Abstract
This meta-analysis aims to determine the clinical outcomes, complications, and fusion rates in endoscopic assisted intra-foraminal lumbar interbody fusion (iLIF) and minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) for lumbar degenerative diseases. The MEDLINE, Embase, and Cochrane Library databases were searched. The inclusion criteria were: five or more consecutive patients who underwent iLIF or MI-TLIF for lumbar degenerative diseases; description of the surgical technique; clinical outcome measures, complications and imaging assessment; minimum follow-up of 12 months. Surgical time, blood loss, and length of hospital stay were extracted. Mean outcome improvements were pooled and compared with minimal clinically important differences (MCID). Pooled and direct meta-analysis were evaluated. We identified 42 eligible studies. The iLIF group had significantly lower mean intra-operative blood loss, unstandardized mean difference (UMD) 110.61 mL (95%CI 70.43; 150.80; p value < 0.0001), and significantly decreased length of hospital stay (UMD 2.36; 95%CI 1.77; 2.94; p value < 0.0001). Visual analogue scale (VAS) back, VAS leg and Oswestry disability index (ODI) baseline to last follow-up mean improvements were statistically significant (p value < 0.0001), and clinically important for both groups (MCID VAS back > 1.16; MCID VAS leg > 1.36; MCID > 12.40). There was no significant difference in complication nor fusion rates between both cohorts. Interbody fusion using either iLIF or MI-TLIF leads to significant and clinically important improvements in clinical outcomes for lumbar degenerative diseases. Both procedures provide high rates of fusion at 12 months or later, without significant difference in complication rates. iLIF is associated with significantly less intraoperative blood loss and length of hospital stay.
Study registration: PROSPERO international prospective register of systematic reviews: Registration No. CRD42020180980, accessible at https://www.crd.york.ac.uk/prospero/ April 2020.
Similar content being viewed by others
Introduction
Transforaminal lumbar interbody fusion (TLIF) has gained wide popularity among the surgical spine community due to its efficacy, safety, and reproducibility, namely in the treatment of lumbar degenerative diseases that failed conservative treatment. Several published studies favor minimally invasive TLIF (MI-TLIF) regarding intraoperative blood loss, length of stay, complication rates, and clinical outcomes over open TLIF (O-TLIF), despite higher radiation exposure1,2.
Minimally invasive spine surgeries have been developed to reduce tissue trauma, decrease complication rates, and improve functional recovery3,4,5,6,7,8. The advances in endoscopic spine surgery made way for new opportunities to minimize tissue aggression further. Recently published meta-analyses regarding the treatment of lumbar disc herniations favored endoscopic discectomy (ED) over microdiscectomy (MD) in clinical outcomes (Oswestry disability index), duration of surgery, length of hospital stay, and lower risk of overall complications. These results opened the perspective that ED could take over the place of MD as the gold standard of care in the management of lumbar disc disease7,8. Technological innovations in endoscopic spine surgery have widened its range of applications beyond lumbar disc herniations. Endoscopic treatment of central and lateral recess stenosis, as well as endoscopic assisted lumbar interbody fusion (LIF) for degenerative lumbar diseases, are increasingly common9,10.
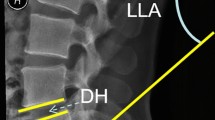
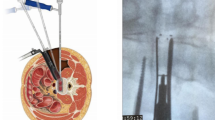
The proposed benefits of an even less invasive technique than MI-TLIF would be further improvement in the advantages over O-TLIF and obviating general anesthesia. Encouraged by the success of ED, endoscopic assisted intraforaminal LIF (iLIF) has been increasing its popularity. Even though several surgical techniques have been described, Kambin’s triangle approach through an intraforaminal facet sparing technique is the most usual and the one that has greater potential to reduce iatrogenic soft and bone tissue trauma10,11,12,13,14,15. Recent studies have shown promising results regarding reduced blood loss, decreased length of stay, clinical outcomes, complications and fusion10,14. However, the comparison between MI-TLIF and iLIF is sparse in the literature, and concerns about the safety and effective benefits of the endoscopic technique remain unanswered.
This systematic review and meta-analysis of MI-TLIF and iLIF were conducted to synthetize and compare the available data in the literature on clinical outcomes, complications and fusion rates.
Methods
Literature research
This review and meta-analysis were performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines16. The study protocol was registered in April 2020 with the PROSPERO international prospective register of systematic reviews (Registration No. CRD42020180980, accessible at https://www.crd.york.ac.uk/prospero/).
Electronic systematic research of MEDLINE, Embase, and Cochrane Library databases was performed to identify all relevant studies published from the date of inception to June 15, 2020. The following search strategy was used: (((“spine fusion” OR “lumbar fusion”) AND (“Endoscopy” OR “Endoscopic”)) OR ((“MI” OR “Minimally invasive”) AND (“TLIF” OR “transforaminal lumbar interbody fusion”))). The language of the included studies was restricted to English.
Selection criteria and data extraction
For this review, “iLIF” procedures were defined as endoscopic assisted lumbar interbody fusion performed through a uniportal intraforaminal access to Kambin’s triangle11,12,13,17. Intraforaminal access implied that minimal, partial, or total resection of the superior articular process (SAP) was performed to allow disc space preparation and cage deployment, while the inferior articular process (IAP) had to be preserved. Studies reporting percutaneous LIF or endoscopic assisted transforaminal lumbar interbody fusion (TLIF) approaches with complete facetectomy were excluded. “MI-TLIF” procedures were defined as surgery performed through a muscle-sparing surgical corridor, created by serial dilators that allowed for a tubular or cylindrical retractor to be docked on the facet joint complex, as reported by Foley and Schwender18,19. Besides anterior support, both techniques implied supplementary same level screw fixation.
The following inclusion criteria were used: 5 or more consecutive patients who underwent iLIF or MI-TLIF for lumbar degenerative diseases; description of the surgical technique; clinical outcome measures reported at a minimum follow-up of 12 months; complications assessment; and imaging assessment of fusion at a minimum follow-up of 12 months. The corresponding authors of studies with insufficient data (i.e., reported mean, standard deviation (SD), number of subjects or events) to extract and pool the predefined primary endpoints were contacted via email for clarification, if otherwise the studies were excluded. Systematic reviews, meta-analyses, technical notes, surgical techniques, biomechanical studies, case reports, and editorials were also excluded.
Two review authors (J.M.S. and H.R.) independently retrieved and screened all titles and abstracts to determine study eligibility. Full-text articles of the relevant abstracts were reviewed by the same two authors. The reference lists of the eligible studies were hand searched for potentially relevant publications. When studies with overlapping samples and outcomes were identified, only the most complete reports included for analysis. Data extraction of the selected studies was performed independently by two review authors (J.M.S. and H.R.) using a standardized data extraction Microsoft Excel form (Microsoft, Redmond, WA). Study characteristics (number of patients, age, body mass index, disease, follow-up, number of levels operated) and outcomes were extracted. The number of subjects, mean, and SD of continuous variables, and cross tabulated frequencies of dichotomous outcomes were also extracted. The following outcomes of interest were defined: a) primary outcomes: clinical outcomes measures at baseline and last follow-up; overall complications; fusion rate; b) secondary outcomes: average surgical time, intraoperative blood loss and hospital length of stay. Clinical outcomes measures reported in at least three studies of each surgical technique were pooled for meta-analysis. Any disagreements related to study selection or data extraction were settled through discussion and consensus with a third reviewer (J.G.C.).
Risk-of-bias
Two review authors (J.M.S. and H.R.) independently assessed the methodologic quality of the studies according to the methodological index for non-randomized studies (MINORS)20. Items were scored as 0, 1, or 2, whether they were not reported, reported but inadequate or reported and adequate, respectively. For non-comparative studies eight items were evaluated (maximum score of 16), and for comparative studies all 12 items were scored (maximum score of 24). Non-comparative studies with MINORS score \(\le\) 12 and comparative studies with MINORS score \(\le\) 20 were considered at high risk of bias. Any disagreements were resolved through discussion and consensus with a third reviewer (J.G.C.).
Statistical analysis
Intraclass correlation coefficient (ICC) was calculated to quantify inter-rater reliability of the MINORS scores20,21.
Unstandardized mean differences (UMD) were pooled and calculated for continuous outcomes—Visual analogue scale (VAS) back, VAS leg, ODI, surgical time, intraoperative blood loss, hospital length of stay—as follows:\(UMD\left(di\right)={\overline{x}}_{1i}-{\overline{x}}_{2i}, var\left(di\right)=\frac{{sd}_{1i}^{2}}{{n}_{1i}}+\frac{{sd}_{2i}^{2}}{{n}_{2i}}, {w}_{i}= \frac{1}{var({d}_{i})}\), where \({w}_{i}\) is the weighting factor, \(di\) is the unstandardized difference of means, \({n}_{1i}\) and \({n}_{2i}\) are number of subjects in groups 1 and 2, \({n}_{i}\) is \({n}_{1i}\) + \({n}_{2i}\), \({sd}_{i}\) is the pooled standard deviation, \(var\left(di\right)\) is the variance of difference, and subscript \(i\) corresponds to study \(i\).
Pooled prevalence of the dichotomous outcomes was calculated as: \(\overline{p}=\frac{\sum {w}_{i}{p}_{i}}{\sum {w}_{i}}\), \({w}_{i}= \frac{1}{var({p}_{i})}\) , where \(\overline{p}\) was the pooled prevalence, \({p}_{i}\) was the prevalence of the event in each study, and \({w}_{i}\) was the weight of each study22,23.
Heterogeneity was estimated using Q statistics. I2 statistics was used to estimate inconsistency among the studies’ results due to heterogeneity rather than chance. Values greater than 50% were considered as substantial heterogeneity. If heterogeneity was present, between studies variation was estimated by calculating \(\tau^{2}\), which was then used to calculate \({w}_{i}^{*}=\frac{1}{var\left({p}_{i}\right)+\tau 2}\), a weight term that accounted for variations between studies. Sub-group analysis was performed to explore causes of heterogeneity24,25.
Baseline to last follow-up mean differences of outcome measures reported in at least three studies in each group were calculated and compared to the minimal clinically important difference (MCID) of each outcome: 1.16 for VAS back, 1.36 for VAS leg, and 12.40 for ODI26.
UMD and odds ratio (OR) were estimated using a random effects model, with a 95% confidence interval. Statistical significance was set at p value > 0.05. Meta-analysis was performed between the pooled studies and the comparative study. Pooled analyses were performed using Microsoft Excel (Microsoft, Redmond, WA), the remaining statistical analyses and forest plots were performed using Cochrane Review Manager, version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark)27.
Results
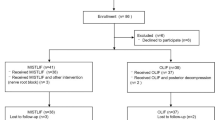
The literature search is illustrated in the PRISMA flow diagram (Fig. 1). Forty-two studies remained for qualitative and quantitative analyses14,15,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67. The mean MINORS score was 12.7 \(\pm\) 1.29 (10–14) for non-comparative studies, and 20.9 \(\pm\) 1.9 (17–24) for comparative studies. Six non-comparative studies (MINORS score \(\le\) 12) and 13 comparative studies (MINORS score \(\le\) 20) were assessed as a high risk of bias. The intra-class correlation coefficient (ICC) to quantify the degree of agreement between the raters was 0.997.
The corresponding author of the study by Shen14 was contacted by email and provided study’s mean and SD of operative time, estimated blood loss and length of hospital stay.
Baseline characteristics
The characteristics of the studies are summarized in Table 1. Thirty-six studies, with a total of 2076 patients, were included in the MI-TLIF group; five studies, with a total of 170 patients, were included in the iLIF group; and one prospective cohort study (PCS) with 75 patients comparing MI-TLIF to iLIF was identified.
The mean age was 57.9 ± 11.0 years and 63.4 ± 10.9 years, for MI-TLIF and iLIF, respectively. The proportion of females was 61% for MI-TLIF, and 52% for iLIF. Thirty-two studies reported single-level surgery, two studies (MI-TLIF) reported two level surgeries, while eight studies (six MI-TLIF; two iLIF) included single-level and two or more levels surgeries.
Operations parameters
Summary changes of surgical time, blood loss and length of hospital stay are portrayed in Tables 2 and 3.
In the pooled studies, surgical time was significantly increased in the MI-TLIF group (UMD 38.1; 95% CI 33.01; 43.23; p value < 0.0001). However, in the meta-analysis no statistically significant difference was observed between both groups (Table. 4).
The mean intra-operative blood-loss of the pooled studies was significantly increased by 128.8 mL (95% CI 118.01; 139.59; p value < 0.0001) in MI-TLIF. In the meta-analysis, there was also a statistically significant mean increase of intra-operative blood-loss (UMD 110.61 mL; 95% IC 70.43; 150.80; p value < 0.0001) in MI-TLIF (Table. 4, Fig. 2).
In the pooled studies, the length of hospital stay had a significantly mean increase of 2.6 days (95% CI 2.27; 2.95; p value < 0.0001) in the MI-TLIF group. In the meta-analysis, there was also a statistically significant mean difference of 2.36 days (95% IC 1.77; 2.94; p value < 0.0001) favoring the iLIF group (Table. 4, Fig. 2).
Clinical Outcomes
VAS back (33 MI-TLIF studies, n = 1946 vs 4 iLIF studies, n = 70), VAS leg (26 MI-TLIF studies, n = 1518 vs 3 iLIF studies, n = 52), and ODI (36 MI-TLIF studies, n = 2050 vs 6 iLIF studies, n = 170) were reported in three or more studies in each technique and are summarized in Tables 5 and 6. UMD of the pooled results and meta-analysis are summarized in Table 7.
There was no difference in baseline VAS back between pooled MI-TLIF and iLIF groups. At the last follow-up, VAS back was significantly lower in the iLIF group (UMD 0.96; 95% CI 0.81; 1.11; p value < 0.0001). However, the meta-analysis showed no statistically significant difference at either time-point.
VAS leg at baseline was significantly higher in the iLIF group (UMD 1.0; 95% CI 0.73; 1.29; p value < 0.0001), with significant heterogeneity. This statistically significant increase was also observed in the meta-analysis (UMD 0.86; 95% CI 0.37; 1.34; p value = 0.0005). At the last follow-up, VAS Leg was significantly lower in the pooled iLIF group (UMD 0.54; 95% CI 0.38; 0.79; p value = 0.027). However, the meta-analysis showed no statistically significant difference at last follow-up.
VAS back and VAS leg baseline to last follow-up mean improvement was statistically significant (p value < 0.0001), and clinically important (MCID > 1.16 and MCID > 1.36, respectively)26 for both groups.
ODI scores at baseline and last follow-up were significantly higher in the MI-TLIF group (UMD 17.1; 95% CI 15.09; 19.19; p value < 0.0001 and UMD 2.27; 95% CI 0.07; 4.47; p value = 0.042, respectively. Conversely, the meta-analysis showed no statistically significant difference at either time-point.
ODI baseline to last follow-up mean improvement was statistically significant (p value < 0.0001), and clinically important (MCID > 12.40) for both groups26.
Complications and fusion
The overall complication rate was 4.7% for iLIF studies versus 9.6% for MI-TLIF. The specific complications identified in each technique are summarized in Table 6. Screw malpositioning (1.6%), adjacent segment degeneration (1.5%), and dural tears (1.3%) were the main complications in MI-TLIF, while in the iLIF group cage migration (1.1%), screw malpositioning (0.6%), and infection (0.6%) were the most prevalent complications (Table 8). The pooled OR was 2.15 (95% CI 1.04, 4.43; p value 0.033) when comparing MI-TLIF and iLIF. The meta-analysis showed a borderline OR (OR 2.03; 95% CI 1.01, 4.12; p value = 0.05) favoring iLIF (Table 9).
The pooled fusion rate had a statistically significant OR favoring iLIF (OR 0.05; 95% CI 0.00, 0.78; p value < 0.0001). However, the meta-analysis revealed no difference between iLIF and MI-TLIF fusion rates.
There was no significant difference (OR 2.39; 95% CI 0.58, 9.87; p value 0.23) in overall revision rate, 1.2% and 2.4% in iLIF and MI-TLIF, respectively (Table 9).
Subgroup analysis
We performed subgroup analysis according to the number of levels operated. Even though ODI at baseline, and VAS back and VAS leg at last follow-up, had statistically significant differences in one level versus two level MI-TLIF, they were not clinically significant. There was no significant difference regarding complications and fusion rates.
Blood loss was significantly increased when two or more levels were operated in either technique (UMD 41.3 mL; 95% CI 27.63, 55.03; p value < 0.0001 for MI-TLIF; UMD 20.55 mL; 95% CI 1.5, 39.6; p value < 0.036 for iLIF). Both one level and two level iLIF subgroups had significantly less blood loss than one level MI-TLIF subgroup (UMD 122.02 mL; 95% CI 110.38, 133.66; p value < 0.0001 for one level iLIF; and UMD 101.47 mL; 95% CI 83.72, 119.22; p value 0.0012 for two level iLIF).
Length of hospital stay was significantly higher in the MI-TLIF subgroup of two or more levels when compared to one-level MI-TLIF (UMD 3.49 days; 95% CI 2.98; 4.0; p value < 0.0001). There was no significant difference between one-level subgroups of MI-TLIF and iLIF (UMD 0.33 days; 95% CI -0.008, 0.74; p value 0.65). Unlike surgical time and blood loss, there was no available data for length of hospital stay in two level iLIF. Comparison between one level MI-TLIF and overall iLIF length of hospital stay revealed a statistically significant decrease favoring iLIF (UMD 2.17 days; 95% CI 1.79; 2.55; p value < 0.0001).
Discussion
We conducted a systematic review and meta-analysis of the available literature reporting MI-TLIF and iLIF for the treatment of lumbar degenerative diseases. We derived our conclusions based on the meta-results, given the discrepancy in some outcomes of the pooled results and the meta-analysis.
The main finding is that both MI-TLIF and iLIF provide significant clinical improvement and high rates of fusion at a minimum follow-up of 12 months. No significant difference in complication rates was identified. Furthermore, iLIF was associated with significantly less intraoperative blood loss, and reduced length of hospital stay.
Previous studies documented similar benefits of MI-TLIF over open TLIF (O-TLIF), while achieving the same fusion rates, operative time, and decreased complication rates1,2. On the downside, MI-TLIF was associated with increased radiation exposure. According to our findings, iLIF further enhances most of the benefits of MI-TLIF over O-TLIF. The data reported in the retrieved studies did not allow for a comparative analysis on radiation exposure.
Besides efficacy, concerns about procedure-related complications are among the major setbacks for adopting emerging surgical techniques. The present meta-analysis revealed no significant difference in complication rates. The intraforaminal route allows an anatomic approach to the disc, with total or partial preservation of the articular processes. On the one hand, facet preservation provides dural protection, reducing the risk of dural tear, on the other, direct endoscopic visualization of the nerve root allows assessing the need for further foraminal decompression, reducing the risk of nerve root damage. By providing an ultra-minimally invasive approach, trauma to the soft and bone tissues is reduced, which may account for the residual infection rates reported.
The anesthesia protocol and neuromonitoring might also play a role in the sparse number of neurologic complications reported in iLIF procedures, by allowing intra-operative neurological monitoring. Wu15 and Ao62 used general anesthesia and neuromonitoring, Shen14 and Kolcun56 operated consciously sedated patients, Jin65 used local anesthesia supplemented with neuroleptic analgesia for the decompression procedure, with the aim of sensory-motor separation, and epidural anesthesia when the patient complained about unbearable pain during bone harvest, cage insertion, and percutaneous pedicle screw placement procedures. Yang59 used a low-dose epidural anesthesia combined with local anesthesia or general anesthesia based on physical condition and willingness of patients. The vast majority of the retrieved MI-TLIF studies either did not disclosure the anesthesia protocol or used general anesthesia without neuromonitoring. Gao44 reported 75 patients operated under epidural anesthesia.
Studies using a strictly percutaneous approach (pTLIF) similar to iLIF have reported increased rates of post-operative radiculopathy, dysesthesia and transitory muscle weakness68,69,70. However, once endoscopic assistance was precluded, these studies were not included in our analysis. The absence of endoscopic assessment does not allow nerve root visualization or foraminal revision after cage deployment, which may justify the increased complication rates reported. From the 205 patients included in the iLIF studies, only Ao62 reported a patient with decreased muscular strength of quadriceps femoris, grade 4, after surgery at L4L5 level. The patient had significant relief of symptoms after one month of neurotrophic drug treatment and functional exercise.
Even though endplate preparation might be technically challenging in iLIF due to access restraints, direct endoscopic visualization of the endplates allows confirming adequate subchondral exposure62,65, contributing to the high rates of fusion.
In the iLIF publications, the type of cage used varied across studies. Wu15 and Ao62 used static cages, Kolcun56 used mesh cages, and Shen14, Yang59 and Jin65 used expandable cages. In most MI-TLIF studies, static cages were used or the type of cage was not disclosed, except in the study by Kim45 that used expandable cages. Data on sagittal alignment was insufficient, namely on iLIF studies, to derive any conclusions.
Despite overlapping and additional benefits of iLIF compared to MI-TLIF, it is worth mentioning that endoscopic spine surgeries have a steep learning curve. Knowledge and understanding of foraminal anatomy and its landmarks are of utmost importance. Besides the anesthetic technique and neuromonitoring, continuous spatial orientation of surgical instruments during the procedure is mandatory to avoid nerve root injury, either by direct trauma or excessive retraction. Pre-operative planning with magnetic resonance imaging (MRI) is crucial to assess the nerve root trajectory and eventual anatomic variations, mostly at L5S1 level where the dorsal root ganglion may be particularly endangered10,14. Proper training and previous experience in lumbar transforaminal endoscopic discectomy and decompression are advised.
Strengths and limitations
Intraforaminal endoscopic assisted fusion is an emerging surgical technique. No systematic review and meta-analysis that synthesizes the available data comparing it to MI-TLIF has been published. Recently, Wagner10 published a review and technical note on uniportal endoscopic assisted fusion. However, despite providing a comprehensive description of the endoscopic assisted intraforaminal transkambin technique, the studies’ procedures were heterogeneous and not standardized. Our review followed the PRISMA guidelines, with a prospective design that specified the main technical details of the procedures and the outcomes and minimum follow-up to be included. Statistical analysis was performed according to previously validated statistical methodology22,23,24,25.
The endoscopic assisted intraforaminal transkambin approach for LIF has been termed as percutaneous endoscopic LIF (abbreviated as PELIF15,65 or PETLIF59,62) or endoscopic MIS-TLIF56. However, the term TLIF is misleading once endoscopic assisted intraforaminal transkambin LIF is a facet sparing procedure, as opposed to the TLIF procedure where a total facetectomy is performed. Also, the term PELIF is ambiguous once it may accommodate different approaches to perform LIF, as long as it is done percutaneously and with endoscopic assistance, either uniportal or biportal. We believe that by referring to the intra-foraminal route instead of the traditional transforaminal route, the term iLIF is more accurate and allows a comprehensive definition of the procedure.
The main limitations of our study are a consequence of the quality of the literature retrieved: low levels of evidence and heterogeneity bias. Only one prospective cohort study comparing both techniques was included, and no randomized control trials. Also, the difference in fusion definition across studies, either by evaluating X-rays or computerized tomography (CT) scans, is a source of heterogeneity and eventual bias on fusion rate assessment. The main clinical outcomes reported were pain scales (VAS back, VAS leg) and a functional/disability scale (ODI). The low prevalence of Health-Related Quality of Life (HRQoL) questionnaires prevented further evaluation and comparison of both techniques.
Conclusion
iLIF and MI-TLIF for the treatment of lumbar degenerative diseases provide significant clinical improvement and high fusion rates at 12 months or later, without significant difference in complication rates. iLIF has significantly less intraoperative blood loss and reduced length of hospital stay in comparison to MI-TLIF.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Phan, K., Rao, P. J., Kam, A. C. & Mobbs, R. J. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: Systematic review and meta-analysis. Eur. Spine J. 24, 1017–1030 (2015).
Khan, N. R. et al. Surgical outcomes for minimally invasive vs open transforaminal lumbar interbody fusion: An updated systematic review and meta-analysis. Neurosurgery 77, 847–874 (2015).
Ward, S. R. et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J. Bone Joint Surg. Am. 91, 176–185 (2009).
Gibson, J. N. A., Subramanian, A. S. & Scott, C. E. H. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur. spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 26, 847–856 (2017).
Bresnahan, L., Ogden, A. T., Natarajan, R. N. & Fessler, R. G. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976) 34, 17–23 (2009).
Dietz, N. et al. Enhanced recovery after surgery (ERAS) for spine surgery: A systematic review. World Neurosurg. 130, 415–426 (2019).
Chen, X., Chamoli, U., Vargas Castillo, J., Ramakrishna, V. A. S. & Diwan, A. D. Complication rates of different discectomy techniques for symptomatic lumbar disc herniation: A systematic review and meta-analysis. Eur. Spine J. 29, 1752–1770 (2020).
Muthu, S., Ramakrishnan, E. & Chellamuthu, G. Is endoscopic discectomy the next gold standard in the management of lumbar disc disease? Systematic review and superiority analysis. Glob. Spine J. https://doi.org/10.1177/2192568220948814 (2020).
Ahn, Y. Current techniques of endoscopic decompression in spine surgery. Ann. Transl. Med. 7, S169–S169 (2019).
Wagner, R. & Haefner, M. Uniportal endoscopic lumbar interbody fusion. Neurospine 17, S120–S128 (2020).
Kambin, P. Arthroscopic microdiskectomy. Mt. Sinai J. Med. 58, 159–164 (1991).
Fanous, A. A., Tumialán, L. M. & Wang, M. Y. Kambin’s triangle: definition and new classification schema. J. Neurosurg. Spine https://doi.org/10.3171/2019.8.SPINE181475 (2019).
Hoshide, R., Feldman, E. & Taylor, W. Cadaveric analysis of the Kambin’s triangle. Cureus 8, e475 (2016).
Shen, J. Fully endoscopic lumbar laminectomy and transforaminal lumbar interbody fusion under local anesthesia with conscious sedation: A case series. World Neurosurg. 127, e745–e750 (2019).
Wu, J. et al. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. Biomed Res. Int. 2018, 5806037 (2018).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 6, e1000100 (2009).
Kambin, P. Arthroscopic microdiscectomy. Arthrosc. J. Arthrosc. Relat. Surg. 8, 287–295 (1992).
Foley, K. T., Holly, L. T. & Schwender, J. D. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 28, S26–S35 (2003).
Schwender, J. D., Holly, L. T., Rouben, D. P. & Foley, K. T. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. J. Spinal Disord. Tech. 18, S1–S6 (2005).
Slim, K. et al. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J. Surg. 73, 712–716 (2003).
Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 20, 37–46 (1960).
Thakkinstian, A. et al. Systematic review and meta-analysis of the association between β2-adrenoceptor polymorphisms and asthma: A HuGE review. Am. J. Epidemiol. 162, 201–211 (2005).
Keorochana, G., Setrkraising, K., Woratanarat, P., Arirachakaran, A. & Kongtharvonskul, J. Clinical outcomes after minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion for treatment of degenerative lumbar disease: a systematic review and meta-analysis. Neurosurg. Rev. 41, 755–770 (2018).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Higgins, J. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions. (The Cochrane Collaboration, 2011).
Carreon, L. Y. et al. Differentiating minimum clinically important difference for primary and revision lumbar fusion surgeries. J. Neurosurg. Spine 18, 102–106 (2013).
Review Manager (RevMan). (2020).
Park, Y. et al. Surgical outcomes of minimally invasive transforaminal lumbar interbody fusion for the treatment of spondylolisthesis and degenerative segmental instability. Asian Spine J. 5, 228 (2011).
Rouben, D. et al. Long-term durability of minimal invasive posterior transforaminal lumbar interbody fusion: A clinical and radiographic follow-up. J. Spinal Disord. Tech. 24, 288–296 (2011).
Lee, K. H., Yue, W. M., Yeo, W., Soeharno, H. & Tan, S. B. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur. Spine J. 21, 2265–2270 (2012).
Lee, J. C., Jang, H.-D. & Shin, B.-J. Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: Our experience in 86 consecutive cases. Spine (Phila Pa 1976) 37, 1548–1557 (2012).
Saetia, K. et al. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J. Med. Assoc. Thai. 96, 41–46 (2013).
Seng, C. et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: A matched-pair comparison study. Spine (Phila Pa 1976) 38, 2049–2055 (2013).
Gu, G. et al. Comparison of minimally invasive versus open transforaminal lumbar interbody fusion in two-level degenerative lumbar disease. Int. Orthop. 38, 817–824 (2014).
Lee, K. H., Yeo, W., Soeharno, H. & Yue, W. M. Learning curve of a complex surgical technique. J. Spinal Disord. Tech. 27, E234–E240 (2014).
Min, S.-H., Yoo, J.-S. & Lee, J.-Y. Usefulness of contralateral indirect decompression through minimally invasive unilateral transforaminal lumbar interbody fusion. Asian Spine J. 8, 453–461 (2014).
Shen, X. et al. Unilateral versus bilateral pedicle screw instrumentation for single-level minimally invasive transforaminal lumbar interbody fusion. J. Clin. Neurosci. 21, 1612–1616 (2014).
Adogwa, O. et al. A prospective, multi-institutional comparative effectiveness study of lumbar spine surgery in morbidly obese patients: Does minimally invasive transforaminal lumbar interbody fusion result in superior outcomes?. World Neurosurg. 83, 860–866 (2015).
Brodano, G. B. et al. Transforaminal lumbar interbody fusion in degenerative disk disease and spondylolisthesis grade I. J. Spinal Disord. Tech. 28, E559–E564 (2015).
Kuo, C.-H. et al. Dynamic stabilization for L4–5 spondylolisthesis: comparison with minimally invasive transforaminal lumbar interbody fusion with more than 2 years of follow-up. Neurosurg. Focus 40, E3 (2016).
Li, Y., Wang, X., Yan, H., Hao, D. & Liu, Z. The long-term clinical effect of minimal-invasive TLIF technique in 1-segment lumbar disease. Clin. Spine Surg. A Spine Publ. 30, E713–E719 (2017).
Yang, Y. et al. Microendoscopy-assisted minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative disease: short-term and medium-term outcomes. Int. J. Clin. Exp. Med. 8, 21319–21326 (2015).
Fan, G. et al. Patient-reported and radiographic outcomes of minimally invasive transforaminal lumbar interbody fusion for degenerative spondylolisthesis with or without reduction: A comparative study. J. Clin. Neurosci. 33, 111–118 (2016).
Gao, A., Zhao, P., Zhou, Y., Zhang, Q. & Cheng, L. Efficacy of minimally invasive transforaminal lumbar interbody fusion for single-segment lumbar degenerative disease. Biomed. Res. 27, 1309–1315 (2016).
Kim, C. W. et al. Minimally invasive transforaminal lumbar interbody fusion using expandable technology: A clinical and radiographic analysis of 50 patients. World Neurosurg. 90, 228–235 (2016).
Shen, X. et al. Radiographic analysis of one-level minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) with unilateral pedicle screw fixation for lumbar degenerative diseases. Clin. Spine Surg. A Spine Publ. 29, E1–E8 (2016).
Lv, Y. et al. Three-year postoperative outcomes between MIS and conventional TLIF in1-segment lumbar disc herniation. Minim. Invasive Ther. Allied Technol. 26, 168–176 (2017).
Bin Abd Razak, H. R., Dhoke, P., Tay, K.-S., Yeo, W. & Yue, W.-M. Single-level minimally invasive transforaminal lumbar interbody fusion provides sustained improvements in clinical and radiological outcomes up to 5 years postoperatively in patients with neurogenic symptoms secondary to spondylolisthesis. Asian Spine J. 11, 204–212 (2017).
Serban, D., Calina, N. & Tender, G. Standard versus minimally invasive transforaminal lumbar interbody fusion: A prospective randomized study. Biomed Res. Int. 2017, 7236970 (2017).
Yang, Y. et al. Learning curve of microendoscopy-assisted minimally invasive transforaminal lumbar interbody fusion: 65 consecutive cases of one surgeon. Int. J. Clin. Exp. Med. 10, 9424–9431 (2017).
Yang, Y. et al. Hidden and overall haemorrhage following minimally invasive and open transforaminal lumbar interbody fusion. J. Orthop. Traumatol. 18, 395–400 (2017).
Zhang, D., Mao, K. & Qiang, X. Comparing minimally invasive transforaminal lumbar interbody fusion and posterior lumbar interbody fusion for spondylolisthesis: A STROBE-compliant observational study. Medicine Baltimore 96, e8011 (2017).
Wu, A.-M. et al. Comparison of minimally invasive and open transforaminal lumbar interbody fusion in the treatment of single segmental lumbar spondylolisthesis: Minimum two-year follow up. Ann. Transl. Med. 6, 105 (2018).
Zhao, Y. et al. Comparison of bilateral versus unilateral decompression incision of minimally invasive transforaminal lumbar interbody fusion in two-level degenerative lumbar diseases. Int. Orthop. 42, 2835–2842 (2018).
Goh, G.S.-H. et al. Elderly patients undergoing minimally invasive transforaminal lumbar interbody fusion may have similar clinical outcomes, perioperative complications, and fusion rates as their younger counterparts. Clin. Orthop. Relat. Res. 478, 822–832 (2020).
Kolcun, J. P. G., Brusko, G. D., Basil, G. W., Epstein, R. & Wang, M. Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: Operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg. Focus 46, E14 (2019).
Lin, G.-X. et al. Time course observation of outcomes between minimally invasive transforaminal lumbar interbody fusion and posterior lumbar interbody fusion. Neurol. Med. Chir. (Tokyo) 59, 222–230 (2019).
Mokawem, M., Katzouraki, G., Harman, C. L. & Lee, R. Lumbar interbody fusion rates with 3D-printed lamellar titanium cages using a silicate-substituted calcium phosphate bone graft. J. Clin. Neurosci. 68, 134–139 (2019).
Yang, J. et al. Percutaneous endoscopic transforaminal lumbar interbody fusion for the treatment of lumbar spinal stenosis: Preliminary report of seven cases with 12-month follow-up. Biomed Res. Int. 2019, 3091459 (2019).
Zhao, Y., Liang, Y. & Mao, K. Radiographic and clinical outcomes following MIS-TLIF in patients with adult lumbar degenerative scoliosis. J. Orthop. Surg. Res. 13, 93 (2018).
Zhao, L., Yu, H., Zhang, Y. & Zhen, W. Comparison of the efficacy of MIS-TLIF combined with unilateral or bilateral internal fixation on single-segment lumbar degenerative diseases. Eur. J. Inflamm. 17, 205873921984439 (2019).
Ao, S. et al. Comparison of preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: Is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int. J. Surg. 76, 136–143 (2020).
Chan, A. K. et al. A comparison of minimally invasive and open transforaminal lumbar interbody fusion for grade 1 degenerative lumbar spondylolisthesis: An analysis of the prospective quality outcomes database. Neurosurgery https://doi.org/10.1093/neuros/nyaa097 (2020).
El Mansy, Y., Migliorini, F., Tingart, M. & Madarassy, G. Minimally versus conventional-invasive transforaminal lumbar interbody fusion in patients with failed back surgery syndrome. Musculoskelet. Surg. https://doi.org/10.1007/s12306-020-00659-7 (2020).
Jin, M., Zhang, J., Shao, H., Liu, J. & Huang, Y. Percutaneous transforaminal endoscopic lumbar interbody fusion for degenerative lumbar diseases: A consecutive case series with mean 2-year follow-up. Pain Physician 23, 165–174 (2020).
Kim, J.-E., Yoo, H.-S., Choi, D.-J., Park, E. J. & Jee, S.-M. Comparison of minimal invasive versus biportal endoscopic transforaminal lumbar interbody fusion for single-level lumbar disease. Clin. Spine Surg. https://doi.org/10.1097/BSD.0000000000001024 (2020).
Wang, Y., Zhang, Y., Chong, F., Zhou, Y. & Huang, B. Clinical outcomes of minimally invasive transforaminal lumbar interbody fusion via a novel tubular retractor. J. Int. Med. Res. 48, 300060520920090 (2020).
Morgenstern, C. et al. Full percutaneous transforaminal lumbar interbody fusion using the facet-sparing, Trans-Kambin approach. Clin. Spine Surg. 33, 40–45 (2020).
Morgenstern, R. & Morgenstern, C. Percutaneous transforaminal lumbar interbody fusion (pTLIF) with a posterolateral approach for the treatment of degenerative disk disease: feasibility and preliminary results. Int. J. Spine Surg. 9, 41 (2015).
Syed, H. & Voyadzis, J.-M. True percutaneous transforaminal lumbar interbody fusion: Case illustrations, surgical technique, and limitations. J. Neurol. Surg. A. Cent. Eur. Neurosurg. 77, 344–353 (2016).
Funding
The present publication was funded by Fundação Ciência e Tecnologia, IP national support through CHRC (UIDP/04923/2020).
Author information
Authors and Affiliations
Contributions
J.M.S., H.R., J.L.S. and J.G.C. made substantial contributions to the conception and design of the work; J.M.S. and H.R. performed the literature search and data extraction; All authors made substantial contributions to the analysis and interpretation of data; J.M.S. and P.N. drafted the work; All authors revised it critically for important intellectual content; All authors approved the version to be published; All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sousa, J.M., Ribeiro, H., Silva, J.L. et al. Clinical outcomes, complications and fusion rates in endoscopic assisted intraforaminal lumbar interbody fusion (iLIF) versus minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): systematic review and meta-analysis. Sci Rep 12, 2101 (2022). https://doi.org/10.1038/s41598-022-05988-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05988-0
This article is cited by
-
The novel Kambin Torpedo full-endoscopic lumbar interbody fusion technique: a case series
European Spine Journal (2024)
-
Transforaminal lumbar interbody fusion with a tantalum cage: lumbar lordosis redistribution and sacral slope restoration with a modified posterior technique
Journal of Orthopaedics and Traumatology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.