfflot.mnhn.fr/champuru/) for those sequences that showed multiple double peaks at several positions. All sequences were aligned using the Clustal W method31 as implemented in the software MEGAX32.
In order to test whether the mitochondrial and nuclear fragments could be combined for joint analyses, the incongruence length difference test (ILD)33 as implemented in PAUP* v. 4.0b1034 was used. According to Cunningham35, if p > 0.01, pooling the data improves the phylogenetic accuracy and thus it is admissible to merge the tested datasets into a single matrix. This condition was fulfilled both for the concatenated 12S and ITS2 sequences (p = 0.63), and for the concatenation of all the genetic markers analyzed in the frame of this study (i.e., 12S, ITS2 and 28S; p = 1). Accordingly, two combined mito-nuclear datasets (i.e., the “12S-ITS2 dataset” and the “12S-ITS2-28S dataset”) were analyzed in the frame of this work. The first dataset includes all the available novel 12S and ITS2 sequences; those individuals which were heterozygous at the ITS2 locus are here represented by two concatenated sequences indicated by different letters, bearing the alternative ITS2 haplotype. The second dataset includes a subset of the 12S and ITS2 sequences and the 28S sequences. For both combined datasets the software packages MrBayes v. 3.2.636 and PhyML v. 337 were used for inferring phylogenetic relationships through Bayesian inference of phylogeny (BI) and maximum likelihood (ML) analysis. As support measures for the nodes, bootstrap values (BS)38 were calculated with 1000 replicates in the ML trees, whereas in the BI tree the posterior probability values (PP) reported. The best evolution model for each dataset was selected in the software PartitionFinder ver. 1.0.139 under the “Akaike Information Criterion” (AIC)40. The General Time-Reversible model of sequence evolution with a proportion of invariable sites was used for the 28S dataset (GTR + I). Instead, a GTR with a proportion of invariable sites and gamma-distributed rate variation among sites (GTR + I + Γ; nst = 6) was selected as the best evolutionary model for both the 12S and ITS2 datasets. In the BI analyses, two independent Markov chain Monte Carlo analyses were carried out for 1,000,000 generations (temp.: 0.2; default priors) with sampling every 1000 generations, the first 2,500 trees were discarded as a burn-in process and a consensus tree was constructed (Effective Sample Size (ESS) greater than 200 was reached in all the analyses performed). An individual of Eudiaptomus intermedius (Steuer, 1897) from a pond near the village of Sales, Italy (coordinates: 45.751845 N, 13.726236 E) was included in all the phylogenetic analyses as an outgroup. Bézier curves connecting the haplotypes found co-occurring in heterozygous individuals were added to the phylogenetic tree based on the ITS2 dataset. In addition, a haplotype network of the “28S dataset” was created with HaplowebMaker (https://eeg-ebe.github.io/HaplowebMaker/) with the "Median-Joining" method41.
Species delimitation methods
In the frame of this paper, we followed the ‘unified species concept’ described by De Queiroz42. Accordingly, morphological similarity or identity was not considered per se sufficient evidence for conspecificity, and single-locus DNA taxonomy approaches were implemented to explore the possible presence of groups of putative species rank within each studied morphospecies42,43. We applied two independent methods of species delimitation based on the 12S and ITS2 datasets. The “assemble species by automatic partitioning” (ASAP)44 method was implemented using the online ASAP server (https://bioinfo.mnhn.fr/abi/public/asap) with the following settings: fixed seed value = − 1 (i.e., no fixed seed value was used), and simple distance. The “multiple rate Poisson Tree Processes” (mPTP)45 model was run through the online mPTP server (https://mptp.h-its.org/). ASAP and mPTP analyses were performed on Tropodiaptomus spp. sequences, with the exclusion of the outgroup.
Genetic distances were calculated within and between the Tropodiaptomus clades of putative species-level using the Kimura two-parameter model with pairwise deletion in MEGAX46.
Ethics statement
The present study was approved by the ethics committee of Kasetsart University (approval no. ACKU61-SCI-004) for collecting the Tropodiaptomus specimens.
Results
Morphological analysis
In the frame of this study, six Tropodiaptomus morphospecies were identified in the 23 sampled locations in Thailand. These are Tropodiaptomus oryzanus, T. vicinus, T. cf. lanaonus, Tropodiaptomus sp.1, Tropodiaptomus sp.2, and Tropodiaptomus sp.3 (Table 1). Studied specimens mostly showed variation in i) the ornamentation of the basis and second exopod segment of adult male right P5, ii) the inner margin of the exopod in adult male left P5, and iii) the length of the process on the antepenultimate segment of adult male right antennule (see Table 2). Moreover, Lai et al.16 also reported that in the inner margin of adult male left P5 exopod in T. vicinus two or three lobes might be present; however, Thai specimens consistently showed the presence of two lobes. Conversely, our T. vicinus specimens showed variability in the ornamentation on second exopod of adult male right P5 and length of spinous process on the antepenultimate segment of adult male right antennule.
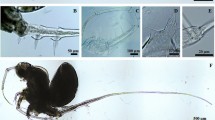
The morphology of the specimens here ascribed to Tropodiaptomus cf. lanaonus do not agree with the original description of the species22 in two characters: (i) the length of the spinous process in the antepenultimate segment of adult male right antennule is longer than segment 21 in the original description, whereas in our specimens (from sites KPK1, NT, NER and NMK4, see Table 1) it is 1/2 to 3/4 of segment 21, and (ii) the ornamentation on the basis of adult male right P5 has one apophysis and one hyaline lamella in the original description, which is in accordance with Thai specimens from KPK1 and NT, whereas studied specimens from NER and NMK4 have only the hyaline lamella and no apophysis. In addition, Thai T. cf. lanaonus also showed variability in the morphology of the basis and second exopod of adult male right P5 (Table 2). Moreover, specimens from NER have a group of spinules near inner margin lobe on the exopod of adult male left P5, this character does not show in specimens from KPK, NMK4 and NT (see Fig. 2).
We refrained from ascribing to any known species the Thai Tropodiaptomus populations from DKT (here referred to as Tropodiaptomus sp.1) and NP2 and NP3 (here referred to as Tropodiaptomus sp.2) because the morphology of adult male P5 and right antennule in Tropodiaptomus sp.1 and Tropodiaptomus sp.2 are different from all the Tropodiaptomus species known to date. Moreover, Tropodiaptomus sp.1 and Tropodiaptomus sp.2 differ one from the other for the following characteristics: the basis of the right P5 of Tropodiaptomus sp.1 has two processes and one hyaline lamella but it has one process and one hyaline lamella in Tropodiaptomus sp.2; the length of the spinous process in antepenultimate segment of adult male right antennule in Tropodiaptomus sp.1 is 3/4 of or equal to segment 21, while it is longer than segment 21 in Tropodiaptomus sp.2; and the shape of second exopod of the right P5 is trapezoidal in Tropodiaptomus sp.1 and rectangular in Tropodiaptomus sp.2. Based on all these characters, we suggest that they are two new putative species pending a formal description.
In addition, a single female whose morphology was not ascribable to any known Tropodiaptomus species was collected from the site KSM1 and here reported as Tropodiaptomus sp.3. Unfortunately, no Tropodiaptomus males were collected from this site.
The most common morphospecies collected in the frame of present study was Tropodiaptomus vicinus, which was found in 13 localities; it was found co-occurring with T. cf. lanaonus (site KPK1, 13 October 2017) and T. oryzanus (sites KDP1 and SNG4, 3 June 2019) (Table 1). All the other Tropodiaptomus taxa and populations were found with no co-occurring congeneric species in each locality in this study.
Molecular analyses
Overall, a total of 62 Tropodiaptomus individuals were molecularly analyzed. The mitochondrial 12S and the nuclear ITS2 markers were successfully amplified in all the 62 individuals; conversely, 28S sequences were produced for a subset of 18 individuals only. See Table 1 for a synopsis of the fragments amplified for each studied specimen and their GenBank Accession Numbers. Unfortunately, no 28S amplicons could be obtained for representatives of clades VI, VIII, and XII (see Fig. 3), which are thus not represented in the analyses which include this fragment. In addition, 12S, ITS2 and 28S sequences were obtained from the Italian Eudiaptomus intermedius specimen used as outgroup (ANs: OL584216 for 12S, OL584119 for 28S, OL630143 for ITS2).
Bayesian phylogram (95% majority rule consensus tree) of Tropodiaptomus spp. based on the concatenated 12S-ITS2 dataset. Eudiaptomus intermedius was used as an outgroup to root the tree. Node statistical support is reported as nodal posterior probabilities (Bayesian Inference of phylogeny, BI)/bootstrap values (maximum likelihood, ML). Asterisks indicate support values lower than 50. Rectangles refer to MOTUs as indicated by ASAP or mPTP (see Supplementary Figs. S2, S3). Square brackets group the samples according to their morphological identification. The analyzed specimens are reported using the location and codes listed in Table 1.
The phylogenetic trees (BI and ML analysis) based on the aligned 899 bp-long fragment of the concatenated “12S-ITS2 dataset” (367 bp for 12S, 533 bp for ITS2; Fig. 3) show a congruent topology, with the occurrence of 13 major clades with strong to moderate node support, hereafter indicated with roman numerals (i.e., clades I–XIII, see Fig. 3). The Thai populations ascribed to the morphospecies Tropodiaptomus vicinus resulted to be paraphyletic, since they were split into two major monophyletic groups with no sister-clades relationship and further subdivided in five clades. “Clade I” includes 15 specimens that belong to five different sites located in the northeastern part of Thailand (KDP1, SNG1, SNG2, SNG3 and TP; PP = 1, BS = 98%). Clade II includes two specimens from a single site located in eastern part of Thailand (RBG13; PP = 1; BS = 100%). Clade III includes four specimens from two locations in eastern and southern part of Thailand (SMR1 and TMP1; PP = 0.99, BS = 80%). Clade IV includes eight specimens from five locations in central, northeastern, and western parts (NH1, NN04, TMG1 and KPK1; PP = 1, BS = 100%). Clade V includes three specimens from one location in the northeastern part of Thailand (KDB; PP = 1, BS = 100%). All the other morphospecies proved to constitute monophyletic groups, although four well-characterized subclades are present within Tropodiaptomus cf. lanaonus (clades VI, VII, VIII, and IX, see Fig. 3).
Based on the 12S-ITS2 dataset, the genetic distance between the 13 clades reported in Fig. 3 ranged between 0.042 (T. cf. lanaonus clade VIII vs. T. cf. lanaonus clade IX) and 0.26 (T. oryzanus vs. T. vicinus clade III); the genetic distance within clades ranged between 0 and 0.013 (Table 3).
The BI and ML phylogenetic trees based on the aligned 1709 bp-long 12S-ITS2-28S dataset (367 bp for 12S, 533 bp for ITS2, 809 bp for 28S; Fig. 4A) group the concatenated sequences in 10 major clades with moderate to strong statistical support (Fig. 4A). These ten clades are consistent with the thirteen clades singled out based on the 12S-ITS2 dataset (Fig. 3), with the exception of clades VI, VIII and XII, which are missing from this analysis due to the failure of 28S amplification (see above).
(a) Bayesian phylogram (95% majority rule consensus tree) of Tropodiaptomus spp. based on the concatenated 12S-ITS2-28S dataset. Eudiaptomus intermedius was used as an outgroup to root the tree. Node statistical support is reported as nodal posterior probabilities (Bayesian Inference of phylogeny, BI)/bootstrap values (maximum likelihood, ML). Asterisks indicate support values lower than 50. Rectangles refer to MOTUs as indicated by ASAP or mPTP (see Supplementary Figs. S2, S3). Square brackets group the samples according to their morphological identification. The analyzed specimens are reported using the location and codes listed in Table 1. (b) Median-joining haplotype network based on a fragment of the nuclear 28S ribosomal DNA. Dashes indicate substitution steps. Each circle represents a haplotype, and its size is proportional to its frequency. Dashed rectangles indicate the morphological identification of the specimens used in the analysis. Colours refer to the clades reported in (a).
The Tropodiaptomus spp. haplotypes network based on the 28S nuclear marker shows the occurrence of seven haplotypes (Fig. 4B). The nine available sequences of T. vicinus belong to three different haplotypes. Tropodiaptomus vicinus specimens belonging to clades II, II and V shared the same haplotype with T. cf. lanaonus (clades VII, IX). One single haplotype was observed for T. oryzanus and Tropodiaptomus sp.1, respectively, whereas two different haplotypes were found within Tropodiaptomus sp.2.
Species delimitation
Based on a 365 bp-long fragment of the mitochondrial 12S gene, ASAP analysis suggested the existence of 12 groups of putative species rank within the ingroup, splitting both Tropodiaptomus vicinus and T. cf. lanaonus in four groups of putative species rank each; conversely mPTP analysis suggested the existence of 13 groups, splitting Tropodiaptomus vicinus and T. cf. lanaonus in five and four groups of putative species rank, respectively (Supplementary Figs. S1, S2, S3). The known geographical distribution of the clades found within T. vicinus and T. cf. lanaonus are reported in Fig. 5. Results of DNA-based classification based on the 12S gene matched morphology rather well, except for the case where putative cryptic species were found, i.e., within T. vicinus (clades I-V) and T. cf. lanaonus (clades VI-IX).
(a) Geographical distribution of the five clades singled out within Tropodiaptomus vicinus s.l. based on DNA taxonomy analyses. Colours refer to the clades reported in Fig. 3. (b) Geographical distribution of the four clades singled out within Tropodiaptomus cf. lanaonus based on DNA taxonomy analyses. Colours refer to the clades reported in Fig. 3. (This map was created using the QGIS software v. 3.18.3 using the layer “ne_10m_admin_0_scale_rank_minor_islands.shp” freely available at www.naturalearthdata.com/downloads/).
Based on a 533 bp-long fragment of ITS2, analyses suggested the existence of five (ASAP) or four (mPTP) groups of putative species rank, lumping in the same group specimens and populations characterized by different morphologies (Supplementary Figs. S4, S5, S6).
Discussion
Morphology-based species diversity of the genus Tropodiaptomus in Thailand
Six Tropodiaptomus morphospecies were found in the frame of present study, bringing to twelve the number of Tropodiaptomus species to date recorded in Thailand12,13,14. The species richness of Tropodiaptomus in Thailand is approximately 15% of the total number of species recorded for the genus worldwide. Based on morphology, Tropodiaptomus diversity to date recorded in Thailand is higher than that observed in other countries in Asia including India (9 species)47, Indonesia (6 species)48, Philippines (4 species)49, Malaysia (3 species)15, and Vietnam (3 species)17. Most species found in present and previous studies seem to prefer habitats characterized by a temporary hydroperiod. However, inhabited habitats might differ. For example, T. oryzanus was recorded from rice fields, swamps and manmade ponds in the present study but it was found also in temporary canals and ponds50, and roadside canals51,52.
The co-occurrence of different Tropodiaptomus species has been seldom observed in Thailand, and it always implied the co-occurrence of T. vicinus with other species, i.e., Tropodiaptomus cf. lanaonus, T. megahyaline and T. oryzanus (Table 1; see also:13,14,51). Conversely, Tropodiaptomus species are routinely observed to co-occur with other diaptomids belonging to the genera Dentodiaptomus Shen & Tai, 1964; Eodiaptomus Kiefer, 1932; Heliodiaptomus Kiefer, 1932; Mongolodiaptomus Kiefer, 1937; Neodiaptomus Kiefer, 1932 and Phyllodiaptomus Kiefer, 1936, with multi-species diaptomid coexistences ranging from two to eight species per site13,52,53,54.
Out of the six morphospecies identified in the frame of present survey, three taxa are putative species new to Science pending a formal description. The finding of such a high incidence of undescribed species suggests the existence of a significant “Linnean shortfall”55 affecting Thai diaptomid fauna, as also confirmed by the recent description of a new species of the genus13 and the report of a further new species pending a formal description by Sanoamuang and Dabseepai14. Current knowledge about Thai and Oriental Tropodiaptomus species is thus likely largely incomplete. Such a shortfall prevents from getting an exhaustive picture of the morphological and genetic diversity patterns of the genus, and of their taxonomical value.
Comparison between morphological and molecular diversity patterns
The existence of a noteworthy morphological variability within the Philippine and Indian species belonging to the genus Tropodiaptomus was reported by Lai et al.16 and Ambedkar18, respectively. They concluded that both intra- and interspecific variations of adult male P5 can be observed. In the frame of the present study, a certain degree of morphological variability was observed within Tropodiaptomus vicinus and Tropodiaptomus cf. lanaonus only, whereas a negligible variability was observed in the other species (Table 2). The molecular analyses carried out on the studied species showed that four of the six morphospecies found in the frame of present study, i.e., Tropodiaptomus oryzanus, Tropodiaptomus sp.1, Tropodiaptomus sp.2, and Tropodiaptomus sp.3, could be consistently identified based on their morphology and DNA sequences. Conversely, the morphospecies T. vicinus and T. cf. lanaonus showed a high level of genetic diversity, which suggests that the resolution of traditional morphological techniques may be insufficient for correctly assess their statuses.
The branching patterns of the phylogenetic trees built upon both the “12S-ITS2” and “12S-ITS2-28S” datasets (Figs. 3, 4a) show a noteworthy structuring within these last two morphospecies, which, along with their morphological variability (Table 2) and the paraphyly of T. vicinus (Fig. 3), is suggestive of the possible existence of multiple taxa lumped under these binomia. The two implemented DNA taxonomy analyses based on 12S sequences suggest the occurrence of four (ASAP) and five (mPTP) groups of putative species rank within Tropodiaptomus vicinus, and of four groups of the same rank within Tropodiaptomus cf. lanaonus for both ASAP and mPTP (Fig. 3, Supplementary Figs. S2, S3). Interestingly, such a deep genetic structure corresponds to the high variability in morphological characters observed in these two morphospecies. Conversely, species delimitation analyses based on ITS2 sequences produced results, which are counter-intuitive and not in agreement with the morphological evidence, largely underestimating the actual diversity of the ingroup. This is likely due to the slow evolutionary rate of this marker, which does not allow to properly resolve species-level relationships within Tropodiaptomus.
Tropodiaptomus vicinus was described from India56, and afterwards reported for Malaysia15, Philippines16, Vietnam17 and India18. Morphological variability was observed within the studied T. vicinus specimens from Thailand (Table 2); however, the observed characters do not allow to consistently distinguish among the clades of putative species rank suggested by DNA taxonomy analyses (Fig. 3, Table 2), and these clades are currently impossible to be told apart based on morphology. However, no clear geographic pattern of the observed molecular diversity pattern is evident (Fig. 5a), so that the existence of a geographical cline of genetic diversity seems not to be supported. Accordingly, the DNA-based clustering of the studied populations in five clades not forming a monophyletic group is neither in agreement with their morphology nor geographical distribution.
Tropodiaptomus lanaonus was described as an endemic species from Lake Lanao in Philippines by Kiefer22. Since then, the species has not been recorded until the twenty-first century, when Sanoamuang23 recorded the occurrence of this species in central and northeastern Thailand, and from Laos. Lopez et al.48 reported that this species has not been found from samples collected in the Philippines during 2008–2015 and hypothesize that the species could be locally extinct. Therefore, until now, this species has been reported only from its type locality (Lake Lanao, Philippines), from Laos, and from the eastern and northeastern parts of Thailand14. In the frame of present work, a Tropodiaptomus species close to T. lanaonus was collected also in southern Thailand, but due to its morphological peculiarities, which are suggestive of a possible species-level differentiation between the studied Thai populations and T. lanaonus s.s., we here conservatively ascribed these populations to Tropodiaptomus cf. lanaonus. A morphological re-analysis of the populations reported as Tropodiaptomus lanaonus in recent Thai literature is advisable in order to compare them with the Tropodiaptomus cf. lanaonus sampled in the frame of this survey. Based on molecular data, Tropodiaptomus cf. lanaonus consists of four clades of putative species rank (Fig. 3) with an allopatric distribution (Fig. 5b) but, as also observed for Tropodiaptomus vicinus (see above), morphological characters do not allow to consistently distinguish among clades.
The spreading of molecular techniques and DNA taxonomy revealed that cryptic or overlooked species are an evolutionary constant also among diaptomids (e.g.3,4,5,6,7,57). The possible occurrence of cryptic, or simply overlooked, species within the widespread, but locally rare, Tropodiaptomus vicinus and T. cf. lanaonus is thus verisimilar and somehow expected. DNA taxonomy methods are powerful tools for the recognition of cryptic species and, according to the International Commission on Zoological Nomenclature (ICZN), “new species can be described on the basis of DNA sequences”58 (ICZN, 1999; accessed 27 July 2020). However, as stressed by Fontaneto et al.29 and Dellicour and Flot43, the clades of putative species rank singled out by DNA taxonomy methods must be considered just as “primary species hypotheses”, and the implementation of an integrative approach including the search for a consensus among the evidence provided by different data sources, e.g., morphology, ecology, biogeography, and ethology, must be adopted before formally proposing their actual species status.
The small inter-clades distances observed for some of the clades of putative species-level found in the frame of present survey (Table 3) suggest caution in order to avoid the risk of an unsupported oversplitting of the taxa. In particular, the possible incomplete sampling of the actual genetic diversity of the Thai populations of Tropodiaptomus vicinus and T. cf. lanaonus might lead to the finding of spurious inter-clades gaps, which would bias subsequent DNA taxonomy analyses (e.g.59,60). This is particularly evident for Tropodiaptomus cf. lanaonus, whose four clades of putative species rank correspond in fact to four clusters of geographically isolated populations (Fig. 5b), so that the occurrence of intermediate haplotypes in geographically intermediate areas, which would reveal a scenario of a clinal distribution of their genetic diversity, cannot be excluded. Moreover, even in the absence of intermediate haplotypes, the genetic differentiation of allopatric populations might be ascribable to local adaptation, genetic drift, or simple isolation by distance phenomena instead of to actual speciation processes.
Conversely, it is not likely that a wider sampling effort dedicated to T. vicinus would change the paraphyletic status suggested for the Thai populations of this morphospecies (Fig. 3), which seems to actually harbour a relevant cryptic diversity, as also suggested by the absence of a clear geographical pattern of molecular diversity (Fig. 5a). In fact, when genetically distinct clades observed within a morphospecies are found in sympatry or constitute a paraphyletic group, this provides strong indirect evidence that these entities are actually independent evolutionary units (cf.9).
Pending other studies and evidence, we thus here refrain from considering the highlighted clades within Tropodiaptomus vicinus and T. cf. lanaonus as taxa of species rank, at the same time stressing the urgency to an in-depth analysis of the diversity pattern observed within these two morphospecies with dedicated surveys covering the whole potential distribution area of these taxa in the Oriental region.
The apparent decoupling between morphological- and molecular-based assessments of Thai Tropodiaptomus diversity is here ascribed to the morphological conservatism of the studied taxa, coupled with an inadequate understanding of the taxonomical value of the characters traditionally used when describing or identifying species. Accordingly, the taxonomic diagnostic morphological characters proposed in recent keys to the genus (e.g.13,18,22,47) should be used with caution and coupled, when possible, with a molecular characterization of the studied populations.
Concluding remarks
Currently knowledge of the distribution of Thai diaptomid species is largely incomplete and more dedicated studies should be realised to get a sound picture of the actual distribution of the studied taxa. This problem affecting biodiversity studies, known as “Wallacean shortfall”55, severely hinders our understanding of the diversity, ecology, and natural history of diaptomids. Present results thus stress that the diversity of Thai diaptomid copepods is currently largely underestimated due both to the Linnean and Wallacean shortfalls. Stabilizing selection and the focus of traditional morphological studies on a few “classical” characters along with the practical difficulties linked with sampling throughout vast and sometimes difficult-to-reach areas, might in fact have been leading to a gross underestimate of the diversity of Diaptomidae fauna of Thailand. The realization of further systematic faunal surveys in currently undersampled areas, coupled with the integrative morphological and molecular study of the collected species, is desirable before any taxonomical act involving cryptic species is done.
We hope that this work might pave the way to inspire future work aimed at a better knowledge of Thai diaptomids.
References
Trontelj, P. & Fišer, C. Perspectives: Cryptic species diversity should not be trivialised. Syst. Biodivers. 7(1), 1–3. https://doi.org/10.1017/S1477200008002909 (2009).
Lajus, D., Sukhikh, N. & Alekseev, V. Cryptic or pseudocryptic: Can morphological methods inform copepod taxonomy? An analysis of publications and a case study of the Eurytemora affinis species complex. Ecol. Evol. 5(12), 2374–2385. https://doi.org/10.1002/ece3.1521 (2015).
Thum, R. A. & Derry, A. M. Taxonomic implications for diaptomid copepods based on contrasting patterns of mitochondrial DNA sequence divergences in four morphospecies. Hydrobiologia 614, 197–207. https://doi.org/10.1007/s10750-008-9506-x (2008).
Marrone, F., Brutto, S. L. & Arculeo, M. M. Molecular evidence for the presence of cryptic evolutionary lineages in the freshwater copepod genus Hemidiaptomus G.O. Sars, 1903 (Calanoida, Diaptomidae)Calanoida, Diaptomidae. Hydrobiologia 644(115–125), 2010. https://doi.org/10.1007/s10750-010-0101-6 (2010).
Marrone, F., Brutto, S. L., Hundsdoerfer, A. K. & Arculeo, M. Overlooked cryptic endemism in copepods: Systematics and natural history of the calanoid subgenus Occidodiaptomus Borutzky 1991 (Copepoda, Calanoida, Diaptomidae). Mol. Phylogenet. Evol. 66, 190–202. https://doi.org/10.1016/j.ympev.2012.09.016 (2013).
Gutiérrez-Aguirre, M. A., Cervantes-Martínez, A. & Elías-Gutiérrez, M. An example of how barcodes can clarify cryptic species: The case of the calanoid copepod Mastigodiaptomus albuquerquensis (Herrick). PLoS ONE 9(1), e85019. https://doi.org/10.1371/journal.pone.0085019 (2014).
Previšić, A., Gelemanović, A., Urbanić, G. & Ternjej, I. Cryptic diversity in the Western Balkan endemic copepod: Four species in one?. Mol. Phylogenet. Evol. 100, 124–134. https://doi.org/10.1016/j.ympev.2016.04.010 (2016).
Pfenninger, M. & Schwenk, K. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol. Biol. 7, 121. https://doi.org/10.1186/1471-2148-7-121 (2007).
Bickford, D. et al. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22(3), 148–155. https://doi.org/10.1016/j.tree.2006.11.004 (2007).
Dussart, B. & Defaye, D. World Directory of Crustacea Copepoda of Inland Waters, I. Calaniformes (Backhuys Publisher, 2002).
Walter, T. C. & Boxshall, G. World of copepods database. Tropodiaptomus Kiefer, 1932. https://www.marinespecies.org/aphia.php?p=taxdetails&id=348143 (2021).
von Daday, E. Untersuchungen über die Copepodenfauna von Hinterindien, Sumatra und Java, nebst einem Beitrag zur Copepodenkenntnis der Hawaii-Inseln. Zool. Jahrb. Syst. 24, 175–206 (1906).
Saetang, T., Sanoamuang, L. & Maiphae, S. A new species of genus Tropodiaptomus Kiefer, 1932 (Crustacea: Copepoda: Calanoida: Diaptomidae) from Thailand. J. Nat. Hist. 54(35–36), 2297–2322. https://doi.org/10.1080/00222933.2020.1843726 (2020).
Sanoamuang, L. & Dabseepai, P. Diversity, distribution, and habitat occurrence of the diaptomid copepods (Crustacea: Copepoda: Diaptomidae) in freshwater ecosystems of Thailand. Water 13(17), 2381. https://doi.org/10.3390/w13172381 (2021).
Lai, H. C. & Fernando, C. H. The malaysian Tropodiaptomus (Copepoda: Calanoida) and its distribution. Hydrobiologia 65, 75–81. https://doi.org/10.1007/BF00032722 (1979).
Lai, H. C., Fernando, C. H. & Mamaril, A. The freshwater Calanoida (Copepoda) of the Philippines. Crustaceana 37(3), 225–240. https://doi.org/10.1163/156854079X00546 (1979).
Defaye, D. A new Tropodiaptomus (Copepoda, Calanoida, Diaptomidae, Diaptominae) from Vietnam. Crustaceana 75(3–4), 341–350 (2002).
Ambedkar, D. Morpho-Taxonomy and Biogeography of Diaptomid Copepods of India, with Descriptions of Four New Species of the Genus Tropodiaptomus Kiefer, 1932 (Crustacea: Calanoida) (Acharya Nagarjuna University, 2012).
Boxshall, G. A. An introduction to Copepod Diversity (The Ray Society, 2004).
Dussart, B. H. & Defaye, D. Introduction to the Copepoda: Guides to the Identification of the Microinvertebrates of the Continental Waters of the World (Backhuys, 2001).
Lai, H. C. & Fernando, C. H. The freshwater Calanoida (Crustacea: Copepoda) of Thailand. Hydrobiologia 76, 161–178. https://doi.org/10.1007/BF00014047 (1981).
Kiefer, F. Comparative studies on morphology, taxonomy and geographical distribution of the species of the genus Tropodiaptomus Kiefer from Asiatic inland waters. Hydrobiologia 93(3), 223–253. https://doi.org/10.1007/BF00012334 (1982).
Sanoamuang, L. Freshwater Zooplankton in Thailand: Calanoid Copepods (Klangnana Publishing, 2002).
García-Morales, A. E. & Elías-Gutiérrez, M. DNA barcoding of freshwater Rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Mol. Ecol. Resour. 13(6), 1097–1107. https://doi.org/10.1111/1755-0998.12080 (2013).
Montero-Pau, J., Gómez, A. & Muñoz, J. Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. Oceanogr. 6(6), 218–222. https://doi.org/10.4319/lom.2008.6.218 (2008).
Vecchioni, L., Arculeo, M., Cottarelli, V. & Marrone, F. Range-wide phylogeography and taxonomy of the marine rock pools dweller Tigriopus fulvus (Fischer, 1860) (Copepoda, Harpacticoida). J. Zool. Syst. Evol. Res. 59(6), 839–857. https://doi.org/10.1111/jzs.12457 (2021).
White, T. J., Bruns, T., Lee, S. & Taylor, J. W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A. et al.) 315–322 (Academic Press Inc, 1990).
Richterich, P. Estimation of errors in “raw” DNA sequences: A validation study. Genome Res. 8(3), 251–259. https://doi.org/10.1101/gr.8.3.251 (1998).
Fontaneto, D., Flot, J. F. & Tang, C. Q. Guidelines for DNA taxonomy, with a focus on the meiofauna. Mar. Biodivers. 45, 433–451. https://doi.org/10.1007/s12526-015-0319-7 (2015).
Flot, J. F. CHAMPURU 10: A computer software for unraveling mixtures of two DNA sequences of unequal lengths. Mol. Ecol. Notes 7(6), 974–977. https://doi.org/10.1111/j.1471-8286.2007.01857.x (2007).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22(22), 4673–4680. https://doi.org/10.1093/nar/22.22.4673 (1994).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Farris, J. S., Källersjö, M., Kluge, A. G. & Bult, C. Testing significance of incongruence. Cladistics 10, 315–319. https://doi.org/10.1111/j.1096-0031.1994.tb00181.x (1995).
Swofford, D. L. Phylogenetic analysis using parsimony (*and other methods) Version 4 (Sinauer Associates Inc, 2002).
Cunningham, C. W. Can three incongruence tests predict when data should be combined?. Mol. Biol. Evol. 14(7), 733–740. https://doi.org/10.1093/oxfordjournals.molbev.a025813 (1997).
Ronquist, F. et al. MrBayes 32: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61(3), 539–542. https://doi.org/10.1093/sysbio/sys029 (2012).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 30. Syst. Biol. 59(3), 307–321. https://doi.org/10.1093/sysbio/syq010 (2010).
Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39(4), 783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x (1985).
Lanfear, R., Calcott, B., Ho, S. Y. W. & Guindon, S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29(6), 1695–1701. https://doi.org/10.1093/molbev/mss020 (2012).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 19(6), 716–723. https://doi.org/10.1109/TAC.1974.1100705 (1974).
Spöri, Y. & Flot, J. F. HaplowebMaker and CoMa: Two web tools to delimit species using haplowebs and conspecificity matrices. Methods Ecol. Evol. 11(11), 1434–1438. https://doi.org/10.1111/2041-210X.13454 (2020).
De Queiroz, K. Species concepts and species delimitation. Syst. Biol. 56(6), 879–886. https://doi.org/10.1080/10635150701701083 (2007).
Dellicour, S. & Flot, J. F. The hitchhiker’s guide to single-locus species delimitation. Mol. Ecol. Resour. 18(6), 1234–1246. https://doi.org/10.1111/1755-0998.12908 (2018).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 21(2), 609–620. https://doi.org/10.1111/1755-0998.13281 (2021).
Kapli, P. et al. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33(11), 1630–1638. https://doi.org/10.1093/bioinformatics/btx025 (2017).
Marrone, F. et al. DNA taxonomy of the potamid freshwater crabs from Northern Africa (Decapoda, Potamidae). Zool. Scr. 49(4), 473–487. https://doi.org/10.1111/zsc.12415 (2020).
Ambedkar, D. & Elia, B. A new species of the genus Tropodiaptomus (Copepoda, Diaptomidae, Diaptominae) from Nepal. Crustaceana 87(4), 430–447. https://doi.org/10.1163/15685403-00003299 (2014).
Alekseev, V. R., Haffner, D. G., Vaillant, J. J. & Yusoff, F. M. Cyclopoid and calanoid copepod biodiversity in Indonesia. J. Limnol. 72(s2), 245–274. https://doi.org/10.4081/jlimnol.2013.s2.e12 (2013).
Lopez, M. L. D. et al. Annotated checklist and insular distribution of freshwater microcrustaceans (Copepoda: Calanoida & Cyclopoida; Cladocera: Anomopoda & Ctenopoda) in the Philippines. Raffles Bull. Zool. 65, 623–654 (2017).
Sanoamuang, L. & Athibai, S. A new species of Neodiaptomus (Copepoda, Diaptomidae) from temporary waters in northeast Thailand. Hydrobiologia 489, 71–82. https://doi.org/10.1023/A:1023202519395 (2002).
Yindee, W. Species Diversity and Distribution of Calanoid and Cyclopoid Copepods in Surin Province (Khon Kaen University, 2002).
Boonsit, B. Species Diversity and Distribution of Calanoid and Cyclopoid Copepods in Songkhram River Basin (Khon Kaen University, 2006).
Sanoamuang, L. Species composition and distribution of freshwater Calanoida and Cyclopoida (Copepoda) of northeast Thailand. In Crustaceans and Biodiversity Crisis: Proceedings of the Fourth International Crustacean Congress (eds Schram, F. R. & Klein, J. V. C.) 217–230 (Brill Academic Publishers, 1999).
Sanoamuang, L. & Yindee, W. A new species of Phyllodiaptomus (Copepoda, Diaptomidae) from northeast Thailand. Crustaceana 72(5), 435–448 (2001).
Hortal, J. et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Ann. Rev. Ecol. Evol. Syst. 46, 523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400 (2015).
Kiefer, F. Neue Ruderfusskrebse von den Sunda-Inseln. Zool. Anz. 86, 185–189 (1930).
Makino, W., Tanabe, A. S. & Urabe, J. The fauna of freshwater calanoid copepods in Japan in the early decades of the 21st Century: Implications for the assessment and conservation of biodiversity. Limnol. Oceanogr. 63(2), 758–772. https://doi.org/10.1002/lno.10667 (2018).
International Code of Zoological Nomenclature. Can DNA be a Type Specimen? https://www.iczn.org/outreach/faqs/ (1999).
Ahrens, D. et al. Rarity and incomplete sampling in DNA-based species delimitation. Syst. Biol. 65(3), 478–494. https://doi.org/10.1093/sysbio/syw002 (2016).
Vecchioni, L., Marrone, F., Rodilla, M., Belda, E. J. & Arculeo, M. An account on the taxonomy and molecular diversity of a marine rock-pool dweller, Tigriopus fulvus (Copepoda, Harpacticoida). Cienc. Mar. 45(2), 59–75. https://doi.org/10.7773/cm.v45i2.2946 (2019).
Acknowledgements
We are grateful to Fabio Stoch (Université libre de Bruxelles, Belgium) for kindly provided the Italian Eudiaptomus intermedius sample used as outgroup in the frame of the molecular analyses. T.S. and S.M. would like to thank Prof. Dr. La-orsri Sanoamuang for giving the idea of studying this genus in Thailand.
Funding
This project was supported by The Capacity Building of KU Students on Internationalization Program, International Affairs Division, Kasetsart University; part of the work has been carried out at the Department of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF) of the University of Palermo, Italy. In addition, it was financially supported by the Centre of Excellence on Biodiversity (BDC) Office of Higher Education Commission (BDC-PG2-161004) and graduate study development scholarship from the National Research Council of Thailand as of 2020 fiscal year. Thanks to all facilities from Department of Zoology, Faculty of Science, Kasetsart University.
Author information
Authors and Affiliations
Contributions
T.S. and S.M. designed the study and carried out the field work. S.M., funding acquisition. T.S. examined the morphological characters and DNA data. T.S. and L.V. conducted the molecular laboratory work and DNA data analysis supervised by F.M. T.S. lead the result interpretation. T.S. and S.M. wrote a first draft of the manuscript. F.M. and L.V. discussed the results and commented on the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saetang, T., Marrone, F., Vecchioni, L. et al. Morphological and molecular diversity patterns of the genus Tropodiaptomus Kiefer, 1932 (Copepoda, Calanoida, Diaptomidae) in Thailand. Sci Rep 12, 2218 (2022). https://doi.org/10.1038/s41598-022-06295-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06295-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.