Abstract
The influence of exposure to hormonal treatments, particularly cyproterone acetate (CPA), has been posited to contribute to the growth of meningiomas. Given the widespread use of CPA, this systematic review and meta-analysis attempted to assess real-world evidence of the association between CPA and the occurrence of intracranial meningiomas. Systematic searches of Ovid MEDLINE, Embase and Cochrane Controlled Register of Controlled Trials, were performed from database inception to 18th December 2021. Four retrospective observational studies reporting 8,132,348 patients were included in the meta-analysis. There was a total of 165,988 subjects with usage of CPA. The age of patients at meningioma diagnosis was generally above 45 years in all studies. The dosage of CPA taken by the exposed group (n = 165,988) was specified in three of the four included studies. All studies that analyzed high versus low dose CPA found a significant association between high dose CPA usage and increased risk of meningioma. When high and low dose patients were grouped together, there was no statistically significant increase in risk of meningioma associated with use of CPA (RR = 3.78 [95% CI 0.31–46.39], p = 0.190). Usage of CPA is associated with increased risk of meningioma at high doses but not when low doses are also included. Routine screening and meningioma surveillance by brain MRI offered to patients prescribed with CPA is likely a reasonable clinical consideration if given at high doses for long periods of time. Our findings highlight the need for further research on this topic.
Similar content being viewed by others
Introduction
Meningiomas are typically slow growing benign tumors arising from the meningothelial cells of the arachnoid membrane encasing the central nervous system1,2. Ninety percent of meningiomas are intracranial, and they account for 38% of all intracranial tumors reported in the United States (US) between 2013 and 20173. These tumors are often revealed incidentally by imaging. When symptoms arise, it is the result of raised intracranial pressure, which vary according to the size and location of the tumor.
The etiology of meningiomas is controversial but unequivocal risk factors are environmental or medical exposure to ionizing radiation4,5,6, and hereditary mutations of the neurofibromatosis type 2 gene7,8,9,10. Strong evidence also suggests a plausible role for sex hormones in meningioma development. These include the predilection for females especially after puberty3, and the well characterized distribution of progesterone, estrogen, and androgen receptors in certain skull base meningiomas11,12,13,14,15,16,17,18. Furthermore, fluctuations in meningioma growth during the menstrual cycle, pregnancy, and breastfeeding have also been well-documented19,20,21,22,23,24,25,26. Benson et al., in a meta-analysis demonstrated that the use of hormone replacement therapy is an independent risk factor for the development of meningiomas26.
Given the hormone-sensitive nature of meningiomas, the influence of exposure to hormonal treatments, particularly cyproterone acetate (CPA), has been theorized to contribute to the growth of meningiomas. CPA is a synthetic progestogen with potent anti-androgenic, progestogenic and antigonadotrophic mechanistic actions27,28. The dose and indications for CPA vary considerably. High dose CPA formulations (> 50 mg/day) are used in persons of male birth sex with inoperable prostate cancer, paraphilia, hirsutism, or male-to-female transsexual hormonal therapy27. Lower doses (2-10 mg/day) are used in combination with estradiol for birth control as well as to treat androgen-associated alopecia or female seborrhea28.
The first signal of an association of prolonged use of high dose CPA with meningioma was raised in a transsexual patient reported by Gazzeri et al.29. In this case, a causal association between the abrupt growth of a giant grade 1 olfactory-groove meningioma and the hormone therapy was suggested by the negative cerebral MRI scan obtained three years before presentation. Since then, several case series30,31,32,33,34,35,36, and adequately powered cohort studies have corroborated these findings37,38,39,40. The presence of progesterone receptors on meningiomas supports the biological plausibility of an association. Furthermore, previous robust in vitro and preclinical studies support the efficacy of progesterone receptor antagonist such as mifepristone (RU 486) in meningiomas41,42,43,44, which supports the argument regarding a causal relationship.
Given the widespread use of CPA, any plausible drug-related risk of meningiomas should be investigated thoroughly. The main objective of this present study was to appraise real-world evidence of the association between CPA and the occurrence of intracranial meningiomas.
Results
Study selection and characteristics
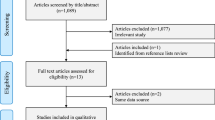
Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram illustrating the number of reviews screened and reasons for exclusion at each stage. Using the designated search terms, a total of 109 articles were retrieved, and four were included in the final dataset37,40,45,46. There were three publications analyzing the same cohort, and the data by Weill et al.37 was chosen over the other two38,39, as it reported the largest patient-year data. Similarly, two publications had analyzed overlapping data from Danish healthcare registers and the more recent study by Mikkelsen et al.46, with larger patient-year data was chosen over the other47. Reliability of study selection between observers was substantial at both the title and abstract screening stage (Cohen’s κ = 1.00) and the full-text review stage (Cohen’s κ = 1.00)48.
All four included studies were retrospective37,40,45,46. Three were cohort studies and one was a case–control study40. The three cohort studies both adopted nation-wide population-based databases (from Denmark, France and Spain)37,45,46. The case–control study identified cases and controls from a large UK primary care database40. Controls were selected at random and frequency-matched to cases by age (within one year), sex and index year (year of newly diagnosed meningioma). Table 1 summarizes the baseline characteristics and outcomes in each included study.
Quality assessment
Using the Joanna Briggs Institute (JBI) checklist for prevalence studies, three studies attained a full score of 11 and one attained a score of 10 (Supplementary Table 3).
Patient characteristics
A total of 8,132,348 patients were reported across the four included studies37,40,45,46. Patient gender was reported in two studies, of which 261,673 of the total 264,522 patients were females (98.9%)37,40. There was a total of 164,006 subjects with usage of CPA. The age of patients at meningioma diagnosis was generally above 45 years in all studies. In the study by Cea-Soriano et al., the mean age at meningioma diagnosis was 62.6 and 62.2 years for female and male patients, respectively40. Gil et al. reported that 403 out of 456 (88.4%) meningioma patients were above the age of 45 years45. Similarly, Weill et al. reported a mean age of 48.1 and 50.5 years at meningioma diagnosis for the exposed and control groups, respectively37.
Exposure and dosages
The dosage of CPA taken by the exposed group (n = 165,988) was specified in three of the four included studies. In the study by Cea-Soriano et al., all female patients had a daily CPA dose of 2 mg or higher, whereas all male patients had a daily dose of 50 mg or higher40. In the study by Weill et al., the cumulative dose of patients within the exposed group was greater than or equal to 3 g (at least three standard packets of 20, 50 mg tablets) within the first six months of the first prescription37. The studies by Cea-Soriano et al. and Gil et al. defined high dose as ever having a daily dose of 50 mg or higher, while low dose was defined in these studies as all daily doses being less than 50 mg, at a markedly lower dose of 2 mg/day (which may likely be for birth control)40,45. The study by Mikkelsen et al., compared the incidence of intracranial meningiomas between groups of high cumulative doses (> 10 g) versus low cumulative doses of CPA (0.1–10 g). Across the three studies (with total sample size being 7,851,805 and number of exposed patients being 26,766), there were a total of 3271 and 23,495 high and low dose patients, respectively40,45,46.
Risk of meningioma associated with use of CPA
All four studies report an increased risk of meningioma associated with high doses of CPA exposure7,40,45,46.
Cea-Soriano et al., Gil et al. and Mikkelsen et al., demonstrated an increased risk of meningioma with use of high dose CPA (defined as above) compared to non-users and use of low dose CPA40,45,46. The distinction between current and past users of CPA was reported in the study by Cea-Soriano et al. and Mikkelsen et al., but not specified in the one by Gil et al. Cea-Soriano et al. found that there was no significantly increased risk of meningioma with past use of CPA, as well as current or ever use (which includes both current and past use) of low dose CPA40. Mikkelsen et al., on the other hand, showed significantly increased risk of meningioma with past and present use of CPA, compared with no use46.
Similarly, Weill et al. found a dose–effect relation between meningioma risk and cumulative dose of CPA, with higher risk associated with a higher cumulative dose37. The hazard ratio (HR) was not significantly different from 1 for exposure to less than 12 g of CPA, and it rapidly increased for higher cumulative doses: 11.3 (95% CI 5.8–22.2) for 36–60 g and 21.7 (95% CI 10.8–43.5) for 60 g or higher. In this study, the exposed group comprised only of current users and does not include past users37.
We pooled the patients across the four included studies to perform a meta-analysis of binary outcome. The total number of patients in the exposed and non-exposed group was 165,988 and 8,997,360, respectively. Meta-analysis demonstrated no statistically significant increased risk of meningioma associated with use of CPA (risk ratio [RR] = 3.78 [95% CI 0.31–46.39], p = 0.190) [Fig. 2]. Study heterogeneity was substantial and statistically significant (I2 = 95.7% [95% CI 91.9–97.8], p < 0.001).
Anatomical location of meningioma
Only Weill et al. reported the anatomical location of CPA-associated meningiomas and hence a pooled subgroup analysis was not possible for anatomical location. Weill et al. demonstrated that the risk of CPA-associated meningioma varied considerably according to their anatomical locations, with a predilection for the anterior base of the skull base (RR 43.6 [95% CI 13.9–137.1] and adjusted HR 47.1 [95% CI 14.9–149.1]).
Discussion
To our knowledge, this is the first meta-analysis to investigate the association between CPA use and intracranial meningioma. Limited current evidence suggests an increased risk of meningioma associated with high dose CPA usage. When high dose users were combined with low dose users, this association becomes statistically insignificant. This meta-analysis underscores the current paucity in evidence about the risk of intracranial meningioma associated with low dose CPA. For example, for the purposes of birth control prescribed at 2 mg or 50 mg for short periods. The included studies had also varied in their definition of low dose CPA prescription. It is still unknown whether or not CPA below a certain threshold may be safe in terms of the risk of meningioma.
Location
A majority of CPA-associated meningiomas have been reported to be preferentially distributed at the anterior (22–75%) and middle base of the skull (25–40%), as opposed to cranial convexity which is the commonest location in the general population16,32,37,38,39. Samarut and colleagues purported that meningiomas located in the anterior and middle skull base appeared to be specific to CPA use, with the risk reducing after termination of CPA38. The predominance of anterior skull base meningiomas may be supported by biological plausibility49. The embryological biology of meninges differs at the convexity (neural crests) and the base of the skull (mesoderm)16,32,50,51,52,53,54,55,56. Molecular and immunohistochemical studies have established that the progesterone receptor distribution in the skull base follows a rostrocaudal gradient14,15,16,57. Thus, we could expect skull base meningiomas to dominate in the anterior cranial fossa with progestogenic CPA exposure.
Risk, causality and interpretation
A causal relationship between high dose CPA and the development of meningioma is tenable. Based on the Bradford Hill criteria58, this may be supported by the strength and dose-dependent association. Our findings suggest a modest magnitude of the association between high dose CPA use and intracranial meningiomas, albeit when high dose users were banded together with low dose users in our pooled analysis, this association became statistically insignificant. Although a three-fold increase in clinically significant risk was found in our meta-analysis, the confidence intervals encompassed the null. This is further supported by the specificity of certain tumor locations (anterior skull base) which are highly dense with progesterone receptors, providing a biological plausibility.
Reverse causality is acknowledged with observational studies especially if the prescription of CPA was linked to an undiagnosed meningioma. However, this bias may be excluded from our meta-analysis because of the temporal aspect of our findings: the risk of meningioma increased with the duration of CPA use and cumulative doses, and not during the initial phase of drug use. Furthermore, reports of rapid spontaneous meningioma regression or stabilization after CPA withdrawal, can be found in the literature30,31,33,36,59. This observation further reinforces the notion of causality58. As progesterone have been postulated to accelerate meningioma growth by vascularization, the biology involved is analogous to the spontaneous regression of meningiomas postpartum60.
Clinical implications and management of CPA-associated meningiomas
Iatrogenic meningioma engendered by high-dose CPA use is a public health issue. Before these results are used to guide clinical decision making, the collective body of data on this safety issue should be scrutinized by drug regulatory authorities and weighed against the benefits of treatment. Nonetheless, patients currently on or previously exposed to high dose CPA should be informed about the increased risk of intracranial meningiomas. The indication of CPA should be clearly defined with the lowest possible daily dose used.
First line management of meningiomas typically involves surgery. Location of the meningioma influences the extent of resection, which, consequently influences outcomes such as recurrence rates61. As shown, CPA-associated meningiomas have a predilection for the skull base, which is of considerable importance because skull base meningioma surgery is associated with poorer prognosis than surgery for non-skull base meningiomas61,62,63,64. Duly, evidence for spontaneous meningioma regression with CPA termination30,31,33,36,59, sustained the notion that invasive treatment may be avoided and conservative management of CPA-associated meningiomas might be treatment of choice30,39,65. However, it must be noted that such cases are exceptional—a patient with clinoidal meningioma and progressive visual loss must be operated on, in spite of previous treatment with CPA. Conservative management, which may be recommended for small and asymptomatic meningiomas, comprises cessation of CPA and close follow-up magnetic resonance imaging (MRI) in the context of current or past history of high dose exposure. As this screening suggestion was not directly investigated in this study, this requires further cost–benefit analysis by guideline groups and/or policymakers. Despite evidence that antiprogesterone treatment reduces the size of meningioma, both in vitro and in vivo, such therapy has not been recommended in the conservative management of meningiomas.
Limitations
Although several factors lend support to the strength of the association, including biological plausibility and consistent epidemiological evidence, our findings must be cautiously interpreted in the context of its known limitations. Limitations of our meta-analysis include the retrospective and observational nature of included studies and the significant heterogeneity among the studies. There were no randomized controlled trials in this study, although conducting one could account for potential biases and confounders, the non-randomized evidence to the risk of meningiomas is so extensive that this would unlikely take place, from practical and ethical standpoints66. A further limitation of the available data is that there is little known about the impact of past exposure or whether there is a cumulative dose effect, and hence we were unable to weigh the effect of historical doses versus current doses differently. Only two studies had defined past exposure40,46. Confounding factors are inevitable in any of our included observational studies. The small number of studies available in the literature could explain the finding of non-significance and limited our ability to perform certain analyses such as meta-regression to explore possible confounders (age and sex) or sources of heterogeneity in our dataset. To minimize the extent of these limitations, we performed sensitivity analyses to attempt to identify outlier studies. Taken together in this light, together with our pooled analysis, we propound that this relationship cannot be proven causal given the aforementioned. Nonetheless, advantages of our meta-analysis include avoiding undue emphasis on individual studies, thus yielding risk estimates that are more reliable.
Conclusion
In light of these results, prescription of high-dose CPA, especially for off label indications, should be considered carefully. Additionally, routine screening and meningioma surveillance by brain MRI offered to patients prescribed with CPA is likely a reasonable clinical consideration if given at high doses for long periods of time. The results obtained herein suggest the necessity for further clinical research on intracranial meningioma associated with CPA.
Methods
The review was conducted according to the PRISMA guidelines67. The protocol was registered on the PROSPERO international prospective register of systematic reviews (registration number CRD42021242120).
Search strategy
Searches of the following three electronic databases were undertaken: Ovid Medline, Ovid Embase, and Cochrane Central Register of Controlled Trials (CENTRAL).
Searches were performed in each database from its inception until 18th December 2021. The concepts of “cyproterone acetate”, and “meningioma”, were used in addition to synonyms and related terms. An example search strategy used for OVID Medline/EMBASE/CENTRAL is presented in Supplementary Table 1.
Eligibility criteria
Any randomized or non-randomized study (cohort study; case–control study) that investigated the association between CPA use regardless of indication, and the risk of intracranial meningiomas were included. As it is the progestogenic effect of CPA that has been purported to contribute to intracranial meningiomas, the controls in this study were limited to patients unexposed to CPA or patients only very slightly exposed who discontinued CPA prematurely, as defined by the included studies. Particularly, in the study by Weill et al. the control group was defined as patients who discontinued treatment rapidly after having received a cumulative dose less than 3 g (one or two standard packs) dispensed within the first six months after this first prescription.
The following designs were excluded: case reports/series; non-English; animal studies. Studies that did not report extractable data including odds ratio (OR), RR, HR, or raw data, were also excluded. Patients were included regardless of gender and ethnicity, or presence of symptoms on presentation. Supplementary Table 2 describes the full list of inclusion and exclusion criteria.
Study selection
All titles and abstracts were screened against the pre-defined eligibility criteria developed independently by two reviewers (KSL and JJYZ). Disagreements were resolved by discussion, and where agreement could not be reached, the senior reviewer assisted with decision making (VDWN). Potentially eligible studies were selected for full-text analysis. At each stage, KSL and JJYZ reviewed 100% of the screened studies for inclusion to ensure reliability of study selection. Disagreements were resolved by consensus or appeal to a third senior reviewer (VDWN). Agreement among the reviewers on study inclusion were evaluated using Cohen’s kappa48.
In the event of multiple publications analyzing the same cohort, the publication that reported the largest patient-year data will be used for evaluation.
The reference lists of included studies were also scrutinized to pursue references of identified citations, in an effort to identify high quality resources in obscure locations that could have been overlooked in our search strategy68.
Risk of bias assessment
The quality of included studies was assessed using the JBI checklist for cohort studies69. In summary, these tools rated the quality of selection, measurement and comparability for all studies and gave a score for cohort studies (maximum of 11). Two researchers (KSL and JJYZ) assessed the quality of all included studies and discussed discrepancies until consensus is reached.
Outcome
The primary outcome of interest was the development of intracranial meningiomas amongst patients who have taken CPA.
Data extraction
A pro forma was developed and piloted to extract data on the following variables to ensure standardization and consistency in this process: (1) study details, (2) study design, (3) participant demographics, (4) country and dataset, (5) selection criteria, (6) controls, (7) indication for CPA, (8) dose of CPA, (9) unadjusted HR or RR or OR, propensity-score adjusted HR, propensity-score matched HR, and covariate-adjusted HR. Two reviewers (KSL and JJYZ) independently and blindly extracted 100% of the data each to ensure reliability. Discrepancies or disagreements about extracted material were resolved by the senior reviewer (VDWN).
Where data was incomplete (e.g. outcomes of interest reported but not specific to CPA exposure), the study authors were contacted via email to obtain full data and were given two weeks to respond.
Statistical analysis
A meta-analysis of binary outcomes was performed to compare the risk of meningioma between the exposed and non-exposed groups. The overall summary estimate was presented as a RR with its 95% confidence interval (CI), and was computed following a weighted analysis of the RR from each individual study. The random effects model was used to account for study heterogeneity, with the overall pooled estimate computed using the inverse variance method. CI for individual studies were calculated using the Wilson Score confidence interval method with continuity correction. The I2 statistic was used to present between-study heterogeneity, where I2 ≤ 30%, between 30 and 50%, between 50 and 75%, and ≥ 75% were considered to indicate low, moderate, substantial, and considerable heterogeneity, respectively70. P values for the I2 statistic were derived from the chi-squared distribution of Cochran Q test.
All statistical analyses were performed using R software version 3.4.3 (R Foundation for Statistical Computing, 2016). P-values less than 0.05 were considered statistically significant.
Ethical approval
Ethical approval was not required for this systematic review and meta-analysis.
Abbreviations
- CI:
-
Confidence interval
- CPA:
-
Cyproterone acetate
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RR:
-
Risk ratio
- US:
-
United States
References
Wiemels, J., Wrensch, M. & Claus, E. B. Epidemiology and etiology of meningioma. J. Neurooncol. 99, 307–314. https://doi.org/10.1007/s11060-010-0386-3 (2010).
Lee, E. J. et al. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J. Neurosurg. 127, 971–980. https://doi.org/10.3171/2016.9.JNS161669 (2017).
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 22, 1–96. https://doi.org/10.1093/neuonc/noaa200 (2020).
Yonehara, S. et al. Clinical and epidemiologic characteristics of first primary tumors of the central nervous system and related organs among atomic bomb survivors in Hiroshima and Nagasaki, 1958–1995. Cancer 101, 1644–1654. https://doi.org/10.1002/cncr.20543 (2004).
Al-Mefty, O., Topsakal, C., Pravdenkova, S., Sawyer, J. R. & Harrison, M. J. Radiation-induced meningiomas: Clinical, pathological, cytokinetic, and cytogenetic characteristics. J. Neurosurg. 100, 1002–1013. https://doi.org/10.3171/jns.2004.100.6.1002 (2004).
Taylor, A. J. et al. Population-based risks of CNS tumors in survivors of childhood cancer: The British Childhood Cancer Survivor Study. J. Clin. Oncol. 28, 5287–5293. https://doi.org/10.1200/JCO.2009.27.0090 (2010).
Hansson, C. M. et al. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genomics 8, 16. https://doi.org/10.1186/1471-2164-8-16 (2007).
Hemminki, K., Tretli, S., Sundquist, J., Johannesen, T. B. & Granström, C. Familial risks in nervous-system tumours: A histology-specific analysis from Sweden and Norway. Lancet Oncol. 10, 481–488. https://doi.org/10.1016/S1470-2045(09)70076-2 (2009).
Torres-Martín, M. et al. Whole exome sequencing in a case of sporadic multiple meningioma reveals shared NF2, FAM109B, and TPRXL mutations, together with unique SMARCB1 alterations in a subset of tumor nodules. Cancer Genet. 208, 327–332. https://doi.org/10.1016/j.cancergen.2015.03.012 (2015).
Kotecha, R. S. et al. Meningiomas in children and adolescents: a meta-analysis of individual patient data. Lancet Oncol. 12, 1229–1239. https://doi.org/10.1016/S1470-2045(11)70275-3 (2011).
Poisson, M. et al. Steroid hormone receptors in human meningiomas, gliomas and brain metastases. J. Neurooncol. 1, 179–189. https://doi.org/10.1007/BF00165601 (1983).
Pravdenkova, S., Al-Mefty, O., Sawyer, J. & Husain, M. Progesterone and estrogen receptors: Opposing prognostic indicators in meningiomas. J. Neurosurg. 105, 163–173. https://doi.org/10.3171/jns.2006.105.2.163 (2006).
Blitshteyn, S., Crook, J. E. & Jaeckle, K. A. Is there an association between meningioma and hormone replacement therapy?. J. Clin. Oncol. 26, 279–282. https://doi.org/10.1200/JCO.2007.14.2133 (2008).
Bouillot, P. et al. Quantitative imaging of estrogen and progesterone receptors, estrogen-regulated protein, and growth fraction: Immunocytochemical assays in 52 meningiomas.Correlation with clinical and morphological data. J. Neurosurg. 81, 765–773. https://doi.org/10.3171/jns.1994.81.5.0765 (1994).
Ülgen, E. et al. Meningiomas display a specific immunoexpression pattern in a rostrocaudal gradient: An analysis of 366 patients. World Neurosurg. 123, e520–e535. https://doi.org/10.1016/j.wneu.2018.11.201 (2019).
Kuroi, Y., Matsumoto, K., Shibuya, M. & Kasuya, H. Progesterone receptor is responsible for benign biology of skull base meningioma. World Neurosurg. 118, e918–e924. https://doi.org/10.1016/j.wneu.2018.07.100 (2018).
Black, P., Carroll, R. & Zhang, J. The molecular biology of hormone and growth factor receptors in meningiomas. Acta Neurochir. Suppl. 65, 50–53. https://doi.org/10.1007/978-3-7091-9450-8_15 (1996).
Carroll, R. S., Zhang, J. & Black, P. M. Expression of estrogen receptors alpha and beta in human meningiomas. J. Neurooncol. 42, 109–116. https://doi.org/10.1023/a:1006158514866 (1999).
Claus, E. B. et al. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J. Neurosurg. 118, 649–656. https://doi.org/10.3171/2012.9.JNS12811 (2013).
Lambe, M., Coogan, P. & Baron, J. Reproductive factors and the risk of brain tumors: A population-based study in Sweden. Int. J. Cancer 72, 389–393. https://doi.org/10.1002/(sici)1097-0215(19970729)72:3%3c389::aid-ijc2%3e3.0.co;2-l (1997).
Michaud, D. S. et al. Reproductive factors and exogenous hormone use in relation to risk of glioma and meningioma in a large European cohort study. Cancer Epidemiol. Biomarkers Prev. 19, 2562–2569. https://doi.org/10.1158/1055-9965.EPI-10-0447 (2010).
Wigertz, A. et al. Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol. Biomarkers Prev. 17, 2663–2670. https://doi.org/10.1158/1055-9965.EPI-08-0406 (2008).
Zong, H. et al. Reproductive factors in relation to risk of brain tumors in women: an updated meta-analysis of 27 independent studies. Tumour Biol. 35, 11579–11586. https://doi.org/10.1007/s13277-014-2448-1 (2014).
Benson, V. S. et al. Hormone replacement therapy and incidence of central nervous system tumours in the Million Women Study. Int. J. Cancer 127, 1692–1698. https://doi.org/10.1002/ijc.25184 (2010).
Lee, E. et al. Association of meningioma with reproductive factors. Int. J. Cancer 119, 1152–1157. https://doi.org/10.1002/ijc.21950 (2006).
Benson, V. S., Kirichek, O., Beral, V. & Green, J. Menopausal hormone therapy and central nervous system tumor risk: Large UK prospective study and meta-analysis. Int. J. Cancer 136, 2369–2377. https://doi.org/10.1002/ijc.29274 (2015).
Schröder, F. H. et al. Metastatic prostate cancer treated by flutamide versus cyproterone acetate: Final analysis of the “European Organization for Research and Treatment of Cancer” (EORTC) Protocol 30892. Eur. Urol. 45, 457–464. https://doi.org/10.1016/j.eururo.2003.11.016 (2004).
Van der Spuy, Z. M. & le Roux, P. A. Cyproterone acetate for hirsutism. Cochrane Database Syst. Rev. 1, 001125. https://doi.org/10.1002/14651858.CD001125 (2003).
Gazzeri, R., Galarza, M. & Gazzeri, G. Growth of a meningioma in a transsexual patient after estrogen-progestin therapy. N. Engl. J. Med. 357, 2411–2412. https://doi.org/10.1056/NEJMc071938 (2007).
Bernat, A. L. et al. Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: A case series of 12 patients. Acta Neurochir. 157, 1741–1746. https://doi.org/10.1007/s00701-015-2532-3 (2015).
Passeri, T. et al. Spontaneous regression of meningiomas after interruption of nomegestrol acetate: A series of three patients. Acta Neurochir. 161, 761–765. https://doi.org/10.1007/s00701-019-03848-x (2019).
Portet, S. et al. Histomolecular characterization of intracranial meningiomas developed in patients exposed to high-dose cyproterone acetate: An antiandrogen treatment. Neurooncol. Adv. 1, 003. https://doi.org/10.1093/noajnl/vdz003 (2019).
Cebula, H., Pham, T. Q., Boyer, P. & Froelich, S. Regression of meningiomas after discontinuation of cyproterone acetate in a transsexual patient. Acta Neurochir. 152, 1955–1956. https://doi.org/10.1007/s00701-010-0787-2 (2010).
Ter Wengel, P. V., Martin, E., Gooren, L., Den Heijer, M. & Peerdeman, S. M. Meningiomas in three male-to-female transgender subjects using oestrogens/progestogens and review of the literature. Andrologia 48, 1130–1137. https://doi.org/10.1111/and.12550 (2016).
Mancini, I. et al. Presentation of a meningioma in a transwoman after nine years of cyproterone acetate and estradiol intake: Case report and literature review. Gynecol. Endocrinol. 34, 456–459. https://doi.org/10.1080/09513590.2017.1395839 (2018).
Botella, C., Coll, G., Lemaire, J. J. & Irthum, B. Intra cranial meningiomas and long term use of cyproterone acetate with a conventional dose in women: A report of two cases of tumor decrease after treatment withdrawal. Neurochirurgie 61, 339–342. https://doi.org/10.1016/j.neuchi.2015.05.002 (2015).
Weill, A. et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: Cohort study. BMJ 372, n37. https://doi.org/10.1136/bmj.n37 (2021).
Samarut, E. et al. Meningiomas and cyproterone acetate: A retrospective, monocentric cohort of 388 patients treated by surgery or radiotherapy for intracranial meningioma. J. Neurooncol. https://doi.org/10.1007/s11060-020-03683-6 (2021).
Champeaux-Depond, C., Weller, J., Froelich, S. & Sartor, A. Cyproterone acetate and meningioma: A nationwide-wide population based study. J. Neurooncol. 151, 331–338. https://doi.org/10.1007/s11060-020-03672-9 (2021).
Cea-Soriano, L., Blenk, T., Wallander, M. A. & Rodríguez, L. A. Hormonal therapies and meningioma: Is there a link?. Cancer Epidemiol. 36, 198–205. https://doi.org/10.1016/j.canep.2011.08.003 (2012).
Olson, J. J., Beck, D. W., Schlechte, J. & Loh, P. M. Hormonal manipulation of meningiomas in vitro. J. Neurosurg. 65, 99–107. https://doi.org/10.3171/jns.1986.65.1.0099 (1986).
Koper, J. W., Foekens, J. A., Braakman, R. & Lamberts, S. W. Effects of progesterone on the response to epidermal growth factor and other growth factors in cultured human meningioma cells. Cancer Res. 50, 2604–2607 (1990).
Blankenstein, M. A., van der Meulen-Dijk, C. & Thijssen, J. H. Effect of steroids and antisteroids on human meningioma cells in primary culture. J. Steroid Biochem. 34, 419–421. https://doi.org/10.1016/0022-4731(89)90119-2 (1989).
Olson, J. J., Beck, D. W., Schlechte, J. A. & Loh, P. M. Effect of the antiprogesterone RU-38486 on meningioma implanted into nude mice. J. Neurosurg. 66, 584–587. https://doi.org/10.3171/jns.1987.66.4.0584 (1987).
Gil, M. et al. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: Evidence from a population-based cohort study. Br. J. Clin. Pharmacol. 72, 965–968. https://doi.org/10.1111/j.1365-2125.2011.04031.x (2011).
Mikkelsen, A. P., Greiber, I. K., Scheller, N. M., Hilden, M. & Lidegaard, Ø. Cyproterone acetate and risk of meningioma: A nationwide cohort study. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2021-326138 (2021).
Giraldi, L. et al. Male hormone-interfering drugs and meningioma development. Neurooncol. Adv. 1, 046. https://doi.org/10.1093/noajnl/vdz046 (2019).
Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Measur. 20, 37–47 (1960).
Maiuri, F. et al. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir. 161, 2553–2561. https://doi.org/10.1007/s00701-019-04084-z (2019).
O’Rahilly, R. & Müller, F. The meninges in human development. J. Neuropathol. Exp. Neurol. 45, 588–608 (1986).
Mack, J., Squier, W. & Eastman, J. T. Anatomy and development of the meninges: Implications for subdural collections and CSF circulation. Pediatr. Radiol. 39, 200–210. https://doi.org/10.1007/s00247-008-1084-6 (2009).
Peyre, M. et al. Progestin-associated shift of meningioma mutational landscape. Ann. Oncol. 29, 681–686. https://doi.org/10.1093/annonc/mdx763 (2018).
Boetto, J., Apra, C., Bielle, F., Peyre, M. & Kalamarides, M. Selective vulnerability of the primitive meningeal layer to prenatal Smo activation for skull base meningothelial meningioma formation. Oncogene 37, 4955–4963. https://doi.org/10.1038/s41388-018-0328-7 (2018).
Savardekar, A. R. et al. Differential tumor progression patterns in skull base versus non-skull base meningiomas: A critical analysis from a long-term follow-up study and review of literature. World Neurosurg. 112, e74–e83. https://doi.org/10.1016/j.wneu.2017.12.035 (2018).
Boetto, J., Peyre, M. & Kalamarides, M. Meningiomas from a developmental perspective: Exploring the crossroads between meningeal embryology and tumorigenesis. Acta Neurochir. 163, 57–66. https://doi.org/10.1007/s00701-020-04650-w (2021).
Dasgupta, K., Chung, J. U., Asam, K. & Jeong, J. Molecular patterning of the embryonic cranial mesenchyme revealed by genome-wide transcriptional profiling. Dev. Biol. 455, 434–448. https://doi.org/10.1016/j.ydbio.2019.07.015 (2019).
Perrot-Applanat, M., Groyer-Picard, M. T. & Kujas, M. Immunocytochemical study of progesterone receptor in human meningioma. Acta Neurochir. 115, 20–30. https://doi.org/10.1007/BF01400586 (1992).
Fedak, K. M., Bernal, A., Capshaw, Z. A. & Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 12, 14. https://doi.org/10.1186/s12982-015-0037-4 (2015).
Vadivelu, S., Sharer, L. & Schulder, M. Regression of multiple intracranial meningiomas after cessation of long-term progesterone agonist therapy. J. Neurosurg. 112, 920–924. https://doi.org/10.3171/2009.8.JNS09201 (2010).
Kerschbaumer, J. et al. Hormone-dependent shrinkage of a sphenoid wing meningioma after pregnancy: Case report. J. Neurosurg. 124, 137–140. https://doi.org/10.3171/2014.12.JNS142112 (2016).
Magill, S. T. et al. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus 44, E4. https://doi.org/10.3171/2018.1.FOCUS17752 (2018).
Morokoff, A. P., Zauberman, J. & Black, P. M. Surgery for convexity meningiomas. Neurosurgery 63, 427–433. https://doi.org/10.1227/01.NEU.0000310692.80289.28 (2008).
Liouta, E., Koutsarnakis, C., Liakos, F. & Stranjalis, G. Effects of intracranial meningioma location, size, and surgery on neurocognitive functions: A 3-year prospective study. J. Neurosurg. 124, 1578–1584. https://doi.org/10.3171/2015.6.JNS1549 (2016).
Güdük, M., Özduman, K. & Pamir, M. N. Sphenoid wing meningiomas: Surgical outcomes in a series of 141 cases and proposal of a scoring system predicting extent of resection. World Neurosurg. 125, e48–e59. https://doi.org/10.1016/j.wneu.2018.12.175 (2019).
Zairi, F. et al. Close follow-up after discontinuation of cyproterone acetate: A possible option to defer surgery in patients with voluminous intracranial meningioma. J. Neurosurg. Sci. 61, 98–101. https://doi.org/10.23736/S0390-5616.16.03243-4 (2017).
Yu, J. et al. Design, conduct, and analysis of surgical randomized controlled trials: a cross-sectional survey. Ann. Surg. 270, 1065–1069. https://doi.org/10.1097/SLA.0000000000002860 (2019).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Greenhalgh, T. & Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 331, 1064–1065. https://doi.org/10.1136/bmj.38636.593461.68 (2005).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 8, 2–10. https://doi.org/10.1111/jebm.12141 (2015).
Higgins, J. P. T. G. S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011).
Acknowledgements
A special thank you to Kenneth Kek Wee Lee (Papa), Lena Lim (Mama) and Chio Tee Koh (Ahma) back home for their unwavering support and belief in me, without which, I would not be able to achieve my educational goals. Love truly overcomes all obstacles (different time zones and distance).
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publications. K.S.L.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Visualization, Funding acquisition. J.J.Y.Z.: Methodology, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Visualization. R.K.: Writing—review & editing, Supervision. T.S.: Writing—review & editing, Supervision. V.D.W.N.: Writing—review & editing, Supervision. T.T.Y.: Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, K.S., Zhang, J.J.Y., Kirollos, R. et al. A systematic review and meta-analysis of the association between cyproterone acetate and intracranial meningiomas. Sci Rep 12, 1942 (2022). https://doi.org/10.1038/s41598-022-05773-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05773-z
This article is cited by
-
Neues aus der hormonellen Kontrazeption
Gynäkologische Endokrinologie (2024)
-
Hormone therapies in meningioma-where are we?
Journal of Neuro-Oncology (2023)
-
Meningioma in patients exposed to progestin drugs: results from a real-life screening program
Journal of Neuro-Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.