Abstract
Depression is very common after stroke, causing multiple sequelae. We aimed to explore the efficacy of escitalopram for poststroke depression (PSD). PubMed, Embase, Scopus, Cochrane Central Register of Controlled Trials, Clinical trials. gov, Wan fang Data (Chinese), VIP (Chinese) and CNKI (Chinese) were retrieved from inception to May 2021. We recruited Randomized Controlled Trials (RCTs) which met the inclusion criteria in our study. The depression rating scores, the incidence of PSD, adverse events as well as functional outcomes were analyzed. 11 studies and 1374 participants were recruited in our work. The results were depicted: the reduction of depression rating scores was significant in the escitalopram groups and the standard mean difference (SMD) was − 1.25 (P < 0.001), 95% confidence interval (95% CI), − 1.82 to − 0.68; the risk ratio (RR) of the incidence of PSD was 0.52 (95% CI, 0.29 to 0.91; P = 0.007 < 0.05), which was significantly lower in the escitalopram groups; Escitalopram is safe for stroke patients; there was improvement of the motor function. However, in sensitivity analyses, the conclusions of the motor function and the incidence of drowsiness were altered. The study suggests that escitalopram has a potentially effective role compared with control groups and demonstrates escitalopram is safe. However, the results of the motor function and the incidence of drowsiness should be considered carefully and remain to be discussed in the future.
Similar content being viewed by others
Introduction
Approximately 79,5000 people suffer a new or recurrent stroke each year1. Additionally, an epidemiology meta-analysis revealed 31% of patients developed depression during 5 years following stroke2. Frustratingly, poststroke depression (PSD) could impair the cognitive level and activities of daily living (ADL), cause negative sequelae on the recovery of patients, and increase the burden of caregivers3.
So far, the etiological mechanisms of PSD have not been revealed clearly. Psychological, social, and biological factors contributed to PSD together4,5. Stroke survivors with the homozygous short variation allele genotype of the serotonin transporter-linked polymorphic region (5-HTTLPR) have a higher risk of PSD6. Both stroke and depression are associated with increased inflammation7. Antidepressants can lower the levels of pro-inflammatory cytokines8. These new promising methods show that, in terms of the physical consequences of stroke, these drugs can reduce bad mood9. Escitalopram is a selective serotonin reuptake inhibitor (SSRI) with few drug interactions, and is thus suitable for stroke patients who are prescribed multiple medications10. In recent years, SSRI escitalopram has been proved to be effective for the treatment and prevention of PSD, but there are still controversy11,12.
It demonstrates that escitalopram is safe in a randomized controlled trial (RCT) for prevention of PSD, and decreases effectively the incidence of PSD, as well as improving ADL and social function11. Moreover, it also shows that there are no significant differences on cognitive function compared with problem-solving therapy (PST) and placebo. However, a study of escitalopram by Kim et al.12 shows that the occurrence of moderate or severe depressive symptoms and adverse events are not statistically significant except diarrhea, ADL improvement, cognitive function, motor function and neurological defects.
Two SSRI systematic reviews13,14 enrolled RCTs of escitalopram have been found, to our knowledge, which both only included a study of Robinson et al.11. Recently, new RCTs of escitalopram are pouring out15,16,17,18,19,20,21,22,23, which were conducted in different circumstances, and the integration effects of these studies was vague.
Therefore, we aimed at conducting a systematic review and meta-analysis of RCTs about escitalopram arm compared with the placebo/the blank control arm, evaluating the depression rating scores, the occurrence of depression along with depressive symptoms, the frequency of adverse events and other significant clinical outcomes.
Methods
Search strategy and study selection
8 databases were searched (search strategy in online supplemental data), Medline via PubMed, Embase, Scopus, Cochrane Central Register of Controlled Trials, Clinical Trials.gov, CNKI (Chinese), Wan fang (Chinese), and VIP database (Chinese), from inception to May 2021. In addition, we scrutinized references of relevant papers and also contacted with authors to get the detailed data if necessary.
Inclusion criteria: ① RCTs were enrolled for participants with a clinical diagnosis of stroke; ② The experimental group was treated with escitalopram at any dose, by any mode of delivery and the control arm was included a placebo or the blank control; ③ The primary outcomes: depression rating scores, in which the Hamilton Depression Scale (HAMD) was preferred, the incidence of PSD, and adverse events including gastrointestinal side effects, sexual side events, cardiovascular adverse effects, and other adverse events. The secondary outcomes: neurological deficit scores, ADL, cognitive impairments, and motor function. For functional outcomes, we gave preference to the National Institutes of Health Stroke Scale (NIHSS), the Barthel Index (BI), Fugl—Meyer motor scale (FM), Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA).
Exclusion criteria: ① The type of study was a non-randomized controlled study; ② The subjects were not stroke patients or no clear diagnostic criteria; ③ The experimental group was not treated with escitalopram, or the control arm was not included a placebo or the blank control, or drugs and therapies with mixed effects; ④ Outcome indicators were not required in this study; ⑤ Intervention methods were not expressed clearly and could not be verified by the authors.
Two team members exacted data of each literature independently. A third investigator was discussed with if necessary.
Quality assessment
Study quality was independently assessed by two reviewers based on the Cochrane Collaboration’s risk of bias tool including randomization, allocation concealment, blinding, incomplete outcome data, and selective reporting. An opinion was sought from a third reviewer if the first two reviewers could not reach an agreement.
Statistical analysis
Pooled analyses were carried out at any follow-up point by RevMan 5.3 software (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Risk ratio (RR) with 95% confidence interval (CI) was described by categorical data. Standardized mean difference (SMD) with 95% CI was used for continuous outcomes. P < 0.05 was used as a cutoff for statistical significance. Statistical heterogeneity of trials was evaluated by I224. We used a random-effect model to calculate the pooled estimates if we observed I2 > 50% or P < 0.10, and on the contrary, we used a fixed-effect model.
Subgroup analyses were conducted based on different rating scales, depression or not at recruitment and follow-up duration (< 3 months vs 3 ~ 6 months vs > 6 months).
In sensitivity analyses, the trails with high heterogeneity were excluded. Publication bias was assessed by a funnel plot and Egger statistical test that was carried out by Stata 12.0 and P < 0.10 was considered as statistically asymmetry25.
Our study was conducted according to the PRISMA 2020 guidelines. We analyzed the data about previous studies which were published early in our research, so ethical approval and patient consent were not necessary and therefore not provided.
Results
Study selection and characteristics
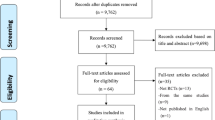
We searched 5 English databases (27 from Medline via PubMed, 72 from Embase, 123 from Scopus, 12 from Cochrane Central Register of Controlled Trials, 3 from Clinical Trials.gov) and 3 Chinese databases (100 from CNKI, 99 from Wan fang, 87 from VIP database) from inception to May 2021. After removing duplicates, there existed 285 records and 51 full texts were obtained. Finally, 11 articles were recruited (Fig. 1), in which 1374 participants were randomly enrolled in the escitalopram or the control11,12,15,16,17,18,19,20,21,22,23. Most of the papers excluded the participants with severe comprehension deficits, aphasia, and unstable medical conditions.
The follow-up was at treatment end in 9 RCTs11,12,15,17,18,19,20,21,22,23. There were 2 RCTs of which the follow-up duration was beyond treatment end12,16, however, we could not obtain the detailed data of Kim et al.12 at 6 months and Mikami et al.16 at 18 months. Participants suffered from depression at recruitment in 5 papers12,19,20,22,23. In 6 papers, participants were with no diagnosis of depression at recruitment11,15,16,17,18,21. Table 1 shows the detailed characteristics of each paper.
Depression rating scores
Figure 2 shows that the SMD of depression rating scores was − 1.25 (95% CI, − 1.82 to − 0.68; 7 trials; I2 = 90%) among participants allocated escitalopram compared with control. But there was moderate heterogeneity among participants who were with depression (SMD = -1.32; 95% CI, − 1.74 to − 0.90; I2 = 57%) or not depression (SMD = -1.15; 95% CI, − 2.21 to − 0.09; I2 = 95%) at recruitment and with no heterogeneity between subgroups (I2 = 0%; P = 0.77). It was reported that the antidepressant efficiency was obvious statistical significance (P < 0.05) in escitalopram group (88.9%) compared with the control (64.7%) in one trail, but we could not obtain the detailed scores23.
Figure 3 shows that there was obvious statistical significance in the subgroup where follow-up duration was the group of < 3 months (SMD = -1.78; 95% CI, − 2.78 to − 0.77; I2 = 91%) and the group of 3 ~ 6 months (SMD = -1.23; 95% CI, − 1.50 to − 0.97; I2 = 0%), however, there were no advantage of escitalopram in the subgroups, follow-up duration ≥ 6 months, but only one trial was included. Significant heterogeneity was among subgroups (I2 = 93.1%; P < 0.001).
The incidence of poststroke depression
The incidence of PSD was higher in control compared with escitalopram and with moderate heterogeneity (RR = 0.52; 95% CI, 0.29 to 0.91; 5 trials; I2 = 72%; Fig. 4).
Safety
No statistical significance was between escitalopram and control among trials for gastrointestinal side events. For nausea, diarrhea, abdominal pain and constipation, the RR was 1.31 (95% CI, 0.86 to 1.99; 7 trials; Fig. 5) with moderate heterogeneity (I2 = 59%, P = 0.02) among trials. There was also no statistical significance for other gastrointestinal side events: the dry mouth (RR = 0.73; 95% CI, 0.52 to 1.03; 3 trials; I2 = 46%; Supplemental Fig. 1), the anorexia (RR = 1.66; 95% CI, 0.95 to 2.90; 3 trials; I2 = 2%; Supplemental Fig. 2), the indigestion (RR = 1.26; 95% CI, 0.75 to 2.11; 3 trials; I2 = 0%; Supplemental Fig. 3), the bleeding (RR = 1.02; 95% CI, 0.15 to 7.07; 2 trials; I2 = 0%; Supplemental Fig. 4).
There was no significant cardiovascular adverse effects in escitalopram groups. The RR was 1.14 (95% CI, 0.44 to 2.96; 3 trials; I2 = 0%, P = 0.65; Fig. 6) for palpitation. For tachycardia, the RR was 1.07 (95% CI, 0.90 to 1.28; 2 trials; Supplemental Fig. 5) without heterogeneity (I2 = 0%; P = 0.65) among trials. Only 2 trails reported the chest pain, and the RR was 1.35 (95% CI, 0.68 to 2.70; 2 trials; I2 = 0%, P = 0.57; Supplemental Fig. 6).
Escitalopram did not affect sexual function versus control (RR = 1.39; 95% CI, 0.94 2.05; I2 = 0%, P = 0.72; 3 trials; Fig. 7) among trials for sexual side events.
The escitalopram was safe for the other adverse events, except for the drowsiness (RR = 6.95; 95% CI, 1.61 to 30.09; 3 trials; I2 = 31%, P = 0.23; Supplemental Fig. 7), and there was no or low heterogeneity among all enrolled trials: the insomnia (RR = 0.82; 95% CI, 0.48 to 1.39; 4 trials; I2 = 0%, P = 0.71; Supplemental Fig. 8), the dizziness (RR = 1.09; 95% CI, 0.90 to 1.32; 3 trials; I2 = 0%, P = 0.95; Supplemental Fig. 9), the fatigue (RR = 1.25; 95% CI, 0.90 to 1.74; 3 trials; I2 = 0%, P = 0.73; Supplemental Fig. 10), the increased sweating (RR = 1.78; 95% CI, 0.99 to 3.20; 3 trials; I2 = 0%, P = 0.80; Supplemental Fig. 11), the falls (RR = 1.02; 95% CI, 0.15 to 7.07; 2 trials; I2 = 0%, P = 0.97; Supplemental Fig. 12), the pain (RR = 0.88; 95% CI, 0.48 to 1.63; 2 trials; I2 = 24%, P = 0.25; Supplemental Fig. 13), the dysuria (RR = 1.38; 95% CI, 0.51 to 3.77; 2 trials; I2 = 0%, P = 0.85; Supplemental Fig. 14), the anxiety (RR = 1.98; 95% CI, 0.37 to 10.61; 2 trials; I2 = 48%, P = 0.16; Supplemental Fig. 15). It was no statistical significance (P > 0.05) in escitalopram group compared with the control, including the incidence of the paraesthesia, tremor, pruritus and peripheral oedema, however, they were only reported in one trail12.
Neurological deficit scores
The SMD was − 0.97 (95% CI, − 1.97 to 0.03; 4 trials; Supplemental Fig. 16) with high heterogeneity among trials (I2 = 97%), regarding different scales NFI (Neurologic Function Impairment) (SMD = -3.25; 95% CI, − 3.86 to − 2.64) vs NIHSS (SMD = -0.15; 95% CI, − 0.46 to 0.17; I2 = 91%, P = 0.15) vs MESSS (SMD = -0.35; 95% CI, − 0.69 to − 0.01). It was reported that the recovery rate of neurological function was obvious statistical significance (P < 0.05) in escitalopram group (86.1%) compared with the control (58.8%) in one trail, but we could not obtain the detailed scores23.
Activities of daily living
The pooled analysis was not in favor of the escitalopram compared with the control (SMD = 0.42; 95% CI, − 0.32 to 1.16; I2 = 94%; Supplemental Fig. 17).
Cognitive impairments
The SMD was 0.56 (95% CI, − 0.23 to 1.34; 3 trials; Supplemental Fig. 18) with high heterogeneity among trials (I2 = 94%; P < 0.001).
Motor function
There was a better effect in the escitalopram versus the control (SMD = 0.47; 95% CI, 0.02 to 0.93; 4 trials, Supplemental Fig. 19) with high heterogeneity among trials (I2 = 83%; P = 0.0005), between different motor function scales FM (SMD = 0.65; 95% CI, 0.25 to 1.06; I2 = 54%, P = 0.11) vs Hemispheric Stroke Scale (SMD = 0.00; 95% CI, − 0.18 to 0.18).
Quality assessment and sensitivity analyses
The quality of studies enrolled was shown in Fig. 8. In the sensitivity analyses, studies of the low quality were eliminated19,20,22, and the conclusions of pooled analyses were robust, except for the motor function (SMD = 0.36; 95% CI, − 0.40 to 1.13; I2 = 90%, P = 0.002) and the drowsiness (SMD = 4.70; 95% CI, 0.17 to 127.25; I2 = 64%, P = 0.09). Moreover, the I2 was decreased from 94 to 79% in the pooled analysis of the ADL. The inverted funnel plot of visual examination depression score (Fig. 9) was symmetrical. Moreover, the Egger tests showed that the outcome of depression rating scores (t = -0.77; P = 0.478 > 0.10) was not affected by publication bias.
Discussion
The systematic review and meta-analysis give an up-to-date and detailed description of the efficacy of escitalopram for PSD, in which 11 papers and 1374 participants were enrolled. Excitingly, participants allocated to the escitalopram were more improved than the control, including depression rating scores, the incidence of PSD and motor function, but the participants enrolled in the escitalopram group did not experience more improved in aspect of the ADL, neurological function and cognitive function. Furthermore, the participants in the escitalopram groups did not suffer more adverse events compared with the control groups in our research, except for the drowsiness. However, in sensitivity analyses, the conclusions of motor function and the drowsiness were not stable, which should be considered carefully.
Our research reveals escitalopram reduces effectively the depression rating scores and the incidence of PSD, which demonstrates the escitalopram is effective in the treatment and prevention of PSD. Escitalopram is safe for stroke patients in our meta-analysis. The pooled results show the participants treated with escitalopram are well tolerated for adverse events, including the gastrointestinal, cardiovascular, sexual and other adverse events but the drowsiness which only 2 trails were enrolled, however, the escitalopram groups did not experience more drowsiness in the sensitivity analyses, which is consistent with our previous meta-analysis26 and is different from two previous meta-analyses13,27, due to different types of antidepressants enrolled in that researches, especially tricyclic antidepressants included in Xu et al.27. It is negative for the functional indexes we analyzed except for the motor function. In the sensitivity analyses, only the result of motor function is altered. The conclusions of functional indexes are not consistent with previous meta-analyses26,27,28, which maybe only ≤ 4 papers are enrolled in each functional index and the validity should be interpreted cautiously and proved in the future.
One of the potential weaknesses was the high heterogeneity among trials in our research, except for the incidence of PSD and adverse events. The possible reasons were analyzed. Firstly, it may be small samples of most trials we enrolled and low quality of some trails, which may lead to high risk of bias and overestimation29. So in the sensitivity analyses, the I2 was decreased from 94 to 79% in the pooled analysis of the ADL. Secondly, different rating scales were used in the studies included, and one study shows the occurrence of PSD is different by different depression scales (HAM-D17 vs. HAM-D6)30, which proved different scales could lead to different outcomes and conclusions. It is testified in the subgroup analyses of neurological function (Supplemental Fig. 16) and motor function (Supplemental Fig. 19). Thirdly, after removing the paper16, the I2 was decreased from 90 to 80% in the pooled analysis of the depression rating scores, and the reason maybe the intervention duration (12 months) was obviously longer than other trails enrolled. Fourthly, after removing the trail12, heterogeneity (I2 = 22%, P = 0.26) decreased significantly in the pooled analysis of cognitive function, which maybe the weight of the trail was too large and the studies enrolled were too few.
The main problems of the serious cognitive impairment, aphasia, and the severe stroke about the participants were excluded in the studies recruited, therefore we could not know whether those patients could be treated with escitalopram, which was also a limitation of our research. Another limitation was that the studies enrolled were too few in some pooled analyses and related original studies should be conducted to clarify and testify the results.
The strengths also existed in our study. An extensive search was conducted, including online papers, references and unpublished trials, as well as a fairly wide range of important clinical results. Additionally, sufficient sensitivity analyses and enough subgroup analyses were performed to ensure the reliability and robustness of the results. In the future, it should be necessary to develop more detailed and rigorous basic experiments on mechanisms and clinical trials to make a better choice for clinicians and patients.
Conclusions
Taken together, our findings prompt escitalopram is safe and effective for PSD. However, the pooled analyses of the motor function and the incidence of drowsiness should be explained cautiously. Moreover, limitations and inspirations are provided for further researches in our study. Therefore, more multicenter, larger sample, more rigorous and more result indexes designed RCTs are needed to evaluate the protective role of escitalopram on PSD.
Change history
06 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-10039-9
References
Mozaffarian, D. et al. Heart disease and stroke statistics–2016 update. Circulation 133, e38–e360 (2016).
Hackett, M. L. & Pickles, K. Part I: Frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int. J. Stroke 9, 1017–1025 (2014).
Ayerbe, L., Ayis, S., Wolfe, C. D. A. & Rudd, A. G. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br. J. Psychiatry 202, 14–21 (2013).
De Ryck, A. et al. Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J. Neurol. Sci. 347, 159–166 (2014).
Hackett, M. L., Yapa, C., Parag, V. & Anderson, C. S. Frequency of depression after stroke: A systematic review of observational studies. Stroke 36, 1330–13340 (2005).
Mak, K. K., Kong, W. Y., Mak, A., Sharma, V. K. & Ho, R. C. Polymorphisms of the serotonin transporter gene and post-stroke depression: A meta-analysis. J. Neurol. Neurosur. Psychiatry 84, 322–328 (2013).
Geng, H. H. et al. The relationship between c-reactive protein level and discharge outcome in patients with acute ischemic stroke. Int. J. Environ. Res. Public Health 13, 636 (2016).
Lu, Y. et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 12(10), e0186700 (2017).
Chollet, F. et al. Use of antidepressant medications to improve outcomes after stroke. Curr. Neurol. Neurosci. Rep. 13, 318 (2013).
Puri, B. K., Ho, R., Hall, A. Revision Notes in Psychiatry; CRC Press: New York, NY, USA (2014).
Robinson, R. G. et al. Escitalopram and problem-solving therapy for prevention of poststroke depression: A randomized controlled trial. JAMA 299, 2391–2400 (2008).
Kim, J. S. et al. Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: A multicentre, double-blind, randomised, placebo-controlled study. Lancet Psychiatry 4, 33–41 (2017).
Mead, G. E. et al. Selective serotonin reuptake inhibitors for stroke recovery: A systematic review and meta-analysis. Stroke 44, 844–850 (2013).
Mead, G. E. et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery (Review). Cochrane Database Syst. Rev. 11, CD009286 (2012).
Jorge, R. E., Acion, L., Moser, D., Adams, H. P. & Robinson, R. G. Escitalopram and enhancement of cognitive recovery following stroke. Arch. Gen. Psychiatry 67(2), 187–196 (2010).
Mikami, K. et al. Increased frequency of first-episode poststroke depression after discontinuation of escitalopram. Stroke 42, 3281–3283 (2011).
Zhan, Y. H. et al. Effect of escitalopram on motor recovery after ischemic stroke. Chin. Hosp. Pharm. J. 34, 752–755 (2014).
Zhan, Y. H., Tong, S. J., An, X. K., Lu, C. X. & Ma, Q. L. Effect of escitalopram for cognitive recovery after acute ischemic stroke. Chin. Gen. Pract. 17, 2422–2425 (2014).
Wang, X. F. Randomized controlled trial of escitalopram on outpatients with post-stroke depression. China Pharmacist. 15, 81–82 (2012).
Jiang, D. D., Peng, C. & Lu, Y. C. Effect of escitalopram oxalate on motor recovery with post-stroke depression. Chin. Manipulat. Rehabilit. Med. 6, 76–77 (2015).
Zhao, X. H., Wen, J. Z. & Yuan, F. Clinical observation of escitalopram oxalate for preventing post-stroke depression. Drugs Clin. 29, 269–272 (2014).
Li, X. Y., Zhang, Z. D. & Zhang, T. Effect of early antidepressant treatment on prognosis of patients with post-stroke depression. Shaanxi Med. J. 45, 1344–1346 (2016).
Lin, L. Effect of escitalopram on hypothalamic-pituitary-adrenergic axis function in patients with post-stroke depression. Chin. J. Pharmacoepidemiol. 21, 417–419 (2012).
Higgins, J. P. T., Green, S.: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration; 2011.
Egger, M. & Smith, G. D. Meta-analysis: Bias in location and selection of studies. BMJ 316, 61–66 (1998).
Feng, R. F. et al. Effect of sertraline in the treatment and prevention of poststroke depression: A meta-analysis. Medicine 97, e13453 (2018).
Xu, X. M. et al. Efficacy and feasibility of antidepressant treatment in patients with post-stroke depression. Medicine 95, e5349 (2016).
Chen, Y., Guo, J. J., Zhan, S. & Patel, N. C. Treatment effects of antidepressants in patients with post-stroke depression: A meta-analysis. Ann. Pharmacother. 40, 2115–2122 (2006).
Wickenberg-Bolin, U., Goransson, H., Fryknas, M., Gustafsson, M. G. & Isaksson, A. Improved variance estimation of classification performance via reduction of bias caused by small sample size. BMC Bioinf. 7, 127–127 (2006).
Rasmussen, A. et al. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics 44(3), 216–221 (2003).
Acknowledgements
Rong-fang Feng and Rui Ma collected and analyzed the data, and wrote the paper. Li Guo, Peng Wang and Xu Ji participated in writing of the manuscript. Rong-fang Feng, Rui Ma, Li Guo and Peng Wang conceived and designed this study. Zhen-xiang Zhang extracted the data and modified the paper. Meng-meng Li and Jia-wei Jiao extracted the data. All authors reviewed the paper, read and approved the final manuscript.
Funding
This work is supported by the Innovative Talent Project of Colleges and Universities in Henan Province (20HASTIT047), Philosophy and social science planning project of Henan (2021BSH017), Foundation of Co-constructing Project of Henan Province and National Health Commission (SBGJ202002103), the Medical Education Research Project of Henan Health Commission (Wjlx2020362), Teaching program of Zhengzhou University (2021ZZUKCLX025, 2021ZZUJGLX194 and 2021-32), the Project of Social Science association in Henan Province (SKL-2021-472 and SKL-2021-476) and Zhengzhou (2021-0651), the Science and Technology Project of Henan Science and Technology Department (202102310069 and 192102310531).
Author information
Authors and Affiliations
Contributions
R.-f.F., and R.M. collected and analyzed the data, and wrote the paper. L.G., P.W., and X.J. participated in writing of the manuscript. R.-f.F., R.M., L.G., and P.W. conceived and designed this study. Z.-x.Z. extracted the data and modified the paper. M.-m.L. and J.-w.J. extracted the data. All authors reviewed the paper, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of the Article contained an error in the Author list, where the authors Rong‑fang Feng and Rui Ma were erroneously listed as equally contributing authors.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, Rf., Ma, R., Wang, P. et al. Efficacy of escitalopram for poststroke depression: a systematic review and meta-analysis. Sci Rep 12, 3304 (2022). https://doi.org/10.1038/s41598-022-05560-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05560-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.