Abstract
The ability of animals to produce endogenous heat provides a buffer against environmental changes but also incurs high energetic costs. Especially small endothermic mammals have high energy demands. Some temperate-zone species (heterotherms) regularly use torpor, which slows down their entire metabolism but also potentially delays reproduction, to compensate for this. We used a unique experimental approach to test the consequences of extended low and high ambient temperatures on the trade-off in energy allocation to body mass maintenance, thermoregulation effort and seasonal sexual maturation in temperate zone male bats. We showed that long exposure to low ambient temperature shifts energy allocation away from sexual maturation to self-maintenance and results in a delay of sperm maturation by as much as an entire month. This effect was partially buffered by higher body mass. Heavier bats were able to afford more intensive thermoregulation and consequently speed up maturation. Interestingly, bats at constant high temperatures avoided deep torpor and matured faster than those at low temperatures, but sperm production was also slower than under natural conditions. Our results show that not only low, but also constant high ambient temperatures are detrimental during seasonal sexual maturation and the trade-off between investing into self-maintenance and fitness is a finely tuned compromise.
Similar content being viewed by others
Introduction
Reproductive performance and survival are determined by fundamental energetic trade-offs. Animals are often faced with unpredictable environments due to variation in environmental conditions, such as food availability or weather, which makes balancing their energy budget challenging1,2. Many animals possess mechanisms to cope with such environmental variation. These mechanisms can be physiological (such as changes in metabolic rate, body temperature, or digestive function) or behavioural (including foraging behaviour, movement or roost choice)3,4,5. For example, endogenous heat production buffers the effects of variation in ambient temperature on important biological functions and reduces dependence on weather. Yet, endothermy is energetically demanding6,7. This demand is particularly high in small animals, including bats, as they have high metabolisms and lose heat rapidly due to their high surface to volume ratio8. Bats especially, are prone to heat and water loss due to the large naked surface of their wing membranes and the poor insulation properties of their pelage9,10.
Bats commonly compensate for energetic constraints by using torpor. Torpor is characterized by controlled metabolic suppression and lower body temperature11,12. Torpor reduces energy expenditure, thus allowing conservation of fat reserves13, which improves self-maintenance and survival during energetic bottlenecks11,14,15. Yet, torpor is also costly. A low metabolic rate down-regulates other physiological processes including those related to reproduction (e.g.,16,17,18). For example, testicle tissue becomes unresponsive to endocrine signals at low temperatures, and thus spermatogenesis proceeds at a slower pace19,20. In the large order of bats, the effects of torpor on reproduction have been studied almost exclusively in females (e.g.17,21,22) where time spent at low body temperatures negatively influences milk production and foetal development17,22. The effects of torpor on male reproductive performance remain poorly understood.
In bats from the temperate zone, sperm production and mating are temporally separated23. In spring, the testes grow in size due to enlargement of the tubular diameter24,25 and reach their maximum size during summer25. Afterwards, the spermatozoa are transferred to the cauda epididymis for storage until mating, and the testes regress again1,25. This process is repeated every year in mature males, which can be referred as “seasonal sexual maturation”26. Spermatogenesis is costly; due to intensive tissue growth of the seminiferous epithelium, testes can reach as much as 8% of body mass27. Moreover, the need for nutrients to proliferate germ cells increases (reviewed by Pescovitz et. al28 and Sharpe et. al29). At the same time, male bats should limit torpor use (e.g.30). Indeed, a reduced metabolic rate is suggested to slow down sexual maturation31. It was previously demonstrated that male bats exposed to both high and low food treatments all avoided torpor use during spermatogenesis26. However, males on a restricted diet reached full sexual maturation later than well-fed males because they were forced to allocate energy into maintaining their body mass, resulting in slower maturation. Thus, avoiding torpor is necessary to keep sperm production going, but may need to be balanced against survival32.
Several strategies for balancing energy allocation between thermoregulation and spermatogenesis in bats have been proposed. For example, bats can increase foraging activity and hence food intake14,30,33,34. Another suggested strategy to optimize energy budget during spermatogenesis is to become social. The males of some species of bats form temporary bachelor colonies during summer35, probably to benefit from more efficient foraging on ephemeral insects and or social thermoregulation36. Additionally, social thermoregulation reduces heat loss. Finally, if conditions are harsh, males may still use torpor (e.g.26,36,37,38) but only during the coldest time of the day, when the cost of heat production is the highest36,37. This compromise should promote survival, but at the cost of reduced or slowed-down sperm production31,39,40.
It is challenging to disentangle the effects of ambient temperature and food availability on the pace of sexual maturation in free ranging animals, but especially on insectivorous male bats. Insect activity, and thus food availability covary with weather conditions and affect bats' behaviour36,41, but the direct effect of temperature on sexual maturation remains untested. In this study, we experimentally tested the trade-off between energy allocated to thermoregulation and to seasonal sexual maturation in male parti-coloured bats, Vespertilio murinus. We exposed bats to two different ambient temperatures in order to mimic the upper and lower range of temperatures they may experience in their natural environment. The "warm" treatment temperature (25 °C) was close to the bats’ thermoneutral zone. The "cold" treatment (10 °C) was in the lower range of local temperature usually recorded during the corresponding time period (average minima and maxima for natural ambient temperatures in Białowieża in years 2014—2016: June : 6.9–27.9 °C, July: 6.6–32.4 °C, August: 5.05—32 °C; local weather stationin Białowieża). Additionally, we controlled for food intake to avoid complex confounding effects on sexual maturation rate26. Vespertilio murinus is one of the species where males aggregate in colonies during summer35. Colonies are formed during the time of spermatogenesis, and it has been hypothesized that colonial males profit from social thermoregulation, similar to reproductive females, to promote sperm maturation rate and to be ready for mating sooner35,42. Thus, this species presents an ideal system to test the trade-offs in energy allocation to self-maintenance versus reproduction.
There are two potential scenarios how low ambient temperature might affect seasonal sexual maturation rate in male bats. In scenario one, we hypothesized that low ambient temperatures should increase torpor use, but males in both treatments should avoid body temperatures close to ambient temperatures and actively thermoregulate in order to maintain sperm production. However, costs of thermoregulation should then be relevantly higher for individuals kept at low ambient temperature. As a result, to balance their energy budget, bats kept at low temperature may gain less or even lose mass, but sexual maturation would not be significantly delayed. Heavier individuals should have larger energy resources, facilitating investment into reproduction. In scenario two, we hypothesized that male bats should prioritize self-maintenance and body mass should not decrease. They should then use torpor and thermoconform to low ambient temperature at the expense of slowed-down or even suspended sexual maturation.
Results
Body mass
The best model for body mass (Mb) included forearm length (FA) as a proxy for size, time (day since capture) grouped by treatment, and treatment as independent variable (Table S1). There was no significant effect of FA or treatment itself on Mb (P > 0.05; Table 1). However, changes within each treatment over time were significant (T25°C P < 0.001, T10°C P < 0.001; Fig. 1, Table 1). In the T25°C treatment, Mb of bats decreased during the first ten days of being exposed to 25 °C (Table 1) from an initial mean of 11.4 g (range: 10.1–12.6 g) to 10.2 g (range: 9.2–11.1 g; Fig. 1). After 20 days (ten days of experiment, in the beginning of July), we observed a small but constant increase of Mb (Fig. 1). The final Mb did not differ from the initial values (mean 11.0 g, range: 9.3–13.5 g). In contrast, mean Mb of bats in the T10°C treatment increased during the first seven days of being kept at 10 °C (Table 1) from 11.7 g (range: 9.9–13.1 g) to 12.0 g (range: 9.7–13.9 g). After day 20, this trend switched to a constant decrease until the end of the experiment (mid-August). The final mean Mb was 10.7 g (range: 8.6–13.4 g) (Fig. 1, Table 1).
Thermoregulation
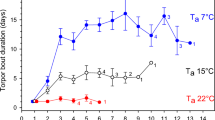
The mean daily skin temperature (Tsk) was different between treatments. In the T25°C treatment the mean daily Tsk increased from an initial 28.1 °C (range: 25.5–30.7 °C) up to a mean 30.9 °C (range 29.0–32.0 °C) after 20 days of treatment and then slowly decreased to 28.2 °C (range 27.0–30.4 °C). In the T10°C treatment the mean daily Tsk constantly decreased throughout the course of the experiment, from an initial 24.8 °C (range: 22.9–27.3 °C) down to 12.2 °C (range: 10–15.7 °C) at the end of the experiment. In the T25°C treatment, skin temperature fluctuated during the day (with the range between 24.2 °C and 30.6 °C). The mean Tsk—onset (the threshold Tsk below which we considered bat to be in torpor) was 32.2 °C (range 31.7–32.7 °C, established for each individual separately). There was an average of 2.6 torpor events per day (range: 1–7) and mean torpor event duration was 14.7 h (range: 4.7–17.8 h). At T10°C there was an average of one torpor event per day (range: 1–3) and mean torpor event duration was 17.6 h (range: 6.3–17.8 h) at a mean Tsk of 10.7 °C (range: 8.6–24.4 °C). Once bats decreased their Tsk to almost as low as Ta after evening handling, they remained torpid until evening arousal.
The best model for mean daily difference (ΔT) between ambient temperature (Ta) and Tsk, (i.e., estimated thermoregulation effort), included time grouped by treatment, Mb grouped by treatment and treatment as an independent variable (Table S2). There was no difference in ΔT between treatments (P > 0.05; Fig. 2A, Table 2). However, there were significant changes within each treatment over time (T25°C P < 0.001, T10°C P < 0.001; Table 2). After 18 days at T25°C, ΔT increased from 2.5 to 5.7 °C (Fig. 2A) and remained elevated but decreased slowly (Fig. 2A, Table 2). In contrast, T10°C animals had an average ΔT of 8.6 °C at the beginning of treatment, which decreased within 10 days down to 3.9 °C. Later there were two peaks of ΔT: a stronger one around day 28 (5.8 °C) and a smaller one around day 52. (4.7 °C) (Fig. 2A, Table 2). Mb was significantly positively correlated with ΔT in the T25°C treatment (P > 0.05; Fig. 2B, Table 2).
Sexual maturation rate of bats in the two treatments. The y-axis shows the average experimental day on which T25°C bats reached the next consecutive maturation class. (A) Delay in time until T10°C bats reached the same threshold. The y-axis shows the average day since the beginning of the experiment on which T25 °C bats reached the next consecutive maturation class. The x-axis shows the delay in time until T10 °C bats reached the same class. Each delay is presented with its 95% credible intervals (CI). (B) The effect of Mb (body mass) on the time of transition to consecutive classes. (C) The effect of ΔT (difference between Tsk and Ta) on the transition time to consecutive classes. Asterisks indicate significant effects.
Seasonal sexual maturation
Only one bat in the entire experiment reached seasonal sexual maturation class 4. This male was from the T25°C treatment. Thus, posterior distributions of the effects related to the transition from sexual maturation class 3 to 4 represented the prior distributions rather than any effect calculated from the data. We thus did not consider the fourth threshold. At T10°C only two males reached class 3.
Bats kept at T25°C reached sexual maturation class 2 on day 39 (end of July) on average, while T10°C bats were significantly delayed by 34 days on average (Table 3; Fig. 3A). The time needed to switch to class 3 was 62 days (counting from the beginning of the captivity) for T25°C on average (mid-August). Bats from the T10°C treatment required significantly more time: 37 days (end of September) (Table 3, Fig. 3A). At T25°C there was no significant effect of Mb or ΔT on sexual maturation rate at any of the transition time points (Table 3). At T10°C both Mb and ΔT had a significant effect only on the transition from class 1 to 2 (Table 3, Fig. 3B, C). The heavier an individual, the less time it needed to reach class 2 (Table 3, Fig. 3B). Similarly, the higher ΔT and thus thermoregulation effort, the faster the transition from sexual maturation class 1 to 2 (Table 3, Fig. 3C).
Discussion
In nature, balancing the energetic trade-off between self-maintenance and reproduction can become challenging when animals face harsh environmental conditions. We investigated how heterothermic males prioritize energy allocation during seasonal sexual maturation.
As expected, we found that male bats exposed to T25°C seasonally sexually matured faster and reached a more advanced stage than those kept at T10°C. Extrapolating from this results, bats from the T10°C treatment would have reached sexual maturity almost a month later than those from T25°C treatment. Sexual maturation rate is expected to depend on the energy balance between food intake, self-maintenance, and investment in thermoregulation26. We thus expected that heavier bats would have more energy to allocate to thermoregulation while still maintaining sperm production. We did find a significant effect of body mass and thermoregulation on seasonal sexual maturation rate, but only among bats exposed to low ambient temperatures. Above a certain level ambient and thus body temperature might be high enough to maintain a high rate of sperm production, but it is not possible to further speed up this physiological process without additional food2.
Despite being exposed to extremely different ambient temperatures, males did not show significant differences in body mass between the two treatments although weights of bats from the T10°C treatment decreased slightly. This small but constant loss of weight indicates that food intake was not sufficient to balance the energy budget. Bats can deplete their fat reserves if are not able to balance their energy budget, in this case due to increased demands of sexual maturation30,31,37.
Individuals kept at low ambient temperature spent most of the time thermoconforming, i.e., their body temperature remained close to ambient temperature. For those individuals, the mean difference between body and ambient temperature was mainly a consequence of morning cooling and evening arousals. Morning cooling was a passive process and probably depended strongly on the bats’ digestion intensity. Nevertheless, even these short term increases in energy expenditures may have exceeded the energy intake of the bats and caused the observed weight loss. In contrast, bats from the T25°C treatment actively thermoregulated almost continuously, even though they did not completely maintain normothermia either, and were in shallow torpor. This was a consequence of high ambient temperature, which made deeper torpor impossible. However, even shallow torpor can enable animals to save some energy43.
Partly in line with our prediction, we found a significant correlation between body mass and ΔT, but interestingly only in the T25°C treatment. In this treatment, heavier individuals may have allocated energy to thermoregulation as well as spermatogenesis, while maintaining mass, similar to what was found in ad libitum fed bats in a food deprivation experiment26. Our results correspond to other observations44,45 which showed that torpor use in other small heterothermic mammals decreases with an increase in body mass.
Sexual maturation of individuals exposed to low ambient temperature was strongly slowed down. The short periods of raised metabolic rate and body temperature during feeding and digesting may have been sufficient to drive marginal testicular growth. Similarly, spermatogenesis of ground squirrels is likely restricted to arousal periods from torpor19, as spermatogenesis proceeds more slowly at lower body temperatures20. Heavier bats which likely had more fat reserves, may have been able to use the time of elevated body temperature to invest more in sexual maturation, despite similar thermoregulation effort in all "cold" bats.
Decreased thermoregulation effort and higher body mass significantly reduced the time for transition from maturation class 1 to 2 (at T10°C). During the seasonal sexual maturation process, this particular stage corresponds to intense production of gametes (reviewed by 25,46). The seminiferous epithelium of the testis is sensitive to cooling47. In mammals including heterothermic species, such as hedgehogs48 the enzyme system in the testes works most efficiently at a very narrow temperature range49,50. Mammalian cell growth and gonadal steroidogenesis are generally strongly reduced at body temperatures below 20 °C (e.g.49,50). Consequently, deep and prolonged torpor may cause stagnation of spermatogenesis at various stages of meiosis19,20. Our results support the assumption that some heterothermic species appear to have mechanisms which enable at least partial continued gametogenesis and endocrine responsiveness at low temperatures19. Low temperatures are favourable for sperm storage51, which suggests that later thermoregulation can be relaxed during the transfer of spermatozoa to the cauda epididymis.
Free-ranging colonies of male V. murinus break up after reaching sexual maturation stage 236,42,52,53. After this stage, according to our results, the need for maintaining high body temperature is lessened. Our results thus provide evidence to support the social warming hypothesis35,42 explaining why male bats form colonies.
We extrapolated from our results that bats from the T25°C treatment would not reach sexual maturity until late September, if continued to be kept at 25 °C. Thus, bats exposed to constant elevated temperature would reach sexual maturity more than one month after wild individuals36,53 or captive ones maintained under naturally fluctuating ambient temperatures and provided with high amounts of food26 (Fig. 4). This coincides with the delay in food-restricted males26. Our results can suggest that at least brief periods of torpor use may favour in process of seasonal sexual maturation. This might be a result of the need for small energy savings to maintain spermatogenesis (see also above) or limiting water loss11,43. During periods of increased energy requirements, such as spermatogenesis, fine temperature tuning may be thus required to facilitate physiological processes. In nature bats can manipulate their body temperature in part by choosing suitable roosts according to their current needs54,55. However the role of brief periods of torpor in the process of seasonal sexual maturation requires more detailed studies.
Effect of high and low ambient temperature or food availability on seasonal sexual maturation rate. The proportion of male bats at each sexual maturation classes over the course of the study and under different treatments. Data from the “High food” treatment are from an experiment, where V. murinus males were fed with a large amount of food and exposed to natural ambient temperature26. Both experiments started on the same calendar days and the numbering of experimental days is the same in both studies.
Mating readiness and synchronisation with female receptivity should be under strong selective pressure in promiscuous species56. Males that encounter low ambient temperatures or abnormally high temperatures may not be ready for mating in a given year, which would have serious consequences for their reproductive success. Spermatogenesis takes places in spring shortly after hibernation in heterothermic species (e.g., chipmunks Tamias striatus and ground squirrels) which have higher reproductive success if they use less torpor during this period19,57. This delay in readiness for reproduction might be seen as a non-energetic cost of hibernation58 but also heterothermy in general. Male sociality during critical stages of seasonal sexual maturation has likely evolved to facilitate sperm production in species relying on ephemeral food resources.
Methods
Ethical statement
All experimental procedures were approved by the General Director for Environmental Protection in Poland (authorization no. DZPWg.6401.09.2.2014, DZP-Wg.6401.09.1.2015, DZPWg.6401.09.5.2016) and by the Local Ethical Commissions in Białystok and Olsztyn (authorization no. 11/2014, 14/2015, 120/2015, 150/2015, 15/ 2015, 45/2015). All methods were carried out in accordance with relevant guidelines and regulations. The study was performed in accordance with the ARRIVE guidelines.
Experimental animals and housing conditions
We captured 36 bats from a free-ranging colony of approximately 100 males of Vespertilio murinus in mid-June 2017 in the Bieszczady region (SE Poland, 49°10′36″N 22°26′01″E, 644 m.a.s.l.). We caught bats during their evening emergence from the roost using a harp trap (Austbat, Bat Conservation and Management) and a custom-built funnel trap. We marked all individuals with subcutaneous PIT Tags (Trovan ID-100B /1.4, 8 mm), weighed them (± 0.1 g; Pesola PTS3000 General Electronic Scale) and measured their forearm length (± 0.1 cm) as a proxy for body size. Body mass (Mb) at capture ranged from 9.4—12.8 g (mean 11.1 g) and forearm length (FA) from 34.0—46.9 mm (mean 44.0 mm). Bats were transported to the Mammal Research Institute of the Polish Academy of Sciences in Białowieża (MRI PAS), where the experiment took place. During an initial 10-day acclimation period, we trained bats to feed independently on mealworms (2 g/day each throughout the captive period based on previous experience26). This diet was supplemented with vitamins (solBiosupervit, Biofaktor, Skierniewice, Poland) added to the water (offered at libitum) every fourth day26,59. During the acclimation period, bats were housed in a closed flight room26. Bats were provided with hollow tree-trunks as roosts60 and were allowed to freely choose group size and composition. During the acclimation time, bats were allowed to fly freely for three hours every day. Upon completion of the experiment, we released the bats in the evening at the capture site. We defined the entire time when bats were in MRI PAS as “captivity” and as “experiment” when they were under temperature treatment ten days into captivity. We numbered days consecutively starting from the day of capture.
Experimental design
Our experiment started at the end of June and lasted until mid-August (52 days), and thus overlapped with the social and solitary periods of V. murinus. Indeed, in eastern Poland, male V. murinus briefly form colonies from the beginning of June until the beginning of July36,53. We caught the males when they were at the early stage of testicle growth and kept them until seasonal sexual maturation should be strongly advanced if not completed53.
We exposed bats to two experimental temperature treatments. Half of the bats were kept at 10 °C (T10°C) and the others at 25 °C (T25°C). The 10 °C treatment was chosen as this is the lowest temperature usually recorded in the region during the experimental period. We chose 25 °C as the high temperature treatment to keep the bats below their thermoneutral zone9. Bats were assigned to treatments according to individual body condition index (FA / Mb) at capture, resulting in two groups with the same BCI distribution. Due to health problems we had to exclude four individuals from the experiment (one from the T25°C and three from the T10°C treatment; final sample size: T25˚C = 17; T10˚C = 15). During the experiment bats from each treatment were kept in groups of three, in wire-mesh cages in temperature-controlled cabinets (Pol-Eko Aparatura, model ST 700 BASIC). This group size was chosen to avoid social isolation stress, while limiting social thermoregulation. Composition of each group remained the same throughout the experiment. We suspended folded paper towels in each cage to offer bats the opportunity to hide. We took bats out of the cages every evening for feeding and weighing. During the experiment, each group was offered the opportunity to fly for three hours every second day in the enclosure where they were kept during the acclimation period.
Body mass and skin temperature
We measured Mb every evening before feeding. We assumed that the increase or decrease in Mb was related to changes in stored fat, and consequently to changes in body condition.
We measured skin temperature (Tsk) as a proxy for body temperature using temperature data logger (iButtons, model DS1922L, Dallas Semiconductors, TX, US; miniaturized61) glued to the skin on the bats' back with tissue adhesive (Sauer Hautkleber, Manfred Sauer, Germany). iButtons weighed approximately 1.6 g (11–18% of individual Mb), but did not disturb the animals or affect their ability to fly, based on our observations and experience26. iButtons recorded temperature every 10 min with a 0.5 °C-resolution. The iButtons detached spontaneously after some time, and duration of attachment varied from two weeks to two months. Once an iButton detached, we replaced it only after a break of two weeks minimum. Throughout the experiment at least one-third of the experimental animals (12 individuals, six from each treatment) were equipped with temperature loggers at the same time. We excluded Tsk recordings from time periods when bats were removed from the cage for everyday feeding and when they were flying.
We describe variation in thermoregulation effort using the mean daily difference (ΔT) between ambient temperature (Ta) and Tsk. ΔT allows us to directly compare energy expenditures, as an increase in body temperature (measured here as Tsk) by one degree is expected to require the same energy input at any Ta below the thermoneutral zone12,62. We also described torpor use by the bats. We determined the torpor onset threshold for each individual following the Eq. 63:
We assumed a bat was using torpor if at least three Tsk recordings in a row were below the threshold.
Sexual maturation
We used a five-step classification based on testicular growth and epididymal filling to describe seasonal sexual maturation stages52. A single observer (EK) assessed stages through external visual examination of the testes and caudae epididymis approximately every ten days. Testicular maturation stages were assigned to five classes from 0 (no to small testes at the penis base with no cauda epididymis visible), through 2 (testes at their maximum size, situated below the penis and cauda epididymis clearly visible as dark patches, but still flat) up to 4 (testes regressed and cauda epididymis completely filled); classes 1 and 3 were intermediate between these classes52 (detailed description in supplementary materials26). Partial filling of cauda epididymis indicates high advancement in spermatogenesis and that spermatozoa are being moved from the testes to cauda epididymis for maturation24,64. We defined the time needed to move from one class to the next as sperm production rate (seasonal sexual maturation rate) and modelled this rate for each individual (see below).
Statistics
Body mass and thermoregulation
We tested for the effects of the two temperature treatments on Mb and ΔT using Generalized Additive Mixed Models (GAMM, mgcv’ package65). Models testing for Mb included three explanatory variables: treatment, FA and time (day since capture). Models for the analysis of ΔT included time, Mb and treatment. In models testing for Mb and ΔT, all variables were continuous except for treatment which was a two-level factor (T25°C and T10°C). We included bat ID nested in group as a random effect. We assumed that the differences in the effect of each factor on Mb and ΔT between treatments were significant when confidence intervals (CI) of treatments did not overlap. We selected the best models for Mb and ΔT based on Akaike’s information criterion (AIC, model selection in Table S1).
Seasonal sexual maturation rate
We estimated the effect of treatments as well as the interaction of Mb and ΔT with treatments, on seasonal sexual maturation over the course of the experiment. We used hierarchical ordinal models using Bayesian inferences66. Mb and ΔT corresponded to values recorded on the day of testes examination (expressed as sexual maturation class). Missing values in ΔT or Mb were modelled from prior Gaussian distributions characterised by single estimated means and precisions. Bats from the T25°C treatment were considered as the baseline. We centred values of ΔT and Mb. Consecutive days since capture were divided by 365 in order to ease the initial convergence of the chains.
A continuous latent sexual maturation variable was modelled and related to the time since capture with a random intercept linear mixed model. Bat ID was included as a random effect. We estimated the days corresponding to switches from one sexual maturation class to the next along the latent variable (defined as “transition time point”). We assumed that bats switched from class 0 to 1 independently from treatments, ΔT or Mb. This class threshold was set at day 10 of captivity (i.e., the onset of the experiment). On that day, half of the bats had already passed the first threshold. We also estimated the effects of treatment, Mb, ΔT and the interactions (treatments * Mb and treatments * ΔT) on the threshold between sexual maturation classes. Effects were considered significant when their posterior distribution 95% credible intervals (CI) did not overlap with 0.
We ran three different Markov chains starting at random initial values in the range of parameter space for 50′000 iterations with a 20′000-iteration burn-in. Markov chains were thinned by a factor of three and the Brooks-Gelman-Rubin criterion (R̂) was used to assess the convergence of chains, indicated when R̂ < 1.167. We used the function ‘jags’ in the package: ‘jagsUI’68 to run the analysis. The model with the description of prior distributions can be found in the supplementary material.
Data availability
Data available from the Open Forest Data Repository: https://doi.org/10.48370/OFD/GEGGMQ.
References
Thomas, D. W., Fenton, M. B. & Barclay, R. M. R. Social-behavior of the little brown bat, myotis-lucifugus. 1. mating-behavior. Behav. Ecol. Sociobiol. 6, 129–136. https://doi.org/10.1007/bf00292559 (1979).
Weiner, J. Physiological limits to sustainable energy budgets in birds and mammals-ecological implications. Trends Ecol. Evol. 7, 384–388. https://doi.org/10.1016/0169-5347(92)90009-z (1992).
Becker, N. I., Encarnação, J. A., Kalko, E. K. V. & Tschapka, M. The effects of reproductive state on digestive efficiency in three sympatric bat species of the same guild. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 162, 386–390. https://doi.org/10.1016/j.cbpa.2012.04.021 (2012).
Becker, N. I., Encarnação, J. A., Tschapka, M. & Kalko, E. K. V. Energetics and life-history of bats in comparison to small mammals. Ecol. Res. 28, 249–258. https://doi.org/10.1007/s11284-012-1010-0 (2012).
Ruf, T. & Bieber, C. Physiological, behavioral, and life-history adaptations to environmental fluctuations in the edible dormouse. Front. Physiol. https://doi.org/10.3389/fphys.2020.00423 (2020).
Scholander, P. F., Hock, R., Walters, V. & Irving, L. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol. Bull. 99, 259–271. https://doi.org/10.2307/1538742 (1950).
Geiser, F. & Ruf, T. Hibernation versus daily torpor in mammals and birds-physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966. https://doi.org/10.1086/physzool.68.6.30163788 (1995).
Aschoff, J. Thermal conductance in mammals and birds-its dependence on body size and circadian phase. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 69, 611–619. https://doi.org/10.1016/0300-9629(81)90145-6 (1981).
McNab, B. K. The economics of temperature regulation in neotropical bats. Comp. Biochem. Physiol 31, 227–268. https://doi.org/10.1016/0010-406X(69)91651-X (1969).
Speakman, J. R. & Thomas, D. W. in Bat ecology (ed Thomas H. Kunz and M. Brock Fenton) 430–490 (University of Chicago Press, 2003).
Wang, L. C. H. & Wolowyk, M. W. Torpor in mammals and birds. Can. J. Zool.-Rev. Can. Zool. 66, 133–137. https://doi.org/10.1139/z88-017 (1988).
Geiser, F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. https://doi.org/10.1146/annurev.physiol.66.032102.115105 (2004).
Geiser, F. & Masters, P. Torpor in relation to reproduction in the mulgara, dasycercus-cristicauda (dasyuridae, marsupialia). J. Therm. Biol. 19, 33–40. https://doi.org/10.1016/0306-4565(94)90007-8 (1994).
Wojciechowski, M. S., Jefimow, M. & Tęgowska, E. Environmental conditions, rather than season, determine torpor use and temperature selection in large mouse-eared bats (Myotis myotis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 828–840. https://doi.org/10.1016/j.cbpa.2006.06.039 (2007).
Ruf, T. & Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 90, 891–926. https://doi.org/10.1111/brv.12137 (2015).
Tuttle, M. D. Population ecology of the gray bat (Myotis grisescens): factors Iifluencing growth and survival of newly volant young. Ecology 57, 587–595. https://doi.org/10.2307/1936443 (1976).
Racey, P. A. & Swift, S. M. Variations in gestation length in a colony of Pipistrelle bats (Pipistrellus pipistrellus) from year to year. J. Reprod. Fertil. 61, 123–129. https://doi.org/10.1530/jrf.0.0610123 (1981).
Audet, D. & Fenton, M. B. Heterothermy and the use of torpor by the bat Eptesicus fuscus (Chiroptera, Vespertilionidae)-a field study. Physiol. Zool. 61, 197–204. https://doi.org/10.1086/physzool.61.3.30161232 (1988).
Barnes, B. M., Kretzmann, M., Licht, P. & Zucker, I. The influence of hibernation on testis growth and spermatogenesis in the golden mantled ground squirrel, Spermophilus lateralis. Biol. Reprod. 35, 1289–1297. https://doi.org/10.1095/biolreprod35.5.1289 (1986).
Gagnon, M. F., Lafleur, C., Landry-Cuerrier, M., Humphries, M. M. & Kimmins, S. Torpor expression is associated with differential spermatogenesis in hibernating eastern chipmunks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 319, R455–R465. https://doi.org/10.1152/ajpregu.00328.2019 (2020).
McLean, J. A. & Speakman, J. R. Energy budgets of lactating and non-reproductive Brown Long-Eared Bats (Plecotus auritus) suggest females use compensation in lactation. Funct. Ecol. 13, 360–372. https://doi.org/10.1046/j.1365-2435.1999.00321.x (1999).
Wilde, C. J., Knight, C. R. & Racey, P. A. Influence of torpor on milk protein composition and secretion in lactating bats. J. Exp. Zool. 284, 35–41. https://doi.org/10.1002/(sici)1097-010x(19990615)284:1%3c35::aid-jez6%3e3.0.co;2-z (1999).
Racey, P. A. The prolonged storage and survival of spermatozoa in Chiroptera. J. Reprod. Fertil. 56, 391–402. https://doi.org/10.1530/jrf.0.0560391 (1979).
Racey, P. A. The reproductive cycle in male noctule bats, Nyctalus noctula. J. Reprod. Fertil. 41, 169–182. https://doi.org/10.1530/jrf.0.0410169 (1974).
Gustafson, A. W. Male reproductive patterns in hibernating bats. J. Reprod. Fertil. 56, 317–0 (1979).
Komar, E., Dechmann, D. K. N., Fasel, N. J., Zegarek, M. & Ruczyński, I. Food restriction delays seasonal sexual maturation but does not increase torpor use in male bats. J. Exp. Biol. https://doi.org/10.1242/jeb.214825 (2020).
Wilkinson, G. S. & McCracken, G. F. in Bat ecology (eds Thomas H. Kunz & M. Brock Fenton) 128–155 (University of Chicago Press, 2003).
Pescovitz, O. H., Srivastava, C. H., Breyer, P. R. & Monts, B. A. Paracrine control of spermatogenesis. Trends Endocrinol. Metab. 5, 126–131. https://doi.org/10.1016/1043-2760(94)90094-9 (1994).
Sharpe, R. M., Kerr, J. B., McKinnell, C. & Millar, M. Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J. Reprod. Fertil. 101, 193–198 (1994).
Becker, N. I., Tschapka, M., Kalko, E. K. V. & Encarnacao, J. A. Balancing the energy budget in free ranging male Myotis daubentonii bats. Physiol. Biochem. Zool. 86, 361–369. https://doi.org/10.1086/670527 (2013).
Entwistle, A. C., Racey, P. A. & Speakman, J. R. The reproductive cycle and determination of sexual maturity in male brown long eared bats, Plecotus auritus (Chiroptera: Vespertilionidae). J. Zool. 244, 63–70. https://doi.org/10.1111/j.1469-7998.1998.tb00007.x (1998).
Fasel, N. J., Kołodziej-Sobocińska, M., Komar, E., Zegarek, M. & Ruczyński, I. Penis size and sperm quality, are all bats grey in the dark?. Curr. Zool. 65, 697–703. https://doi.org/10.1093/cz/zoy094 (2018).
Dietz, M. & Kalko, E. K. V. Reproduction affects flight activity in female and male Daubenton’s bats, Myotis daubentoni. Can. J. Zool.-Rev. Can. Zool. 85, 653–664. https://doi.org/10.1139/z07-045 (2007).
Encarnação, J. A. Spatiotemporal pattern of local sexual segregation in a tree dwelling temperate bat Myotis daubentonii. J. Ethol. 30, 271–278. https://doi.org/10.1007/s10164-011-0323-8 (2012).
Safi, K. & Kerth, G. Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am. Nat. 170, 465–472. https://doi.org/10.1086/520116 (2007).
Hałat, Z., Dechmann, D. K. N., Zegarek, M. & Ruczyński, I. Male bats respond to adverse conditions with larger colonies and increased torpor use during sperm production. Mamm. Biol. 22, 2109 (2020).
Dietz, M. & Horig, A. Thermoregulation of tree dwelling temperate bats-a behavioural adaptation to force live history strategy. Folia Zool. 60, 5–16. https://doi.org/10.25225/fozo.v60.i1.a2.2011 (2011).
Ruczyński, I., Zahorowicz, P., Borowik, T. & Hałat, Z. Activity patterns of two syntopic and closely related aerial-hawking bat species during breeding season in Bialowieza Primaeval Forest. Mammal Res. 62, 65–73. https://doi.org/10.1007/s13364-016-0298-5 (2017).
Jolly, S. E. & Blackshaw, A. W. Prolonged epididymal sperm storage, and the temporal dissociation of testicular and accessory gland activity in the common sheath-tail bat, Taphozous georgianus, of tropical Australia. J. Reprod. Fertil. 81, 205–211. https://doi.org/10.1530/jrf.0.0810205 (1987).
Boyles, J. G., Dunbar, M. B., Storm, J. J. & Brack, V. Energy availability influences microclimate selection of hibernating bats. J. Exp. Biol. 210, 4345–4350. https://doi.org/10.1242/jeb.007294 (2007).
Ruczyński, I., Hałat, Z., Zegarek, M., Borowik, T. & Dechmann, D. K. N. Camera transects as a method to monitor high temporal and spatial ephemerality of flying nocturnal insects. Methods Ecol. Evol. https://doi.org/10.1111/2041-210x.13339 (2020).
Safi, K. Social bats: the males’ perspective. J. Mammal. 89, 1342–1350. https://doi.org/10.1644/08-mamm-s-058.1 (2008).
Webb, P. I., Speakman, J. R. & Racey, P. A. The implication of small reductions in body temperature for radiant and convective heat loss in resting endothermic brown long eared bats (Pecotus auritus). J. Therm. Biol. 18, 131–135. https://doi.org/10.1016/0306-4565(93)90026-p (1993).
Boratyński, J. S., Iwińska, K. & Bogdanowicz, W. An intrapopulation heterothermy continuum: notable repeatability of body temperature variation in food deprived yellow necked mice. J. Exp. Biol. 222, 197152. https://doi.org/10.1242/jeb.197152 (2019).
Christian, N. & Geiser, F. To use or not to use torpor? Activity and body temperature as predictors. Naturwissenschaften 94, 483–487. https://doi.org/10.1007/s00114-007-0215-5 (2007).
Smith, L. B. & Walker, W. H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 30, 2–13. https://doi.org/10.1016/j.semcdb.2014.02.012 (2014).
Macdonald, J. & Harrison, R. G. Effect of low temperatures on rat spermatogenesis. Fertil. Steril. 5, 205–216 (1954).
Fowler, P. A. & Racey, P. A. Relationship between body and testis temperatures in the European hedgehog, Erinaceus europaeus, during hibernation and sexual reactivation. Reproduction 81, 567. https://doi.org/10.1530/jrf.0.0810567 (1987).
Davis, J. R., Firlit, C. F. & Hollinger, M. A. Effect of temperature on incorporation of l-lysine-U-C14 into testicular proteins. Am. J. Physiol. 204, 696–698. https://doi.org/10.1152/ajplegacy.1963.204.4.696 (1963).
LeVier, R. R. & Spaziani, E. The influence of temperature on steroidogenesis in the rat testis. J. Exp. Zool. 169, 113–120. https://doi.org/10.1002/jez.1401690113 (1968).
Geiser, F. & Brigham, R. M. in Living in a seasonal world (eds Thomas Ruf, Claudia Bieber, Walter Arnold, & Eva Millesi) 109–121 (Springer, 2012).
Safi, K. Die Zweifarbfledermaus in der Schweiz: Status und Grundlagen zum Schutz. (Haupt Verlag, 2006).
Hałat, Z., Dechmann, D. K. N., Zegarek, M., Visser, A. F. J. & Ruczyński, I. Sociality and insect abundance affect duration of nocturnal activity of male parti-colored bats. J. Mammal. 99, 1503–1509. https://doi.org/10.1093/jmammal/gyy141 (2018).
Ruczyński, I. Influence of temperature on maternity roost selection by noctule bats (Nyctalus noctula) and Leisler’s bats (N-leisleri) in Biaowieza Primeval Forest, Poland. Can. J. Zool. 84, 900–907. https://doi.org/10.1139/z06-060 (2006).
Ruczyński, I. & Bartoń, K. A. Seasonal changes and the influence of tree species and ambient temperature on the fission-fusion dynamics of tree-roosting bats. Behav. Ecol. Sociobiol. 74, 63. https://doi.org/10.1007/s00265-020-02840-1 (2020).
Linton, D. M. & Macdonald, D. W. Phenology of reproductive condition varies with age and spring weather conditions in male Myotis daubentonii and Myotis nattereri (Chiroptera: Vespertilionidae). Sci. Rep. 10, 6664. https://doi.org/10.1038/s41598-020-63538-y (2020).
Dammhahn, M., Landry-Cuerrier, M., Reale, D., Garant, D. & Humphries, M. M. Individual variation in energy-saving heterothermy affects survival and reproductive success. Funct. Ecol. 31, 866–875. https://doi.org/10.1111/1365-2435.12797 (2017).
Boyles, J. G., Johnson, J. S., Blomberg, A. & Lilley, T. M. Optimal hibernation theory. Mammal. Rev. 50, 91–100. https://doi.org/10.1111/mam.12181 (2020).
Boratyński, J. S., Willis, C. K. R., Jefimow, M. & Wojciechowski, M. S. Huddling reduces evaporative water loss in torpid Natterer’s bats, Myotis nattereri. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 179, 125–132. https://doi.org/10.1016/j.cbpa.2014.09.035 (2015).
Ruczyński, I., Kalko, E. K. V. & Siemers, B. M. The sensory basis of roost finding in a forest bat, Nyctalus noctula. J. Exp. Biol. 210, 3607–3615. https://doi.org/10.1242/jeb.009837 (2007).
Lovegrove, B. G. Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J. Comp. Physiol. B 179, 451–458. https://doi.org/10.1007/s00360-008-0329-x (2009).
Willis, C. K. R., Lane, J. E., Liknes, E. T., Swanson, D. L. & Brigham, R. M. Thermal energetics of female big brown bats (Eptesicus fuscus). Can. J. Zool. 83, 871–879. https://doi.org/10.1139/z05-074 (2005).
Willis, C. K. R. An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol. Biochem. Zool. 80, 643–651. https://doi.org/10.1086/521085 (2007).
Krutzsch, P. H. in Reproductive Biology of Bats (ed Academic Press) 91–155 (2000).
Wood, S. N. Generalized Additive Models: An Introduction With R. Vol. 66 (2006).
Jackman, S. Bayesian Analysis for the Social Sciences. (Wiley, 2009).
Brooks, S. P. & Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455. https://doi.org/10.2307/1390675 (1998).
Kellner, K. jagsUI: A Wrapper Around 'rjags' to Streamline 'JAGS' Analyses. v.R package version 1.5.1. (2019).
Acknowledgements
We thank our students and volunteers for much appreciated help: J. Szlachetka, A. Grabek, E. Obłuska, K. Guzowska, M. Wirowska, I. Cameron and E. Collin. We are grateful to Z. Hałat for her overall support as well as Jenna Kohles for correcting the English in the manuscript.
Funding
This work was funded by the National Science Centre, Poland, on the basis of decision number DEC-2013/10/E/NZ8/00725.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.K., N.J.F., P.A.S., D.K.D., I.R.; Methodology: E.K., N.J.F., I.R.; Formal analysis: E.K., N.J.F.; Investigation: E.K., M.Z., I.R.; Writing—original draft: E.K., N.J.F., P.A.S., D.K.D., I.R.; Writing—review & editing: E.K., P.A.S., N.J.F., D.K.D., M.Z., I.R.; Supervision: I.R.; Project administration: I.R.; Funding acquisition: I.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Komar, E., Fasel, N.J., Szafrańska, P.A. et al. Energy allocation shifts from sperm production to self-maintenance at low temperatures in male bats. Sci Rep 12, 2138 (2022). https://doi.org/10.1038/s41598-022-05896-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05896-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.