Abstract
Since the discovery of the breast cancer susceptibility genes, BRCA1 and BRCA2, various other genes conferring an increased risk for breast cancer have been identified. Studies to evaluate sequence variants in cancer predisposition genes among women of African ancestry are limited and mostly focused on BRCA1 and BRCA2. To characterize germline sequence variants in cancer susceptibility genes, we analysed a cohort of 165 South African women of self-identified African ancestry diagnosed with breast cancer, who were unselected for family history of cancer. With the exception of four cases, all others were previously investigated for BRCA1 and BRCA2 deleterious variants, and were negative for pathogenic variants. We utilized the Illumina TruSight cancer panel for targeted sequencing of 94 cancer susceptibility genes. A total of 3.6% of patients carried a pathogenic/likely pathogenic variant in a known breast cancer susceptibility gene: 1.2% in BRCA1, 0.6% in each of BRCA2, ATM, CHEK2 and PALB, none of whom had any family history of breast cancer. The mean age of patients who carried deleterious variant in BRCA1/BRCA2 was 39 years and 8 months compared to 47 years and 3 months among women who carried a deleterious variant in other breast cancer susceptibility genes.
Similar content being viewed by others
Introduction
Breast cancer is an increasing public health problem worldwide. It is the most commonly diagnosed cancer and the leading cause of cancer deaths in women. Breast cancer incidence and mortality rates are rising in transitioning countries in Africa, with some of the most rapid increases occurring in sub-Saharan Africa1,2. The GLOBOCAN 2020 database of the International Agency for Research on Cancer (IARC), estimated the current age standardised breast cancer incidence per 100,000 women in Southern (50.4), Western (41.5), Eastern (33), and Central Africa (32.7) with associated mortality rates estimated at 15.7, 22.3, 17.9, and 18, respectively3. Female breast cancer represents 25.8% of all cancer diagnoses in sub-Saharan Africa1,2. Newly diagnosed breast cancer cases in South Africa accounts for 27.1% of female cancers in 2020, with age-standardized (World) incidence and mortality rates of 52.6 and 16 (per 100,000 women) respectively3. Cancer results from a process of genetic changes, some inherited, some induced by environmental exposures and some occurring by chance. Early age of onset and a family history is a hallmark of hereditary breast cancer that is associated with germline variants in the high-penetrance genes, BRCA1 and BRCA24,5,6. An association with breast cancer susceptibility has also been reported for a further eleven high- to moderate-penetrance genes (TP53, PALB2, PTEN, STK11, CDH1, ATM, BRIP1, CHEK2, RAD51B, RAD51C, and RAD51D)7,8. In addition, pathogenic variants in genes from the mismatch repair pathway (MLH1, MSH2, MSH6 and PMS2) have been identified in breast cancer and ovarian cancer patients7. With the advent of next-generation sequencing (NGS), simultaneous sequencing of multiple cancer susceptibility genes is available through multiplex panels. Several studies utilizing NGS gene panels have been carried out, mainly on breast cancer cases from west European and Asian populations9,10. Some studies have included African-Americans, but this data can be difficult to interpret in an African context due to the fact that they are an admixed population. The estimated proportion of African, European and Native American ancestry in African-American groups vary from 76 to 85% African, 14% to 21% European and 1% to 3% Native American ancestry11.
Studies of breast cancer susceptibility genes in African populations are scarce and have mainly focused on BRCA1 and BRCA212. In addition, there is a paucity of data on sequence variants in cancer predisposition genes among women of African descent/ancestry with breast cancer, who are unselected for age at diagnosis, or family history of cancer. To date only two studies in Africa, one on Nigerian women13, and one on women from Uganda and Cameroon14, have used multigene panel sequencing to test for germline variants in patients, unselected for family history or age at diagnosis. In the present study, we included South African women of African ancestry (self-identified) diagnosed with breast cancer, who were unselected for age at diagnosis or family history of cancer. With the exception of four cases, all others were previously investigated for BRCA1 and BRCA2 pathogenic variants, and were negative for pathogenic/likely pathogenic BRCA1/BRCA2 variants We used targeted next-generation sequencing of a multigene panel, comprised of 94 cancer susceptibility genes (Illumina TruSight cancer panel) in order to assess the frequency of deleterious germline variants in this cohort.
Results
A total of 165 breast cancer patients of African ancestry (self-reported), unselected for family history or age at diagnosis, were included in this study (Supplementary Table S1). Their mean age (SD) at diagnosis was 41.28 (7.35) years (age range 22 to 54 years). Figure 1 depicts the patients’ age at diagnosis displayed in 5-year intervals. Furthermore, 9% (15/165) of the patients reported either a 1st and/or 2nd degree relative with breast and/or ovarian cancer (Supplementary Table S1).
Information on the histology type was available for 145 of the 165 patients. The most common type was infiltrating ductal carcinoma (81.8%), followed by medullary ductal carcinoma (2.4%), invasive lobular carcinoma (1.2%), and at 0.6% each, tubular ductal carcinoma, papillary carcinoma, infiltrating mucinous carcinoma and carcinoma not otherwise specified. Cancer grade information was unavailable for eight of the 165 patients. Only one of the patients was diagnosed with grade I (0.6%), 40 (24.2%) with grade II, 46 (27.9%) with grade III and 70 (42.4%) with grade IV breast cancer. High-grade tumours (grade III and IV) were by far the most common, accounting for 70.3% of all carcinomas. Information from hospital and histology records regarding hormone receptor status was unavailable.
We performed targeted sequencing of 94 cancer susceptibility genes (Supplementary Table S2) in peripheral blood DNA samples from the 165 patients, using a next-generation sequencing platform. All of the 94 genes were investigated for nonsense, frameshift, or splice-site variants affecting the invariant splice sites. In addition, a subset of nineteen established and candidate breast or ovarian cancer genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11 and TP53) were investigated for all sequence variants.
Variants
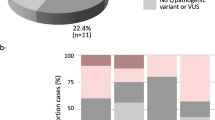
In the final data set, filtering by concordant deleterious effect prediction for missense variants in the breast cancer susceptibility genes (at least 3/5 methods), population allele frequencies (< 1% in African populations of 1000 genomes phase 1 and 3), read depth (≥ 20), resulted in the identification of 52 unique variants in 20 genes. Of these 52 variants, eleven were classified as PV/LPV (Table 1), 27 classified as VUSs (Table 2) and 14 were classified as benign/likely benign (Supplementary Table S3). These variants were present in 76 of the patients (Fig. 2, Supplementary Table S4). Missense variants dominated (39), followed by frameshift variants (3), nonsense variants (3), variants affecting canonical splice sites (3), and in-frame deletions (3).
Matrix of patients vs. genes with sequence variants in breast cancer susceptibility genes and genes exclusively investigated for truncating variants (multiple variants per gene may be present). The genes are sorted from the most to least number of variants per gene as indicated in brackets. Black indicates truncating variants (frameshift, nonsense and splice-site variants affecting the invariant splice sites); Grey indicates an in-frame insertion or deletion. Missense variants are indicated according to the five in silico functional effect predictors, where yellow indicates a deleterious effect predicted by 3/5 methods, orange 4/5 methods and red 5/5 methods (Figure generated using Matplotlib 3.4.2: https://matplotlib.org).
The concordance of the five in silico functional effect predictors is shown in Supplementary Fig. S1 (full concordance for 22 variants), and the number of distinct deleterious variants per predictor is shown in Supplementary Fig. S2.
Deleterious variants
Six patients (3.6%) were found to carry a pathogenic or likely pathogenic (P/LP) variant in one of five known breast cancer susceptibility genes: 1.2% in BRCA1, 0.6% in each of BRCA2, ATM, CHEK2 and PALB. A further seven patients carried deleterious variants in one of five hereditary cancer predisposition genes exclusively investigated for truncating variants, specifically ALK, BUB1B, FANCG, RB1 and XPC (Table 1). None of these patients reported any family history of cancer.
Variants of uncertain clinical significance
A total of 27 variants were detected in 12 genes associated with breast cancer susceptibility (Table 2). VUS were identified most commonly in the ATM gene (eight variants), followed by MSH6 with five variants and BRCA2 (four variants).
Discussion
This study screened 165 South African breast cancer patients of African ancestry (self-identified) for the presence of deleterious germ line sequence variants in 94 genes associated with hereditary cancer. The patients were unselected for age at diagnosis or family history of cancer. With the exception of four cases (BRB130, BRB290, BRC134 and BRC210) all others were previously screened for BRCA1/BRCA2 variants, and found to be negative for pathogenic/likely pathogenic variants.
Although the patients were unselected for family history of breast or ovarian cancer, 9% did report some family history of breast or ovarian cancer. This is higher than that reported for similar studies in breast cancer patients from Cameroon/Uganda (6.6%) and Nigeria (6%)13,14. With regards to tumour stage, 70.3% of patients were diagnosed with stage III/IV at diagnosis. It is thought that low survival rates in sub-Saharan Africa is mostly attributable to late-stage presentation. The stage at presentation of our cohort is similar to that reported in 83 studies across 17 sub_Saharan African countries, with 77% of cases presenting at stage III/IV15.
We identified pathogenic/likely pathogenic variants (P/LP) in 13 patients, in ten different genes (Table1), which represents 7.9% of the cohort. Six of these patients (3.6%) have P/LPVs in genes that are confirmed to confer an increased risk for breast cancer. The mean age of patients who carried deleterious variant in BRCA1/BRCA2 was 39 years and 8 months compared to 47 years and 3 months among women who carried a deleterious variants in other breast cancer susceptibility genes. Pathogenic variants in non-BRCA1/BRCA2 breast cancer susceptibility genes accounted for 1.8% of our cohort. None of these women reported any family history of cancer. In addition, 14 benign/likely benign variants were detected in eight breast cancer genes (Supplementary Table S3) and 27 VUS including six variants not previously described, were detected in 12 established and candidate breast cancer genes (Table 2).
In the studied cohort, variants in the ATM gene were the most frequently identified (Tables 1, 2). Pathogenic ATM variants act in a recessive manner to cause Ataxia telangiectasia (a neurodegenerative disease), whereas heterozygous carriers are at moderately increased risk for breast cancer16,17. Patient BRB14 (Zulu-speaking patient), diagnosed with breast cancer at age 47 years and 8 months, was a carrier of the novel ATM likely pathogenic variant, c.162T > A. It is predicted to be a nonsense variant, p.(Tyr54Ter), that may cause the transcript to be exposed to nonsense-mediated mRNA decay. If ATM is synthesized it will lack most of the protein sequence and thus be non-functional. Interestingly, a recent study that explored the clinico-pathologic characteristics of breast cancers developed by ATM mutation carriers reported the median age at first diagnosis to be 46.9 years in their cohort18. Unfortunately we do not have any further histopathologic information on the breast cancer of BRB14.
There has been some debate on whether mono-allelic truncating ATM variants are associated with increased breast cancer risk. Early on it was hypothesised that some missense variants in ATM might have dominant negative effects and confer a particularly high risk of breast cancer when heterozygous, compared to truncating variants19. In a meta-analysis of ATM variants, a later study found strong evidence that a subset of rare evolutionary unlikely missense variants confer increased cancer risk. They found marginal evidence that protein-truncating and splice-site variants contribute to breast cancer risk20. Goldgar et al.21 further investigated the issue and reported risk estimates that women who carry either a pathogenic missense or truncating variant have a significantly increased risk of breast cancer. To obtain accurate risk estimates require a large sample size, which a recent large study of more than 113,000 women (mostly population-based samples), addressed22. This study identified ATM protein-truncating variants to confer significant disease risks (odds ratio 2.1), compared to rare missense variants (odds ratio 1.06)22.
Two of the four patients (BRB130 and BRB290) who had not previously been screened for BRCA1/BRCA2 variants, were found to carry a BRCA1 or BRCA2 deleterious variant (Table 1). The BRCA1 c.4524G > A p.(Trp1508Ter) variant was identified in BRB130, a Tswana-speaking woman diagnosed with breast cancer at age 45 years and 8 months. The variant is predicted to introduce a stop codon that will produce a transcript that may be targeted for nonsense-mediated mRNA decay (NMD). This nonsense variant has been detected in multiple families with hereditary breast ovarian cancers23,24,25,26,27,28,29,30. Of note, the variant is also designated as 4643G > A in published literature.
BRB264 (diagnosed at 42 years and three months, Tsonga-speaking patient) carried the BRCA1 c.5096G > A p.(Arg1699Gln), intermediate risk variant. It is located in the BRCA1 carboxyl terminal region of the transcriptional transactivation domain. The cancer risks associated with this variant was first defined by the ENIGMA consortium (Evidence-based Network for the Interpretation of Germline Mutant Alleles) in 2012 and in a follow up study in 2017 the risk estimates were confirmed31,32. Functional assays showed this variant to have impaired homology-directed DNA repair activity and it was classified as being a hypomorphic allele33. Interestingly, this pathogenic missense was also found in a Nigerian woman with breast cancer13.
The BRCA2 frameshift variant, c.5771_5774del p.(Ile1924ArgfsTer38), was identified in BRB290 who was diagnosed with breast cancer at 26 years and 6 months of age. The variant is expected to result in loss of function due to an absent or disrupted protein. This alteration has been reported in multiple individuals (of European ancestry) with hereditary breast and ovarian cancer syndrome34 and has been reported as a founder mutation in Bantu-speaking Xhosa women from the Western Cape of South Africa35. BRB290 is however a Bantu-speaking Sotho individual, and at this time it is not possible to do any haplotype analysis to ascertain whether she carries this PV on the same haplotype as that of the Xhosa founder variant.
The pathogenic CHEK2, c.283C > T p.(Arg95Ter), variant detected in BRB121 (diagnosed at 54 years, Zulu-speaking patient) was previously identified in the germline of two Norwegian patients diagnosed with locally advanced breast cancer36. Of interest, both patients were resistant to anthracycline therapy. In vitro assays of the p.(Arg95Ter) variant found the CHEK2 protein to be non-functional in terms of kinase activity and dimerization. Loss of heterogeneity (LOH) analysis of the tumours found that the wild type allele of the CHEK2 gene was lost for both of the patients36. The possibility that this nonsense variant together with LOH is associated with resistance to anthracyclines in cancer patients underlines its potential clinical importance. In a follow up case control study of 7081 incident cancer cases from Norway, Knappskog et al., detected the p.(Arg95Ter) variant in 0.23% breast cancer cases and in 0.16% prostate cancer cases37. This variant is also reported as pathogenic by multiple laboratories in ClinVar (Variation ID: 140772). In our study 0.61% (1/165) of cases carried a pathogenic CHEK2 variant. There is substantial variation in the prevalence of germline CHEK2 pathogenic variants among different populations and ethnicities, with individuals of European ancestry that have the highest prevalence38. A multi-ethnic population-based study of a cohort of breast cancer and ovarian cancer patients found that for breast cancer 2.3% (95% CI 1.8% to 2.8%) of white individuals and only 0.15% (95% CI 0% to 0.82%) of black individuals carried a pathogenic CHEK2 variant39.
The PALB2 variant, c.2835-1G > C, located in a canonical acceptor splice-site (in Intron 8) was identified in a Xhosa-speaking patient (BRB241, diagnosed at 40 years of age). The variant has been reported in the literature in persons affected with breast or ovarian cancer40,41,42,43. Several in silico bioinformatic tools predicted this variant to abolish the 3′-acceptor splice site, which would alter the natural splicing of PALB2. The expected effect is an in-frame deletion in the PALB2 mRNA by skipping exon nine (deletion of 162 bp, 54 amino acids; Ala946 to Gly999). Another possibility is that an alternative cryptic splice site could be used. The strongest alternative site is in exon nine at c.2864, and should this be used, the result would be the loss of 30 bp (10 amino acids; Ala946 to Glu955) from exon nine.
cBROCA analysis of mRNA from patients with the c.2835-1G > C variant showed that it preferentially leads to skipping of exon 9 (r.2835–2996) and is therefore expected to produce an abnormal PALB2 protein, lacking the 54 amino acids44. The deleted section is part of the second and third blades of the WD40 domain of PALB2. This seven bladed region is essential for the interaction of BRCA2 with PALB245,46. When BRCA2 is unable to bind to PALB2, homologous recombination repair is severely disrupted.
Pathogenic variants in five “other” cancer predisposition genes (ALK, BUB1B, FANCG, RB1 and XPC) exclusively investigated for truncating variants, were identified in seven patients (Table 1). Three of the genes (BUB1B, FANCG and XPC) are associated with autosomal recessive conditions, requiring the inheritance of two pathogenic variants for the particular condition to manifest. Deleterious variants, either in the hetero- or homozygous state, in these genes have not been found to confer an increased risk for breast cancer. Pathogenic germline variants of ALK usually are gain-of-function missense variants that are associated with familial neuroblastoma47,48—the novel variant found in our study leads to a loss-of-function effect. Biallelic pathogenic variants in the spindle assembly checkpoint gene, BUB1B, causes the disorder, mosaic variegated aneuploidy (MVA)49. The FANCG frameshift variant, c.637_643del p.(Tyr213LysfsTer6), detected in two breast cancer patients (BRB98 & BRB225), is a founder variant that is present in 82% of Fanconi anaemia subtype G patients from sub-Saharan African populations50. The RB1 gene is the first tumour suppressor gene to be cloned and germline pathogenic variants predispose to hereditary retinoblastomas (childhood retinal cancer)51. It is unknown whether BRB73, carrier of the RB1 donor splice site variant, had retinoblastoma as a child. The XPC splice acceptor site variant, c.2251-1G > C, that was detected in two breast cancer patients (BRB114 & BRB161), is an ancient founder variant that is thought to have occurred ~ 800 years ago in the Bantu population of West-Central Africa52. This variant is present in the homozygous state in many Xeroderma pigmentosum families of African ancestry52.
Two of the VUS detected in the ATM gene (p.Asp44Gly, p.Glu2181Asp) were also found in breast cancer patients from Cameroon and Uganda14. The available evidence is currently insufficient to unequivocally determine the role of the VUS that we detected in established and candidate breast cancer genes (Table 2). However there is one variant of note, the PALB2 N-terminus variant c.23C > T p.(P8L) detected in two patients (BRB55 & BRB89). This variant is near the coiled-coil domain of PALB2 that is involved in hetero-dimerization of BRCA1 with the protein. PALB2 is an essential component in homologous recombination-based DNA repair (HR) and loss of PALB2 function was shown to be synthetic lethal in combination with poly(ADP-ribose) polymerase inhibitors (PARPi)53,54. This has led to the development of tests that exploit this weakness to assess the functional effect of PALB2 sequence variants.
Functional assays that test the vulnerability of PALB2 variants to PARP inhibitors as well as HR functionality were applied to the p.(P8L) variant. Moderate but statistically significant (P < 0.0001) PARPi sensitivity was observed (76% cell survival), whereas wild type PALB2 had 100% cell survival55. The homology-directed repair assay found p.(P8L) to have an intermediate phenotype with a 40% reduction in HR when compared to wild type PALB255,56, all of which appear to indicate that this variant may play a role in breast cancer.
A limitation of this study is that no copy number variation using NGS data or MLPA was used to investigate the genes. Thus large deletions or duplications could be undetected. Furthermore, the relatively small sample size and unavailability of hormone receptor status precluded any investigation of the prevalence of sequence variants by breast cancer subtype.
While precision medicine is currently still mostly out of reach in African countries due to economic reasons, the rapidly declining costs of genomic technologies will in future necessitate population-specific variant information, particularly in diseases such as cancer.
To our knowledge, this is the first study that has investigated South African breast cancer patients of African ancestry for germline sequence variants in a multigene panel.
Although we investigated a relatively small cohort of patients, our study provides some insights towards the genetic breast cancer risk factors in South African women of African ancestry. In conclusion, our study has shown that the 3.6% of women who carry a pathogenic/likely pathogenic variant in a breast cancer susceptibility gene do not necessarily have a family history of breast cancer. In our cohort there was an equal proportion of women who carried a deleterious variant in BRCA1/BRCA2 (1.8%) and women who carried a deleterious variant in other breast cancer susceptibility genes (1.8%). These findings must however be treated with caution because of the small sample size. Further studies of a larger patient cohort is warranted to assess the distribution of variants in clinically relevant cancer susceptibility genes.
Patients and methods
Patients and DNA samples
Peripheral blood samples were previously collected from South African women with breast cancer, who attended the Oncology Clinic at Steve Biko Hospital, Pretoria, between 1993 and 2001. The study population were of self-reported African ancestry, at least 18 years old and were included regardless of age at diagnosis or family history. In total we received blood samples from 286 patients with age at diagnosis ranging from 21 to 85 years (mean 49.52 years ± 12.93 years). DNA was extracted from the blood samples using the method described by Johns and Paulus-Thomas57. For the current study we selected 165 of these patients (Supplementary Table S1) beginning with the youngest patients. With the exception of four cases (BRB130, BRB290, BRC134 and BRC210) all the samples were previously screened for BRCA1/2 deleterious variants using SSCP/Heteroduplex analyses and multiplex ligation-dependent probe amplification (MLPA), and were negative for pathogenic or likely pathogenic variants.
Ethics approval
This study was approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Protocol no. 260/2018). All experiments were performed in accordance with guidelines and regulations. The patients gave written informed consent for participation in the study.
Analysed cancer genes
The Illumina TruSight Cancer sequencing panel, which targets 94 cancer related genes was used (Supplementary Table S2). All 94 genes were assessed for nonsense, frameshift, or splice-site variants affecting the invariant splice sites. A subset of nineteen established and candidate breast or ovarian cancer genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11 and TP53) were further investigated for all sequence variants. The results from the previous BRCA1/BRCA2 screening were also verified.
Library preparation and sequencing
Patient DNA samples were sent to Omega Biotech in Georgia, USA, where DNA libraries were produced with the TruSight Rapid Capture kit (Illumina) and sequenced using the Illumina TruSight Cancer sequencing panel.
Sequencing data analysis
All variant calling and variant filtration codes were executed on a Linux cluster with 10× nodes, each having 28× cores and 128 GB of RAM, running CentoS 7.4. Quality analysis of raw sequences was performed using FastQC (version 0.11.7)58. Reads were subsequently pre-processed with the FastX toolkit (version 0.0.14) to trim five nucleotides from the 5′- and 3′-ends of the 100 bp paired-end reads59. Thereafter, samples were analysed using the GATK best practices60 approach by means of the BCBIO pipeline (June 2021 release, detailed tool versions provided in supplementary information)61. This includes mapping against the UCSC hg19 reference genome with Burrows–Wheeler Aligner-MEM (BWA MEM)62, marking duplicates with Picard and base quality score recalibration. Variant calling was carried out using the HaplotypeCaller in gVCF mode and specified cut off-based filtering of variants done with VariantFiltration using the BCBIO default filtering cut-offs.
Variant annotation
Functional variant annotation was done using Variant Effect Predictor (VEP), the default parameters were used in concordance with documentation63. Quality-filtered variants were uploaded to the VEP web interface, and additional output fields were activated in the dbNSFP section for LRT_pred64, MutationTaster65, PROVEAN66, CADD67 and FATHMM68. Filtering of common variants was not performed in VEP.
Variant filtration and in silico evaluation of variants
In-house Python code (available on request) was developed for the selection of variants for inclusion in this study. Variants with a minor allele frequency (MAF) of ≥ 1% in the 1000 Genomes African database were removed. For missense variants in the breast cancer susceptibility genes, the results of five in silico functional effect predictors were considered, being LRT_pred64, MutationTaster65, PROVEAN66, CADD67 and FATHMM68 with variants being selected if at least 3/5 methods predicted a variant to be deleterious. A threshold of 2.0 for GERP_RS and 10.0 for CADD was used. For the other methods, a prediction of ‘D’ was selected. As VEP provides results for multiple transcripts per gene, canonical transcripts are reported on, as determined by mapping of REFSEQ identifiers to Ensembl canonical transcripts via UCSC tables69, accessed July 2018.
Variant classification
Variants were classified in accordance with the American College of Medical Genetics and Genomics guidelines70, as pathogenic, likely pathogenic, likely benign, benign or as variants of uncertain significance (VUS). For clarification, pathogenic/likely pathogenic variants were defined as “deleterious variants” linked to the condition “hereditary cancer-predisposition syndrome”.
Nomenclature
Variants were described according to the Human Genome Variation Society (HGVS) recommendations71. Reference sequences were obtained from the NCBI database as listed in Tables 1 and 2. For BRCA1 the most common human transcript (NM_007294.3) was used with custom numbering of the exons (missing exon 4).
Data availability
The raw datasets analysed during the study, and filtering scripts are available from the corresponding author on reasonable request.
References
Rebbeck, T. R. Cancer in sub-Saharan Africa. Science 367, 27–28 (2020).
Anyigba, C. A., Awandare, G. A. & Paemka, L. Breast cancer in sub-Saharan Africa: The current state and uncertain future. Exp. Biol. Med. 246, 1–11 (2021).
Ferlay, J. et al. Global Cancer Observatory: Cancer Tomorrow (International Agency for Research on Cancer, 2020).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Wooster, R. et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 378, 789–792 (1995).
Haffty, B. et al. Breast cancer in young women (YBC): Prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann. Oncol. 20, 1653–1659 (2009).
Couch, F. J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3, 1190–1196 (2017).
Samadder, N. J., Giridhar, K. V., Baffy, N., Riegert-Johnson, D. & Couch, F. J. Hereditary cancer syndromes a primer on diagnosis and management: Part 1: Breast-ovarian cancer syndromes. Mayo Clin. Proc. 94, 1084–1098 (2019).
Easton, D. F. et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 372, 2243–2257 (2015).
Castéra, L. et al. Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 HBOC families. Genet. Med. 20, 1677–1686 (2018).
Baharian, S. et al. The great migration and African-American genomic diversity. PLoS Genet. 12(5), e1006059 (2016).
Abbad, A. et al. Genetics of breast cancer in African populations: A literature review. Glob. Health Epidemiol. Genomics 3, e8 (2018).
Zengh, Y. et al. Inherited breast cancer in Nigerian women. J. Clin. Oncol. 36, 2820–2825 (2018).
Adedokun, B. et al. Prevalence of inherited mutations in breast cancer predisposition genes among women in Uganda and Cameroon. Cancer Epidemiol. Biomark. Prev. 29, 359–367 (2020).
Jedy-Agba, E., McCormack, V., Adebamowo, C. & Dos-Santos-Silva, I. Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 4, e923–e935 (2016).
Renwick, A. et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat. Genet. 38(8), 873–875 (2006).
Marabelli, M., Cheng, S.-C. & Parmigiani, G. Penetrance of ATM Gene mutations in breast cancer: A meta-analysis of different measures of risk. Genet. Epidemiol. 40, 425–431 (2016).
Toss, A. et al. Clinicopathologic profile of breast cancer in germline ATM and CHEK2 mutation carriers. Genes 12, 616 (2021).
Gatti, R. A., Tward, A. & Concannon, P. Cancer risk in ATM heterozygotes: A model of phenotypic and mechanistic differences between missense and truncating mutations. Mol. Genet. Metab. 68(4), 419–423 (1999).
Tavtigian, S. V. et al. Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am. J. Hum. Genet. 85, 427–446 (2009).
Goldgar, D. E. et al. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 13, R73 (2011).
Breast Cancer Association Consortium et al. Breast cancer risk genes—Association analysis in more than 113,000 women. N. Engl. J. Med. 384, 428–439 (2021).
Loman, N. et al. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J. Natl. Cancer Inst. 93, 1215–1223 (2001).
Laitman, Y. et al. Germline mutations in BRCA1 and BRCA2 genes in ethnically diverse high risk families in Israel. Breast Cancer Res. Treat. 127, 489–495 (2011).
Walsh, T. et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. U.S.A. 108, 18032–18037 (2011).
Kang, E. et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: Recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res. Treat. 151, 157–168 (2015).
Lynce, F. et al. Deleterious BRCA1/2 mutations in an urban population of Black women. Breast Cancer Res. Treat. 153(1), 201–209 (2015).
Bu, R. et al. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int. J. Cancer 139, 1091–1097 (2016).
Plaskocinska, I. et al. New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: Results of the genetic testing in epithelial ovarian cancer (GTEOC) study. J. Med. Genet. 53(10), 655–661 (2016).
Briceño-Balcázar, I. et al. Mutational spectrum in breast cancer associated BRCA1 and BRCA2 genes in Colombia. Colomb. Med. 48(2), 58–63 (2017).
Spurdle, A. B. et al. BRCA1 R1699Q variant displaying ambiguous functional abrogation confers intermediate breast and ovarian cancer risk. J. Med. Genet. 49, 525–532 (2012).
Moghadasi, S. et al. The BRCA1 c.5096G>A p.Arg1699Gln (R1699Q) intermediate risk variant: breast and ovarian cancer risk estimation and recommendations for clinical management from the ENIGMA consortium. J. Med. Genet. 55(1), 15–20 (2018).
Petitalot, A. et al. Combining homologous recombination and phosphopeptide-binding data to predict the impact of BRCA1 BRCT variants on cancer risk. Mol. Cancer Res. 17(1), 54–69 (2019).
Breast Cancer Information Core (BIC). https://research.nhgri.nih.gov/bic/ (Accessed 9 June 2021).
Van der Merwe, N. C. et al. A founder BRCA2 mutation in non-Afrikaner breast cancer patients of the Western Cape of South Africa. Clin. Genet. 81(2), 179–184 (2012).
Chrisanthar, R. et al. CHEK2 mutations affecting kinase activity together with mutations in TP53 indicate a functional pathway associated with resistance to Epirubicin in primary breast cancer. PLoS ONE 3(8), e3062 (2008).
Knappskog, S. et al. Prevalence of the CHEK2 R95* germline mutation. Hered. Cancer Clin. Pract. 14, 19 (2016).
Stolarova, L. et al. CHEK2 germline variants in cancer predisposition: Stalemate rather than checkmate. Cells 9, 2675 (2020).
Kurian, A. W. et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J. Clin. Oncol. 37, 1305–1315 (2019).
Tischkowitz, M. et al. Rare germline mutations in PALB2 and breast cancer risk: A population-based study. Hum. Mutat. 33(4), 674–680 (2012).
Antoniou, A. C. et al. Breast-cancer risk in families with mutations in PALB2. N. Engl. J. Med. 371(6), 497–506 (2014).
Norquist, B. M. et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2(4), 482–490 (2016).
Eliade, M. et al. The transfer of multigene panel testing for hereditary breast and ovarian cancer to healthcare: What are the implications for the management of patients and families? Oncotarget 8(2), 1957–1971 (2017).
Casadei, S. et al. Characterization of splice-altering mutations in inherited predisposition to cancer. Proc. Natl. Acad. Sci. U.S.A. 116(52), 26798–26807 (2019).
Xia, B. et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol. Cell 22, 719–729 (2006).
Oliver, A. W., Swift, S., Lord, C. J., Ashworth, A. & Pearl, L. H. Structural basis for recruitment of BRCA2 by PALB2. EMBO Rep. 10, 990–996 (2009).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Morgan, N. V. et al. A common Fanconi anemia mutation in black populations of sub-Saharan Africa. Blood 105(9), 3542–3544 (2005).
Lee, W.-H. et al. Human retinoblastoma susceptibility gene: Cloning, identification, and sequence. Science 235, 1394–1399 (1987).
Sarasin, A., Munier, P. & Cartault, F. How history and geography may explain the distribution in the Comorian archipelago of a novel mutation in DNA repair-deficient xeroderma pigmentosum patients. Genet. Mol. Biol. 43, e20190046 (2020).
Shen, Y. et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin. Cancer Res. 19, 5003–5015 (2013).
Smith, M. A. et al. Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673. Pediatr. Blood Cancer 62, 91–98 (2015).
Rodrigue, A. et al. A global functional analysis of missense mutations reveals two major hotspots in the PALB2 tumor suppressor. Nucleic Acids Res. 47, 10662–10677 (2019).
Boonen, R. A. C. M., Vreeswijk, M. P. G. & van Attikum, H. Functional characterization of PALB2 variants of uncertain significance: Toward cancer risk and therapy response prediction. Front. Mol. Biosci. 7, 169 (2020).
Johns, M. B. & Paulus-Thomas, J. E. Purification of human genomic DNA from whole blood using sodium perchlorate in place of phenol. Anal. Biochem. 180(2), 276–278 (1989).
Andrews, S. FastQC (2018). https://www.bioinformatics.babraham.ac.uk/projects/fastqc.
Hannon, G. The FastX Toolkit (2018). http://hannonlab.cshl.edu/fastx_toolkit/.
Van der Auwera, G. A. et al. From FastQ data to high confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 43, 1–33 (2013).
Chapman, B. bcbio: Blue Collar Bioinformatics (2018). https://github.com/bcbio/bcbio-nextgen.
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14), 1754–1760 (2009).
McLaren, W. et al. The ensembl variant effect predictor. Genome Biol. 17(1), 122 (2016).
Chun, S. & Fay, J. C. Identification of deleterious mutations within three human genomes. Genome Res. 19, 1553–1561 (2009).
Schwarz, J. M. et al. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 11(4), 361–362 (2014).
Choi, Y. & Chan, A. P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31(16), 2745–2747 (2015).
Kircher, M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46(3), 310–315 (2014).
Shihab, H. A. et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum. Mutat. 34(1), 57–65 (2013).
Karolchik, D. et al. The UCSC table browser data retrieval tool. Nucleic Acids Res. 32, D493–D496 (2004).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17(5), 405–424 (2015).
den Dunnen, J. T. et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum. Mutat. 37, 564–569 (2016).
Acknowledgements
This study was funded by the University of Pretoria, Genomics Research Institute, the Cancer Association of South Africa, the South African Medical Research Council and the South African National Research Foundation (Grants IFR160218158695, BFG14081491267, CPRR13091237847 and the Rated Researcher Incentive Grant).
Author information
Authors and Affiliations
Contributions
F.J. and E.J.v.R. conceived and designed the study. Funding was obtained by F.J. and E.J.v.R. DNA samples were provided by E.J.v.R. F.J. and E.J.v.R. supervised the project. Analyses were performed by D.E., E.J.v.R. and F.J. The manuscript was written by D.E., E.J.v.R. and F.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eygelaar, D., van Rensburg, E.J. & Joubert, F. Germline sequence variants contributing to cancer susceptibility in South African breast cancer patients of African ancestry. Sci Rep 12, 802 (2022). https://doi.org/10.1038/s41598-022-04791-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-04791-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.