Abstract
To determine whether increasing thrombectomy-capable hospitals with moderate comprehensive stroke center (CSC) capabilities is a valid alternative to centralization of those with high CSC capabilities. This retrospective, nationwide, observational study used data from the J-ASPECT database linked to national emergency medical service (EMS) records, captured during 2013–2016. We compared the influence of mechanical thrombectomy (MT) use, the CSC score, and the total EMS response time on the modified Rankin Scale score at discharge among patients with acute ischemic stroke transported by ambulance, in phases I (2013–2014, 1461 patients) and II (2015–2016, 3259 patients). We used ordinal logistic regression analyses to analyze outcomes. From phase I to II, MTs increased from 2.7 to 5.5%, and full-time endovascular physicians per hospital decreased. The CSC score and EMS response time remained unchanged. In phase I, higher CSC scores were associated with better outcomes (1-point increase, odds ratio [95% confidence interval]: 0.951 [0.915–0.989]) and longer EMS response time was associated with worse outcomes (1-min increase, 1.007 [1.001–1.013]). In phase II, neither influenced the outcomes. During the transitional shortage of thrombectomy-capable hospitals, increasing hospitals with moderate CSC scores may increase nationwide access to MT, improving outcomes.
Similar content being viewed by others
Introduction
Mechanical thrombectomy (MT) is the standard of care for patients with large vessel occlusion (LVO) related to acute ischemic stroke (AIS), but only approximately 3% of patients with AIS underwent MT in the US and Japan in 2016 and 2015, respectively1,2 patients with AIS and LVO could benefit from direct transportation to intervention centers; however, patients with no LVO need rapid intravenous thrombolysis at the nearest center3,4. Patients with AIS admitted directly to comprehensive stroke centers (CSCs) with endovascular treatment capacities may have better outcomes than those receiving drip-and-ship treatment3, but timely MT may be impeded by the distance that patients need to travel to thrombectomy-capable hospitals. Is there a valid alternative to regional centralization of MT to CSCs with high CSC capabilities to achieve better AIS patient outcomes5?
The existence of a single national emergency medical service (EMS) system in Japan provides a unique opportunity to conduct nationwide studies to examine the influence of prehospital time and hospital characteristics on clinical outcomes using linked data6.
Using data from the J-ASPECT (Nationwide survey of Acute Stroke care capacity for Proper dEsignation of Comprehensive stroke cenTer in Japan) study—a hospital-based, Japan-wide stroke registry7,8—we examined whether increasing thrombectomy-capable hospitals with moderate CSC capabilities2,9 is a valid alternative for regional centralization of MT to CSCs with high CSC capabilities3,5. Accordingly, we compared the influence of CSC capabilities and prehospital time on outcomes of MT for patients with AIS during the transitional phase before and after the pivotal trials10.
Methods
Ethics statement
To maintain confidentiality, we used deidentified databases, for which it would have been impracticable to obtain informed consent. The study was approved by the National Cerebral and Cardiovascular Center Institutional Review Board (M29-161-4, M29-161-3, and M29-088-3), which waived the requirement for individual informed consent. We reiterate that all methods were performed in accordance with the relevant guidelines and regulations.
J-ASPECT study
Participation of hospitals in the J-ASPECT study was voluntary, and the study was performed in collaboration with the Japan Neurosurgical Society (JNS) and Japan Stroke Society (JSS)2,7,8,11. This study consisted of two projects: (1) an institutional survey to assess the CSC capabilities of the hospitals (Table 1)9,12 and (2) a retrospective cohort study using the nationwide Diagnosis Procedure Combination (DPC) inpatient database2,7,8,11. Briefly, the DPC is a mixed-case classification system linked with a lump-sum payment system that was launched in 2002 by the Ministry of Health, Labor, and Welfare of Japan. By 2015, the DPC system had been adopted by an estimated 1580 acute care hospitals, representing approximately half of all Japanese hospital beds and encompassing a wide variety of centers, including rural and urban, academic and non-academic, and small and large hospitals7,13. An increasing number of acute stroke cases in Japan are being registered in the J-ASPECT study each year14, with approximately 1,090,000 cases registered as of October 2020.
Assessment of facility capabilities using the institutional survey
We sent out questionnaires in 2014 to assess the CSC capabilities of the facilities using 25 items recommended by the Brain Attack Coalition to the training institutions of the JNS and JSS7,9. The items are classified into five categories, as follows: personnel, diagnostic, specific expertise, infrastructure, and education (Table 1)7,9. A score of 1 point was assigned for each recommended item met by the hospital, yielding a total CSC score of up to 25. The content, development, and predictive validities of this scoring system for measuring CSC capabilities have been reported7,9. Our study group previously showed that the CSC score categorized into either quintiles or quartiles is associated with short-term clinical outcomes of patients with ischemic and hemorrhagic stroke including those who received surgical and endovascular treatment for stroke. In this study, we categorized the participating thrombectomy-capable hospitals based on the CSC scores into low (< 17), moderate (17–21) and high (> 21).
Study design
We conducted a retrospective observational study using data from the J-ASPECT stroke database (specifically those that could be linked to the national EMS records) captured between January 2013 and December 2016. Since the JSS, JNS, and Japanese Society for Neuroendovascular Therapy (JSNET) acted quickly to revise the clinical practice guidelines for MT use in response to the publication of the pivotal trials10, we divided the study period into phases I (2013–2014) and II (2015–2016). After publishing the results of the pivotal trials, the American Heart Association/American Stroke Association (AHA/ASA) updated the clinical guidelines for the endovascular treatment of acute ischemic stroke in 201515. In this guideline, patients should receive endovascular therapy with a stent retriever if they meet all of the criteria. In response to this, the relevant Japanese Society updated guidelines for mechanical thrombectomy16. Since the publication of these guidelines15.16, mechanical thrombectomy has been considered as standard treatment for large-vessel occlusion acute ischemic stroke regardless of geographical locations. Using the J-ASPECT DPC database, we extracted data of 303,719 patients with AIS diagnosed based on the diagnostic criteria of the International Classification of Diseases, 10th Revision (ICD-10) diagnosis code I63. Of those, we included 163,292 records of patients directly transported to facilities by ambulance, in the record linkage analysis. Patients’ consciousness level at admission was measured using the Japan Coma Scale (JCS) for stroke severity (Supplementary Table S1)7,17.
In this study, we defined thrombectomy-capable hospitals as those where MT was performed at least once in each phase. Using government data from 2015, we further categorized hospitals into three classes according to the population density in the regions that they serve, as follows: < 300 persons/km2, 300–1000 persons/km2, and > 1000 persons/km2.18.
We obtained permission to use all EMS data for 2013 and 2016 from the Fire and Disaster Management Agency of the Ministry of Internal Affairs and Communications6. Almost 99% of 771 EMS departments all over Japan participated in this study, which yielded 21,327,841 patient records. Using this database, we calculated the total EMS response time (i.e., from receipt of the emergency call to arrival at the hospital), which includes EMS response time, on-scene time, and transport time.
Data linkage and participant selection
We used one-to-one probabilistic linkage using the relink module in Stata 15.1 to match the J-ASPECT study and EMS records using the following five linkage points: date of incident/admission, hospital code, prefectures code, age, and sex19,20. A participant was included only if there was a linkage across all these characteristics. After linkage, we identified patients with AIS and excluded those who (1) were younger than 18 years; (2) had been transferred from another facility; or (3) had no valid timestamps, had duplicate cases, or had missing care outcomes. We used information from insurance claims to constitute the MT group by identifying all patients treated with any kind of device, including Merci (Concentric Medical, Mountain View, CA, US), Penumbra (Penumbra, Alameda, CA, US), Trevo ProVue (Stryker, Kalamazoo, MI, US), and Solitaire-FR (Covidien, Irvine, CA, US).
Study outcomes
We assessed outcomes using the modified Rankin Scale (mRS) score at discharge.
Statistical analyses
To compare patient characteristics and outcomes between phases I and II, we used t-tests for normally distributed continuous variables and Mann–Whitney U tests for non-normally distributed continuous variables. We compared categorical variables using Fisher’s exact or chi-square tests. After linking the records, we used multiple imputations to handle missing data points regarding the CSC scores, body mass index (BMI), and smoking history21. We used ordinal logistic regression analyses to investigate the associations of total EMS response time and CSC score with the mRS score at discharge, while adjusting for confounding patient-related (age, sex, stroke severity, comorbidities, BMI, smoking history, and total EMS response time) and hospital-related (CSC score9 and geographical category18) factors. We performed all analyses using JMP Statistical Software version 12 (SAS Institute Inc., Cary, NC, US) and Stata version 15.1 (Stata Corp., College Station, TX, US). A p-value < 0.05 was considered significant.
Results
Probabilistic record linkage
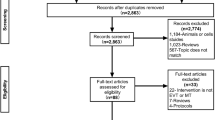
We were able to link 122,457 patients with AIS using the J-ASPECT and EMS data (linkage rate of 75.0%). After implementing the exclusion criteria, we included 113,564 records of patients with AIS in the final analyses (Fig. 1). Among them, 1461 patients with AIS received MT from 170 hospitals in phase I and 3259 patients received MT from 206 hospitals in phase II. From phase I to II, the proportion of patients who received MT according to population density remained unchanged (Table 2).
Comparison of patient characteristics between phases I and II
In the MT group, patients in phase II were significantly older, experienced less severe strokes, had a higher frequency of hyperlipidemia and atrial fibrillation, were treated more frequently with MT (5.5% vs. 2.7%), and had better in-hospital outcomes (30-day mortality 10.5% vs. 14.8%) than those in phase I (Table 2). From phase I to II, the use of stent retrievers significantly increased from 29.6 to 69.8%, whereas the use of aspiration catheters (Penumbra) and Merci retrievers significantly decreased from 73.9 to 65.2% and from 13.4 to 0.2%, respectively. The proportion of patients who received recombinant tissue-plasminogen activator (rt-PA) administration before MT remained the same between both phases. We observed similar findings for the characteristics of all patients with AIS except for a higher frequency of hypertension, a higher baseline mRS score, and a lower rt-PA administration score in phase I.
Prehospital time of the participating hospitals in each phase
In the MT groups, the total EMS response time remained almost the same between phase I and II, despite a shorter on-scene time in phase II (Table 2). We observed no clinically meaningful differences in prehospital time metrics in all AIS patients between phases. Notably, the transport time in the MT groups remained the same from phase I to II and comparable with those of all patients with AIS in each phase.
CSC capabilities of the participating hospitals in each phase
The CSC capabilities based on hospital characteristics are summarized in Table 3. In phases I and II, the percentages of missing CSC score data in the MT groups were 6.0% and 14.7%, respectively. Among all participating hospitals, the proportion of thrombectomy-capable hospitals increased from 45.6 to 58.2% from phase I to II. The median CSC scores of all participating hospitals and thrombectomy-capable hospitals remained unchanged between phases. Although there were no between-phase differences in the CSC scores for thrombectomy-capable hospitals, the difference in the median CSC scores between all participating and thrombectomy-capable hospitals in phase II became smaller than that in phase I (17 vs. 18 in phase I, 18 vs. 19 in phase II). Among the 25 items used to assess CSC capabilities, we observed no between-phase differences in availability in all participating hospitals; however, in the thrombectomy-capable hospitals, availability of the items related to endovascular treatment such as full-time, board-certified endovascular physicians and intra-arterial reperfusion therapy significantly decreased in phase II (p = 0.05, < 0.03) (Table 3).
Associations between total EMS response time and clinical outcomes
No clinically meaningful differences in the median total EMS response time were observed between all patients with AIS and the MT group in each phase (e.g., 33 vs. 32 min in phase II) (Table 2). In the MT group, a longer total EMS response time was associated with worse outcomes in phase I (odds ratio [OR] for each 1-min increase, 1.007 [CI, 1.001–1.013]); however, this association was not observed in phase II (1.003 [0.998–1.007]). The relationships between the total EMS response time and probabilities of an mRS score of 6 at discharge in phases I and II in the MT groups are shown in Fig. 2a, b.
Relationships between the total EMS response time or the CSC scores and probabilities of an mRS score of 6 at discharge (stroke outcomes) in the MT group. Panels (a) and (b) show the effects of total EMS response time (minutes) on the probabilities of an mRS score of 6 at discharge in phases I and II, respectively, in the MT group. Panels (c) and (d) show the effects of the CSC scores on the probabilities of an mRS score of 6 at discharge in phases I and II, respectively, in the MT group. EMS emergency medical services; CSC comprehensive stroke center; mRS modified Rankin Scale; MT mechanical thrombectomy.
Subgroup analyses demonstrated that the effect of total response time on clinical outcomes in phase I was notable for patients aged ≥ 70 years and those who reside in areas with low and intermediate population density (< 300, 300–1000 persons/km2; Fig. 3a). This association was only noted for patients aged < 70 years in phase II (Fig. 3b).
Subgroup analyses of the effect of the total EMS response time and the CSC score on outcomes. The forest plot shows the effect size of a 1-min increase of the total EMS response time on the mRS score at discharge in phases I (panel a) and II (panel b), respectively, in the MT group, analyzed according to ordinal logistic regression across subgroups. Dots indicate point estimates for the effect of the total EMS response time. The forest plot shows the effect size of a 1-point increase of the CSC score on the mRS score at discharge in phases I (panel c) and II (panel d), respectively, in the MT group, analyzed according to ordinal logistic regression across subgroups. Dots indicate point estimates for the effect of the CSC score. EMS emergency medical services; CSC comprehensive stroke center; mRS modified Rankin Scale; MT mechanical thrombectomy.
Associations between CSC score and clinical outcomes
In phase I, there was an association between an increase in the CSC score and better outcomes after MT (each 1-point increase, 0.951 [0.915–0.989]). In phase II, however, the association between a higher CSC score and improved outcomes was no longer observed in the MT group (each 1-point increase, 0.987 [0.965–1.010]). The relationships between the CSC score and probabilities of an mRS score of 6 at discharge in phases I and II in the MT groups are shown in Figs. 2c, d.
Subgroup analyses demonstrated that the effect of CSC scores on clinical outcomes in phase I was notable for men, patients aged ≥ 70 years, those who received preceding rt-PA administration, and those who resided in areas with intermediate population density (300–1000 persons/km2) (Fig. 3c). No influence of the CSC score on clinical outcomes was observed in any subgroup in phase II (Fig. 3d).
Discussion
Linking data from the J-ASPECT stroke database from 2013 to 2016 to the national EMS records, we demonstrated the increased use of MT and better clinical outcomes after MT with less initial stroke severity in an increasing number of thrombectomy-capable hospitals following revisions to the clinical practice guidelines for MT in 2015 by the relevant societies in Japan. Although the availability of endovascular physicians in all participating hospitals remained the same during the study period, fewer endovascular physicians were present in thrombectomy-capable hospitals in phase II, probably because of the rapid nationwide increase of such hospitals in response to the abovementioned revisions.
The influence of the CSC capabilities of the thrombectomy-capable hospitals and prehospital time on the clinical outcomes of patients with AIS who received MT in phase I may support the implementation of regional centralization of thrombectomy-capable hospitals3,5,22. No such clinical influence was observed in phase II; however, we posit that, in the current transitional period, while there is a relative shortage of thrombectomy-capable hospitals, increasing the amount of hospitals with moderate CSC scores may benefit the populations of countries with geographical conditions similar to those of Japan.
Temporal changes in patient characteristics
We demonstrated temporal changes in patient characteristics in the MT group, such as a higher age, decreased stroke severity, and better outcomes in phase II, which were consistent with those reported in previous studies of patients with AIS2,23. MT use in patients with AIS who were directly transported to a suitable facility via an ambulance in phase I (2.7%) was comparable to the findings from the US hospitals that participated in the Get With The Guidelines-Stroke program (MT use 3.3%)1. MT use (5.5%) in patients with AIS in phase II doubled from that in phase I, which may be explained by an increased awareness of the effectiveness of MT and the implementation of thrombectomy-capable hospitals in response to the revised guidelines in Japan24.
In contrast, MT use in patients with AIS who were directly transported via an ambulance to a suitable facility in phase II was almost twice the proportion (3.0%) of all patients with AIS who were urgently hospitalized in Japan from April 2010 to March 20162. This is consistent with the findings of previous studies showing that only 60% of urgently hospitalized patients with AIS are transported via an ambulance7,25, suggesting the underuse of ambulances for patients with AIS who are possible candidates of MT in Japan.
Influence of the CSC capabilities and total EMS response time of thrombectomy-capable hospitals on clinical outcomes in phases I and II
The observed influence of CSC capabilities of thrombectomy-capable hospitals on clinical outcomes of patients with AIS who received MT in phase I may support the concept of regional centralization of thrombectomy-capable hospitals3,5,22. This is consistent with our previous studies using data before 2015. Therein, we demonstrated that hospitals with higher (vs. lower) CSC capabilities were more likely to have lower in-hospital mortality among patients with AIS and to provide timely rt-PA infusion and MT on a 24-h basis2,7. Although the CSC score comprises heterogeneous items of stroke care expertise, it reflects the joint effort of multiple healthcare professionals to manage emergencies7,9,11,12.
One major finding of this study was the lack of association between the CSC capabilities and clinical outcomes of MT in phase II. This unexpected finding may be explained by several observations. First, and most notably, despite the similar CSC scores in phases I and II in the thrombectomy-capable hospitals in this study, the availability of specific items related to endovascular therapy (e.g., full-time availability of board-certified endovascular physicians and intra-arterial reperfusion therapy) decreased. This relative shortage was probably because of the rapid increase in hospitals where MT can be performed. In contrast, in all participating hospitals, the availability of those items remained almost the same from phase I to II, which is consistent with the results in our previous study. Therein, we showed that the implementation of six items, mainly related to endovascular therapy, increased > 20% from 2010 to 2018, especially between 2010 and 201412.
Second, even in thrombectomy-capable hospitals with comparable CSC capabilities in phases I and II, there may be a difference in the quality of in-hospital care related to MT, depending on the availability of endovascular physicians26,27,28,29. For example, thrombectomy-capable hospitals without sufficient in-house endovascular physicians are more likely to make use of those from neighboring hospitals to perform MT, which may increase the onset-to-reperfusion time and worsen the clinical outcomes26,30.
Another key finding of this study was that the total EMS response time was not associated with clinical outcomes of MT in phase II, regardless of the level of urbanization. The influence of the total EMS response time on clinical outcomes in the MT group in phase I is in line with previous studies evaluating the effect of onset-to-treatment time on outcomes of patients who received MT10. The total median EMS response time (phase I, 33 min; phase II, 32 min) in the MT group remained unchanged between phase I and II and was shorter than that in a US study (36 min)31, suggesting that the total EMS response time may not be an effective target in shortening the onset-to-treatment time. However, this may be characteristic of countries, such as Japan, where a greater proportion of the population lives closer to hospitals than that in more expansive countries18. In more expansive countries, driving time exceeding 90 min may be more common. Despite this, we believe that our findings may not be unique to Japan18 (e.g., 79% of adults in the US reside within 60 min of a hospital that provides acute cardiac therapy32).
The influence of the total EMS response time in phase II may be outweighed by other processes involved in the onset-to-treatment time, such as a delay in EMS activation33 and the in-hospital workflow before MT34. In this study, we did not have information on the time from symptom recognition to the ambulance call or on the in-hospital workflow; therefore, we could not quantify the role of those processes on the outcomes of patients with AIS who received MT26,35. A recent study suggested that patients with a lower socioeconomic status may be more likely to delay EMS activation than those with a higher status33; however, educational campaigns raising awareness of the signs and symptoms of stroke have had little effect on the actual response to a stroke event36,37. In contrast, fast reperfusion is a modifiable factor associated with better clinical outcomes when successful reperfusion is achieved34,38. A recent meta-analysis of the pivotal trials that led to the change in guidelines showed that the intermediary outcome, the rate of successful reperfusion, was higher with faster (vs. slower) hospital-arrival-to-groin-puncture time34. This nationwide study lends real-world support to the findings of existing literature on the importance of in-hospital workflow to improve clinical outcomes of patients with AIS who receive MT38,39.
In the US, AIS care and quality may differ between institutions, with CSCs outperforming primary stroke centers (PSCs) in timely acute reperfusion therapy and risk-adjusted mortality33. Recently, we developed the Close The Gap-Stroke (CTGS) program, the first nationwide quality improvement program within the J-ASPECT study; it allows prospective evaluation of the quality of acute stroke care in Japan, using the DPC database and electronic medical records14,29. Further studies are necessary to examine the influence of CSC capabilities on performance in terms of quality indicators and clinical outcomes of patients with AIS who received MT after the JSS started to certify PSCs who are able to perform MT.
Our study suggests that equal accessibility to MT remains an urgent unmet need in real-world situations, which may justify the nationwide implementation of thrombectomy-capable hospitals with moderate CSC capabilities since 201540.
In 2019, in Japan, the JSNET started to certify endovascular physicians who are qualified only to perform MT, and the JSS started to certify PSCs that are encouraged to perform MT. Availability of MT in the PSCs is in line with the increasing availability of endovascular treatment at PSCs in the US40. The current findings may lend support to this certification policy, as it may assist in equalizing the accessibility to MT.
Limitations
First, selection bias and unmeasured residual confounders may exist10. The participating hospitals in the J-ASPECT study were more likely to commit to quality improvement in stroke care than non-participating hospitals; however, the number of MTs performed in phase I corresponded to approximately 74.4% of those reported in the Japanese Registry of Neuroendovascular Therapy—the official registry of the JSNET41. Further, the geographical locations of the thrombectomy-capable and all participating hospitals in this study were comparable with those reported in the previous nationwide study on rt-PA use in Japan18. This suggests that the findings may represent the real-world situation in Japan. Second, the DPC database lacks data regarding several important factors, including the National Institutes of Health Stroke Scale (NIHSS) score, time metrics, and imaging results7,8,13,14. Thus, we used the JCS score, rather than the NIHSS score, as an index of stroke severity. Nationwide implementation of the CTGS program of the J-ASPECT study may solve this issue. Third, the LVO site was not included in the analysis; however, our recent study showed that approximately 86.4% of patients with AIS underwent MT from January 2013 to December 2015 according to the guidelines29. Fourth, we did not examine the effect of CSC capabilities on in-hospital care provision29,39. The result of the CTGS, an ongoing nationwide quality improvement initiative in Japan, may answer this question14,29. Finally, long-term outcomes (≥ 90 days) after AIS were not evaluated. Further studies are necessary to address these issues.
Conclusion
In the current transitional period, while there is a relative shortage of thrombectomy-capable hospitals, increasing the number of hospitals with moderate CSC scores may benefit the general Japanese population by equalizing access to MT in response to AIS. Certification of endovascular physicians qualified to perform MT may also promote such accessibility in thrombectomy-capable hospitals.
Data availability
We have documented the data, methods, and materials used to conduct the research in this report. The individual patient data are not publicly available owing to the memorandum signed by the directors of the participating hospitals and the principal investigator of the J-ASPECT Study group.
References
Smith, E. E. et al. Increase in endovascular therapy in get with the guidelines-stroke after the publication of pivotal trials. Circulation 136, 2303–2310 (2017).
Kada, A. et al. National trends in outcomes of ischemic stroke and prognostic influence of stroke center capability in Japan, 2010–2016. Int. J. Stroke; doi:https://doi.org/10.1177/1747493019884526 (2019).
Ismail, M. et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J. Neurointerv. Surg. 11, 14–19 (2019).
Gerschenfeld, G. et al. Two paradigms for endovascular thrombectomy after intravenous thrombolysis for acute ischemic stroke. JAMA Neurol. 74, 549–556 (2017).
Venema, E. et al. Prehospital triage strategies for the transportation of suspected stroke patients in the United States. Stroke 51, 3310–3319 (2020).
Kitamura, T. et al. Nationwide public-access defibrillation in Japan. N. Engl. J. Med. 362, 994–1004 (2010).
Iihara, K. et al. Effects of comprehensive stroke care capabilities on in-hospital mortality of patients with ischemic and hemorrhagic stroke: J-ASPECT study. PLoS ONE 9, e96819. https://doi.org/10.1371/journal.pone.0096819 (2014).
Kurogi, R. et al. Comparing intracerebral hemorrhages associated with direct oral anticoagulants or warfarin. Neurology 90, e1143–e1149. https://doi.org/10.1212/WNL.0000000000005207 (2018).
Kada, A. et al. Development and validation of a score for evaluating comprehensive stroke care capabilities: J-ASPECT Study. BMC Neurol. 17, 46. https://doi.org/10.1186/s12883-017-0815-4 (2017).
Saver, J. L. et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316, 1279–1288 (2016).
Kurogi, R. et al. Effects of case volume and comprehensive stroke center capabilities on patient outcomes of clipping and coiling for subarachnoid hemorrhage. J. Neurosurg. 134, 929–939 (2021).
Kurogi, A. et al. Temporal trends and geographical disparities in comprehensive stroke centre capabilities in Japan from 2010 to 2018. BMJ Open 10, e033055. https://doi.org/10.1136/bmjopen-2019-033055 (2020).
Yasunaga, H., Ide, H., Imamura, T. & Ohe, K. Impact of the Japanese diagnosis procedure combination-based payment system on cardiovascular medicine-related costs. Int. Heart. J. 46, 855–866 (2005).
Nishimura, A. et al. Development of quality indicators of stroke centers and feasibility of their measurement using a nationwide insurance claims database in Japan—J-ASPECT Study. Circ. J. 83, 2292–2302 (2019).
William, J. P. et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46, 3020–3035 (2015).
Japanese Guideline for the Management of Stroke 2015 (addendum 2017), www.jsts.gr.jp/img/guideline2015_tuiho2017.pdf. (2015)
Shigematsu, K., Nakano, H. & Watanabe, Y. The eye response test alone is sufficient to predict stroke outcome—reintroduction of Japan Coma Scale: a cohort study. BMJ Open 3, e002736. https://doi.org/10.1136/bmjopen-2013-002736 (2013).
Kunisawa, S. et al. Association of geographical factors with administration of tissue plasminogen activator for acute ischemic stroke. J. Am. Heart Assoc. 2, e000336. https://doi.org/10.1161/JAHA.113.000336 (2013).
Zhu, Y., Matsuyama, Y., Ohashi, Y. & Setoguchi, S. When to conduct probabilistic linkage vs. deterministic linkage? A simulation study. J. Biomed. Inform. 56, 80–86 (2015).
Jaro, M. A. Probabilistic linkage of large public health data files. Stat. Med. 14, 491–498 (1995).
Rubin, D. B. Multiple imputation for nonresponse in surveys 485-486 (Wiley, Hoboken, 1989).
Holodinsky, J. K. et al. Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol. 75, 1477–1486 (2018).
Stecksén, A. et al. Thrombolytic therapy rates and stroke severity. Stroke 43, 536–538 (2012).
Takagi, T. et al. Distribution and current problems of acute endovascular therapy for large artery occlusion from a two-year national survey in Japan. Int. J. Stroke 15, 289–298 (2020).
Powers, W. J. et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49, e46–e110. https://doi.org/10.1161/STR.0000000000000158 (2018).
Menon, B. K. et al. Components and trends in door to treatment times for endovascular therapy in get with the guidelines-stroke hospitals. Circulation 139, 169–179 (2019).
Xian, Y. et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA 317, 1057–1067 (2017).
Meretoja, A. et al. Effectiveness of primary and comprehensive stroke centers: PERFECT stroke: a nationwide observational study from Finland. Stroke 41, 1102–1107 (2010).
Ren, N. et al. Measuring quality of care for ischemic stroke treated with acute reperfusion therapy in Japan—the close the gap-stroke. Circ. J. 85, 201–209 (2021).
Seker, F. et al. Time metrics to endovascular thrombectomy in 3 triage concepts: a prospective, observational study (NEUROSQUAD). Stroke 51, 335–337 (2020).
Schwartz, J., Dreyer, R. P., Murugiah, K. & Ranasinghe, I. Contemporary prehospital emergency medical services response times for suspected stroke in the United States. Prehosp. Emerg. Care 20, 560–565 (2016).
Nallamothu, B. K., Bates, E. R., Wang, Y., Bradley, E. H. & Krumholz, H. M. Driving times and distances to hospitals with percutaneous coronary intervention in the United States: implications for prehospital triage of patients with ST-elevation myocardial infarction. Circulation 113, 1189–1195 (2016).
Ader, J. et al. Hospital distance, socioeconomic status, and timely treatment of ischemic stroke. Neurology 93, e747–e757. https://doi.org/10.1212/WNL.0000000000007963 (2019).
Bourcier, R. et al. Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 76, 405–411 (2019).
Janssen, P. M., Venema, E. & Dippel, D. W. J. Effect of workflow improvements in endovascular stroke treatment. Stroke 50, 665–674 (2019).
Rasura, M. et al. Effectiveness of public stroke educational interventions: a review. Eur. J. Neurol. 21, 11–20 (2014).
Boden-Albala, B. et al. Comparison of acute stroke preparedness strategies to decrease emergency department arrival time in a multiethnic cohort: the stroke warning information and faster treatment study. Stroke 46, 1806–1812 (2015).
Kaesmacher, J. et al. Effect of pre- and in-hospital delay on reperfusion in acute ischemic stroke mechanical thrombectomy. Stroke 51, 2934–2942 (2020).
Man, S. et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ. Cardiovasc. Qual. Outcomes 11, e004512. https://doi.org/10.1161/CIRCOUTCOMES.117.004512 (2018).
Yamagami, H. et al. Guidelines for Mechanical Thrombectomy in Japan, the Fourth Edition, March 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol. Med. Chir. (Tokyo) 61, 163–192 (2021).
Sakai, N. et al. Japanese Surveillance of Neuroendovascular Therapy in JR-NET-Part II. Japanese Registry of Neuroendovascular Treatment 3. Main report. Neurol. Med. Chir. 15, 106–115 (2019).
Acknowledgements
We thank all the enrollees of the J-ASPECT study for their contribution to this project. The institutions and names of the J-ASPECT study Collaborators are listed in Supplementary Table S2.
Funding
This study was supported by the Practical Research Project for lifestyle-related diseases, including cardiovascular diseases and diabetes mellitus, managed by the Japan Agency for Medical Research and Development (JP19ek0210088, JP20ek0210129, JP20ek0210147, JP21ek0210147); Grants-in-Aid from the Japanese Ministry of Health, Labour and Welfare (H28-Shinkin-Ippan-011, 19AC1003); KAKENHI grants (25293314, 18H02914) from the Japan Society for the Promotion of Science; and Intramural Research Fund (20–4-10) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center.
Author information
Authors and Affiliations
Consortia
Contributions
AI KUROGI: Drafting/revision of the manuscript for content, including medical writing for content; Analysis or interpretation of data. Daisuke Onozuka: Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; Analysis or interpretation of data. Akihito Hagihara: Study concept or design; Analysis or interpretation of data. Kunihiro Nishimura: Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design. Akiko Kada: Major role in the acquisition of data; Analysis or interpretation of data Manabu Hasegawa: Major role in the acquisition of data Takahiro Higashi: Study concept or design; Analysis or interpretation of data. Takahiro Higashi: Study concept or design. Takanari Kitazono: Major role in the acquisition of data; Analysis or interpretation of data. Tsuyoshi Ohta: Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design. Nobuyuki Sakai: Study concept or design. Hajime Arai: Major role in the acquisition of data. Susumu Miyamoto: Major role in the acquisition of data. Tetsuya Sakamoto: Major role in the acquisition of data. Koji Iihara: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data.
Corresponding author
Ethics declarations
Competing interests
Dr. Iihara is the principal investigator. Dr. Kada reports receiving personal fees from Bayer Yakuhin, Ltd. outside of the submitted work. Dr. Hagihara reports receiving a KAKENHI grant from the Japan Society for the Promotion of Science and grants from the Japan Agency for Medical Research and Development during the conduct of the study. Dr. Nishimura reports receiving grants from the Japanese Ministry of Health, Labour and Welfare and the Japan Agency for Medical Research and Development during the conduct of the study. Dr. Kitazono reports receiving grants from the Japanese Ministry of Health, Labour and Welfare and the Japan Agency for Medical Research and Development during the conduct of the study; personal fees from Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., and Chugai Pharmaceutical Co., Ltd.; and grants from Takeda Pharmaceuticals Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Co, Eisai Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., and MSD KK, outside of the submitted work. Dr. Ohta reports receiving funding for travel to speak at conference, Takeda Pharmaceutical, Tanabe Mitsubishi, Daiichi Sankyo, Otsuka Pharmaceutical, Stryker, Bayer, Kaneka, MSD, Medicos Hirata, Kyorin Pharmaceutical, Kochi Prefecture Kidney bank society, Uji Tokushukai Hospital, The Japanese Congress of Neurological Surgeons, Eisai, Medtronic and Terumo. He also reports Speakers' Bureaus, Takeda Pharmaceutical , Tanabe Mitsubishi, Daiichi Sankyo, Otsuka Pharmaceutical, Stryker, Bayer, Kaneka, MSD, Medicos Hirata, Kyorin Pharmaceutical , Kochi Prefecture Kidney bank society , Uji Tokushukai Hospital, Eisai, Medtronic and Terumo. Dr. Sakai reports receiving grants from Terumo Co., Ltd. and Daiichi Sankyo Co., Ltd.; and personal fees from Biomedical Solutions Co., Ltd., Johnson and Johnson Co., Ltd., Medtronic Co., Ltd., Penumbra Co., Ltd., Stryker Co., Ltd., and Terumo Co., Ltd., outside of the submitted work. Dr. Higashi reports receiving grants from the Japan Agency for Medical Research and Development and a KAKENHI grant from the Japan Society for the Promotion of Science during the conduct of the study. Dr. Sakamoto reports receiving grants from the Japan Agency for Medical Research and Development during the conduct of the study; personal fees from Boehringer Ingelheim Japan, Inc.; and grants from Asahi Kasei Pharma Corporation, Japan Blood Products Organization, and the Japanese Ministry of Health, Labour and Welfare, outside the submitted work. Dr. Iihara reports receiving grants from the Japanese Ministry of Health, Labour and Welfare, the Japan Agency for Medical Research and Development, and a KAKENHI grant from the Japan Society for the Promotion of Science during the conduct of the study; as well as grants from Otsuka Pharmaceutical Co., Ltd., Kaneka Medix Corporation, Eisai Co., Ltd., and Mitsubishi Tanabe Pharma Co., outside the submitted work. All other authors have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurogi, A., Onozuka, D., Hagihara, A. et al. Influence of hospital capabilities and prehospital time on outcomes of thrombectomy for stroke in Japan from 2013 to 2016. Sci Rep 12, 3252 (2022). https://doi.org/10.1038/s41598-022-06074-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06074-1
This article is cited by
-
Optimal allocation of physicians improves accessibility and workload disparities in stroke care
International Journal for Equity in Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.