Abstract
Calcination treatments in the range of 500–900 °C of TiO2 synthesised by the sol–gel resulted in materials with variable physicochemical (i.e., optical, specific surface area, crystallite size and crystalline phase) and morphological properties. The photocatalytic performance of the prepared materials was evaluated in the oxygen evolution reaction (OER) following UV-LED irradiation of aqueous solutions containing iron ions as sacrificial electron acceptors. The highest activity for water oxidation was obtained with the photocatalyst thermally treated at 700 °C (TiO2-700). Photocatalysts with larger anatase to rutile ratio of the crystalline phases and higher surface density of oxygen vacancies (defects) displayed the best performance in OER. The oxygen defects at the photocatalyst surface have proven to be responsible for the enhanced photoactivity, acting as important active adsorption sites for water oxidation. Seeking technological application, water oxidation was accomplished by immobilising the photocatalyst with the highest OER rate measured under the established batch conditions (TiO2-700). Experiments operating under continuous mode revealed a remarkable efficiency for oxygen production, exceeding 12% of the apparent quantum efficiency (AQE) at 384 nm (UV-LED system) compared to the batch operation mode.
Similar content being viewed by others
Introduction
Oxygen is used in numerous industrial processes, for example, synthesis of chemicals, petroleum processing, glass, ceramic, pulp and paper manufacture. It is mainly used to enhance the reaction rates and to ensure the complete oxidation of possible undesired by-products occurring during some processes1. Unfortunately, the transport and delivery of gaseous molecular oxygen pose several treads to its industrial application. Production in situ is undoubtedly an alternative forwarded by many technological strategies.
Therefore, the demand for oxygen production has encouraged the scientific community to expand the concept of artificial photosynthesis that mimics nature to convert solar energy into value-added products. Since the pioneering work of Fujishima and Honda in 19722, reporting the development of a photoelectrochemical cell for the decomposition of water into hydrogen and oxygen under UV light irradiation using the optical semiconductor titanium dioxide (TiO2), research in this field became an important topic both for environmental decontamination and energy conversion. A variety of optical semiconductors (e.g., TiO2, zinc oxide (ZnO), tungsten trioxide (WO3), graphitic carbon nitride (g-C3N4), bismuth-based materials, black phosphorus, and others) have been employed in several photoactivated applications, including degradation of domestic and industrial sewage, and conversion of natural resources, such as carbon dioxide, nitrogen, and water to value-added chemical and fuels3,4,5,6,7,8,9,10.
Concerning water splitting using semiconductor photocatalysis, the process consists of two half-catalytic redox reactions, i.e., the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), which are described by the following Eqs. (1) and (2), respectively:
The OER is viewed as a bottleneck for the energy conversion due to its slow kinetics11, which involves four proton-coupled electron transfer steps. These elementary stages (i.e., charge carrier generation, separation and migration) occur during the photocatalytic water oxidation, followed by the surface catalytic reaction. Many efforts have been pursued on developing photocatalysts to accelerate the water oxidation, such as junction construction of metal oxides and by metal deposition, aiming to decrease the energy barrier10,12. Despite the significant advances in photocatalysts development neat TiO2 continues to receive particular attention due to the nature of the physical interactions between anatase and rutile crystalline phases, which may result in unique trap states and therefore can have a significant impact on the interparticle carrier transport13.
Although the catalyst nature is of high importance concerning the water splitting systems, the major limitation is its relatively low efficiency in the presence of pure water, requiring the addition of sacrificial agents. Thus, many examples exist of sacrificial molecules as electron donors to improve hydrogen production in solar-driven water splitting systems14,15,16. However, only a limited variety of electron acceptors have been considered concerning OER. The frequently used electron acceptors are silver cations (Ag+), yet some problems arise from their use, i.e., generally, the photocatalytic formation of molecular oxygen is accompanied by the deposition of metallic silver (Ag0) nanoparticles, causing irreversible optical changes on the semiconductor properties. Slightly, ferric ions (Fe3+) have been employed as sacrificial electron acceptors to produce oxygen17,18 owing to its lower redox potential in water (+ 0.77 V; vs. NHE) over Ag+ (+ 0.80 V; vs. NHE), and due to the reversible redox couple Fe3+/Fe2+ in the aqueous phase compared with the Ag+ ions. The influence of Fe3+ ions as electron acceptors was documented by Matsumura group17. In this study, the authors used a TiO2 rutile material for water oxidation and found that the reaction is favoured by superior adsorption of Fe3+ ions on TiO2 over the Fe2+ ions.
Therefore, due to the crucial importance of O2 in various processes this work explores in situ oxygen production by water oxidation based on semiconductor photocatalysis technology. The influence of oxygen defects, optical and crystalline properties of TiO2 samples calcined at different temperatures was investigated for oxygen evolution using a low concentration of Fe3+ aqueous solution rather than the typical electron acceptor (Ag+). The lower price, high abundance and broad availability of iron compared to silver, the use of Fe3+/Fe2+ in low concentrations, makes this a cost-effective and sustainable process. In addition, considering the advantages of using continuous mode systems, a selected TiO2 sample was deposited in a glass support, and the rate of oxygen production was assessed. To the best of our knowledge, this is the first study on photocatalytic water oxidation using immobilised TiO2 towards in situ oxygen production under continuous mode, avoiding the need of recovery the catalyst as an additional step.
Results and discussion
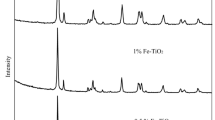
X-ray diffraction (XRD) analysis was performed to determine the crystalline phase and crystallite size of TiO2 samples (Fig. 1, Table 1). The peaks appearing at 25° and 48°, correspond to the lattice planes of 101 and 200 (JCPDS 21-1272) of TiO2 anatase phase. The peaks at 27°, 36° and 54° with lattice planes of 110,101 and 211 (JCPDS 21-1276), respectively reveal the presence of rutile phase. As displayed in Fig. 1 the diffraction peaks of both anatase and rutile phases of TiO2 samples become intense as the calcination temperature increases, suggesting that TiO2 samples are composed of irregular polycrystalline materials, and the crystallinity for both phases is improved by rising the temperatures.
Representative SEM images were obtained to visualise the morphology of the different TiO2 samples (Fig. 2). Results reveal an irregular shape of TiO2 particles with the increase of the calcination temperature. For TiO2-500 and TiO2-600 (Fig. 2a,b), spherical-like TiO2 particles are observed with distinct sizes aggregate into clusters. At temperatures over 700 °C, it seems that small particles coalesce to form larger particles (Fig. 2c), due to the transformation of anatase into rutile phase (Fig. 1). At 900 °C the transformation of anatase–rutile is completed and agglomerations of more a uniform size of TiO2 particles are noticed (Fig. 1e).
The TEM and high-resolution transmission electron microscopy (HRTEM) were used to further investigate the microstructure and composition of representative TiO2 sample (TiO2-700, Fig. 2c). In this case, the particles show a typical size of ca. 100 nm, for its crystallized phase (Fig. 1c-i,c-ii, respectively). In Fig. 2c-iii, the disordered layers on the surface of the TiO2-700 sample are visible and marked with orange dashed lines, suggesting the presence of defects that can provide higher carrier concentration and more active sites19.
HRTEM image revealed well-resolved crystal lattices in a representative TiO2-700 (Fig. 3c-iv), and the inter-planar spacing values of 0.32, 0.35 nm correspond to the (110) and (101) crystal planes of rutile and anatase TiO2, respectively, verifying the co-existence of rutile and anatase in a single nanoparticle, consistent with the XRD results.
Specific surface areas of TiO2 samples were determined by nitrogen adsorption at 77 K (Table 1). As expected, the results show a decrease of SBET with the enhancement of the calcination temperature, agreeing with the SEM observations.
The TiO2 samples were examined by PL spectroscopy at room temperature under an excitation energy of 4.43 eV (280 nm) to investigate the incidence of charge separation upon light excitation (Fig. 3). The intense PL emission in the UV and visible regions is commonly attributed to excitons recombination and the presence of defect sites, respectively20. The samples TiO2-500 (Fig. 3a) and TiO2-600 (Fig. 3b) show a broad peak at 3.0 eV ascribed to the crystallinity of TiO2. By increasing the annealing temperature (samples c, d and e), a noticeable red shift is observed for 2.93 eV, suggesting that the TiO2-700, TiO2-800 and TiO2-900 samples exhibited higher crystallinity, as confirmed by HRTEM observations (Fig. 2). Additionally, these strong peaks (2.93 and 3.00 eV) have been ascribed to the self-trapped excitons in anatase and free excitons in rutile, respectively20. The peaks at 2.63–2.82 eV arise from the excitation electrons/holes recombination via oxygen vacancies and defects in both anatase and rutile phases of TiO2.
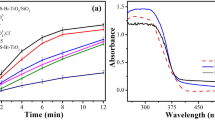
The diffuse reflectance spectra of the TiO2 samples were performed (Fig. 3b) and the respective bandgap values were obtained by indirect Tauc plot analysis (Table 1). As displayed, increasing the calcination temperature the bandgap of TiO2 samples slightly diminish, which may indicate faster electron/hole recombination rates21.
We further have examined the surface chemical states of TiO2 using XPS spectroscopy. Given that XPS allows investigating the surface atomic constituents, it has been used to characterise the degree of oxygen defect according to the relative element contents, and the intensities and positions of peaks22,23. Among the defects identified in TiO2, the presence of oxygen vacancies (Ov) can act as active adsorption sites, and strongly influence the reactivity of the photocatalysts22. Additionally, the formation of Ov commonly leads to the creation of unpaired electrons that can generate donor levels in the electronic structure of the TiO224,25. As observed in Fig. 4 the O 1s XPS spectra of the TiO2 samples were deconvoluted into three main peaks at 528.8 eV, 529.3 eV and 529.9 eV energies. The peak located at 528.8 eV is assigned to Ti–O–Ti bonding and lattice oxygen (OL) within the TiO2 structure24. In contrast, the peaks at 529.3 eV and 529.9 eV energies are associated to surface chemisorbed hydroxyl groups (Oads) and Ov, respectively. As displayed in Table 1, the area proportional to oxygen defects increase with the calcination temperature of TiO2 samples until 700 °C (TiO2-500, TiO2-600, and TiO2-700; 16.8%, 24.5%, and 31.7% respectively). For highest calcination temperatures (TiO2-800, TiO2-900; 30.7% and 26.1%, respectively) the oxygen defects start to decrease, suggesting that surface Ov concentration gradually diminishes with temperatures above 700 °C, being consistent with the literature24,26.
Oxygen evolution reactions
Photocatalytic oxygen evolution was evaluated by TiO2 samples calcined at various temperatures under UV-LED radiation using an aqueous Fe(NO3)3·9H2O solution acting as sacrificial electron acceptor. Although the mechanism of O2 evolution using silver cations (Ag+) as electron acceptors is well known in the literature15,27 the photocatalytic generation of molecular oxygen is followed by the deposition of metallic silver nanoparticles on the surface of the photocatalyst. Irreversible reactions accompany this phenomenon due to the plasmonic adsorption band of the silver particles15. Due to this issue and the high documented studies using Ag+ ions as sacrificial agent in photocatalytic water oxidation preliminary experiments were performed under the same operational conditions using this electron acceptor for comparison purposes (Supplementary Fig. S1). The results revealed a slight increasing efficiency for O2 production in the first 30 min using Ag+ ions solution compared with the Fe3+. However, with the reaction time a progressive reduction of Ag+ into Ag0 and the oxygen evolution rates decreases.
Concerning the blank experiments (i.e., dark conditions, absence of catalyst and sacrificial agent), using a Fe3+ aqueous solution no formation of O2 was noticed. Contrary, photocatalytic water oxidation reactions revealed that all the tested samples were found to generate O2 upon UV-LED irradiation, although the efficiencies were dependent on the calcination temperature of TiO2 samples (Fig. 5). In general, the results show that when the calcination temperature is raised, the efficiency of the photocatalytic process is enhanced. Nevertheless, after a specific temperature (800 °C), a decrease in O2 evolution was observed. Among the photocatalysts, the TiO2-700 sample showed the best performance for water oxidation with 8.95 µmol min−1 gcat−1 of dissolved oxygen being detected.
The calcination treatment commonly affects the physicochemical properties of optical semiconductors, such as crystalline phase and crystallite size, specific surface area, and oxygen surface defects. Therefore, it is commonly accepted that a higher specific surface area (SBET) promotes a significant number of active adsorption sites and improves reactivity. Yet, from the results it is unlikely that the efficiency of the TiO2 samples is related to their specific surface area once was found a decrease in the SBET with the rise of the calcination temperature. This diminution on the SBET was more noticeable for the samples calcined at 800 and 900 °C due to the enhancement of the TiO2 particles size as observed by SEM micrographs (Fig. 2). Additionally, the presence of higher percentage of rutile phase in these samples may indicate faster electron/hole recombination, which may explain the decrease in the efficiency of the water oxidation process24.
Although in terms of oxygen evolution, effect of the particle size and the crystallite phases of the photocatalysts is not clearly understood, some reports suggest that these factors may contribute to the efficiency of photocatalytic water oxidation. Hiroshi et al.28, ascribed the performance of the water oxidation photocatalytic process to the crystallization from anatase to rutile phase. More recently, Maeda et al.29 have attributed the high efficiency of TiO2 catalysts for oxygen evolution to the enhancement of the particle size which seems to hinder the electron/hole recombination. Higher efficiencies for O2 production have been assigned to the TiO2 rutile phase unlike what happens in the degradation of organic compounds using TiO2 anatase30,31. To understand the efficiency of the developed TiO2 samples in terms of crystallization, experiments using commercial 100% TiO2 anatase and 100% rutile were tested under the same experimental conditions (Fig. 5; A and R, respectively). As shown, high efficiency was found with the commercial TiO2 rutile. Nevertheless, for the synthesised TiO2 calcined at 900 °C 100% rutile (Table 1) the performance for O2 production was lower compared with the TiO2-700 and also with TiO2-800 samples, suggesting that the other factors account to the efficiency of the process using the TiO2 samples prepared by sol–gel method. As documented, the ratio between surface anatase and rutile particles increases the photocatalytic activity in water splitting reactions32,33. As displayed in Fig. 6, the performance of the photocatalytic water oxidation for O2 evolution was achieved when the ratio between anatase and rutile crystalline phases are superior (88%, Table 1), corroborating with the reported investigations33,34.
It is recognised that lattice defects such as oxygen vacancies (Ov) can provide intermediate band levels or additional states into the forbidden band of the semiconductor that may trap electrons, which can increase the light absorption capacity and charge separation29,35. Moreover, the high density of Ov (electron donors) is thought to shift the Fermi level of photocatalysts such as TiO2 near the conduction band, enabling the charge separation at the TiO2 particle interface, thus enhancing the degree of band bending at the semiconductor surface. Although the thermal treatments are known to increase the density of Ov, above a certain temperature, the number of Ov is reduced19,24,29. Amano et al.33 investigated the photocatalytic activity of a TiO2 commercial sample treated at 700 °C under H2 flow. The authors ascribed the photocatalytic performance to an increase in the density of the oxygen vacancies at the photocatalyst surface, which enhanced the electrons availability in the conduction band.
Regarding the characterisation results, it can be inferred that the high reactivity of the TiO2-700 sample for water oxidation is due to the higher percentage of oxygen vacancies at the catalyst surface as was found by XPS analysis. This higher density of oxygen vacancies may facilitate the charge carrier diffusion in TiO2, improving the photocatalytic activity for O2 production. Although, the percentage of oxygen vacancies in the TiO2-800 sample (30.7%) is negligible compared with the TiO2-700 sample (31.7%), a decrease in the rutile particle size was observed (Table 1). The same behaviour was detected for TiO2-900, i.e., smaller rutile particle size than the TiO2-700, suggesting that excess thermal treatment is being used to decrease the oxygen vacancies, leading to the photocatalytic decreasing performance, being reliable with literature26. Additionally, tries to obtain electrochemical evidence of the oxidation potential of the photocatalysts were performed by supporting the TiO2 samples on conductive ITO electrode. The TiO2-700 and TiO2-800 samples (Supplementary Fig. S2) revealed an oxidation peak between 0.5 and 0.7 V vs SCE, suggesting that positive holes can be generated in the photocatalysis process, compatible with the observed results for photocatalytic oxygen production36.
As described above, a significant number of investigations have been devoted to understanding the photocatalytic water oxidation using Ag+ cations as sacrificial electron acceptors, yet, using Fe3+ aqueous solutions few reports have been documented. Concerning the results obtained with the TiO2 samples using the Fe3+ ion solutions it seems reasonable to propose that the overall process is governed by the mechanism illustrated in Scheme 1, and by the following equations:
Additionally, as the O2 is being accumulated in the reaction system the oxidation of Fe2+ into Fe3+ may also occur (Eq. (8)). Although in practice the oxidation in acid media is hard, at a moderate pH the iron(III) oxide hydrate (Fe2O3) can precipitate, and a reduction reaction of Fe3+ to Fe2+ can take place quickly (Eq. (9)). As a result, hydrogen peroxide (HOOH) could be formed, occurring again the oxidation of Fe2+ into Fe3+ (Eq. (10)). Nevertheless, this last hypothesis seems to be challenging to occurs, as the maximum pH measured during the reactions was ca. 2.28.
Although the set of Eqs. (3)–(7) seems to describe the results obtained with the TiO2 samples, a maximum amount of oxygen was evaluated after 60 min in the reaction conditions; after that, the reaction system achieved the steady state (Supplementary Fig. S3). This can be ascribed to the reaction system configuration used, i.e., as the process performance was evaluated in terms of in situ O2 generation with the product remaining in reaction, which may indicate the O2 solubility in the aqueous media was reached.
Therefore, considering the above results, a potential technological application for oxygen production was assessed using the TiO2-700 immobilised in a glass slide and tested under continuous mode (Supplementary Scheme S1b). Using this reactor configuration we can overcome the practical problems that arise from using a catalyst in powder form and ensure continuous feed of sacrificial electron acceptors. The apparent quantum efficiency (AQE) was examined for both reaction systems by the following equation:
where n is the amount of O2 molecules, NA the Avogadro constant, h the Plank constant, c the speed of light in vacuum (m/s), A the irradiated area (78.5 and 19.8 cm2, batch, and continuous systems, respectively), I the exposed irradiation intensity in each system, t the time of the reactions (s), and λ the wavelength of the monochromatic LED (384 nm).
As displayed in Fig. 7 a, after 60 min of reaction the concentration of O2 is lower operating in continuous mode (5.46 µmol min−1 gcat−1) comparing with slurry mode (8.95 µmol min−1 gcat−1). Nevertheless, it is important to refer that a larger amount of catalyst per volume is used in the slurry system (0.120 g) in contrast with the continuous mode (0.013 g). Nevertheless, the results reveal that the immobilisation of TiO2-700 as film enables a remarkable efficiency for O2 production, exhibiting AQE exceeding 12% (Fig. 7b) at 384 nm compared with the slurry mode. This configuration system may constitute a step forward for developing efficient and viable systems capable of producing O2 in continuous-flow reactors, reducing the operating costs and allowing large-scale production.
Conclusions
TiO2 photocatalysts were synthesised by the sol–gel method and successfully used for water oxidation. High photocatalytic performance for oxygen evolution was achieved using iron nitride aqueous solution as sacrificial electron acceptors. Among the photocatalyst studied, the TiO2-700 material revealed the highest efficiency with an in situ oxygen production rate of 8.95 µmol min−1 gcat−1 being determined. The results were correlated with the physicochemical and morphological properties of the developed TiO2 photocatalysts. The specific surface area of the resulting photocatalysts was found negligible for O2 production, as well as the bandgap of the materials. The remarkable efficiency of the TiO2-700 for oxygen evolution was correlated with the highest ratio between anatase and rutile phases (88%), which seems to indicate an efficient separation of the photogenerated electron/hole carriers. In addition, the superior reactivity of the TiO2-700 sample was ascribed to the enhancing of the oxygen vacancies at the photocatalyst surface, which act as active sites for water oxidation.
Finally, the TiO2-700 was successfully immobilised over a glass support for a continuous production of oxygen. The continuous-flow reaction system demonstrated promising results for water oxidation revealing a superior AQE at 384 nm compared with the results obtained in the batch system, 0.60 and 0.53, respectively.
Materials and methods
TiO2 rutile (99%) was purchased from Iolitec. Iron(III) nitrate nonahydrate (≥ 98%; Fe(NO3)3·9H2O), titanium butoxide (97%; Ti(OCH2CH2CH2CH3)4), TiO2 anatase (> 99%) and citric acid (≥ 99.5%; HOC(COOH)(CH2COOH)2) were supplied from Sigma-Aldrich. Absolute Ethanol (CH3CH2OH) was purchase from VWR. Ultrapure water was obtained from a Milli-Q water system (resistivity of 18.2 MΩ cm, at 25 °C).
Catalyst preparation and characterisation
Titanium dioxide samples were prepared from the sol–gel method: a solution with ethanol:titanium butoxide (50:3.5; molar ratio) was added drop-by-drop to a solution containing water:ethanol:citric acid (50:60:0.4; molar ratio) under continuous stirring. The final solution was kept mixed for 30 min followed by ageing in the air for several days, then the resulting xerogel was ground and dried at room temperature. The powders were calcined under the air atmosphere. Briefly, 1 g of powder was placed in a quartz crucible in a Microwave Phoenix apparatus (CEM Corporation) under static air conditions for 90 min at a given temperature. The final catalysts were designed as TiO2-T, where T corresponds to the calcination temperature (between 500 and 900 °C).
Uncoated glass supports (7.6 × 2.6 cm) were extensively cleaned under sonication with an anionic detergent in water, followed by the immersion in 2-propanol for 15 min under sonication, and finally dried. A paste of a selected TiO2-T sample was prepared as described in our previous work37. The resulting photocatalyst paste was spread on the washed glass slides using the casting knife applicator (Elcometer 3580, Warren, MI). Then, the TiO2-coated slides were calcined at 500 °C in static air for 3 h. The amount of catalyst immobilised was found from the difference between uncoated and coated slides after the calcination treatment.
X-ray diffraction (XRD) measurements were conducted in a PANalytical X’Pert MPD equipped with an X’Celerator detector and secondary monochromator (Cu Kα λ = 0.154 nm, 40 kV,30 mA).
X-ray photoelectron spectroscopy (XPS) characterisation was carried out on an ESCALAB 250 instrument with Al Kα X-rays (1486.6 eV).
The morphology of the photocatalysts was examined by scanning electron microscopy (SEM) using a FEI Quanta 400 FEG ESEM/EDAX Genesis X4M (15 keV) microscope. High-resolution transmission electron microscopy (HRTEM) was conducted on a probe-corrected transmission electron microscope operating at 200 kV (FEI Themis 60–300).
The N2 adsorption–desorption isotherms at −196 °C were acquired in a Quantachrome NOVA 4200e device. The Brunauer–Emmett–Teller equation was used to obtain the specific surface area (SBET) from the N2 adsorption data in the relative pressure range 0.05–0.20 (Supplementary Fig. S4).
Diffuse Reflectance UV–Visible (DRUV-vis) spectra were obtained within the 220–800 nm wavelength range using a JASCO V-560 spectrophotometer equipped with an integrating sphere at room temperature. Photoluminescence (PL) analyses of the powder photocatalysts were attained at room temperature by a JASCO (FP 82000) fluorescence spectrometer with a 150 W Xenon lamp as a light source, using bandwidths of 10 nm for emission and excitation.
Photocatalytic water oxidation
Water oxidation experiments were conducted in a slurry glass-immersion reactor filled with 120 mL of 5 mM Fe(NO3)3·9H2O solution acting as a sacrificial agent. Preliminary experiments were performed varying the initial concentration of the Fe(NO3)3·9H2O solution between 1.0 and 50.0 mM (Supplementary Fig. S5).The photocatalytic system was maintained at ca. 20 ℃ using a cooling water system. In a typical experiment, TiO2 powders were dispersed in the Fe(NO3)3·9H2O solution and the resulting suspension (1 g L−1) was completely deaerated by saturation with argon (50 mL min−1) for 30 min. Then, the reactor headspace was maintained under the argon atmosphere (10 mL min−1) to hinder oxygen gas–liquid transfer through the interface. The oxygen concentration was measured by an O2-sensor FOXY probe (Ocean Optics NeoFOX) dipped in the suspensions. The photocatalytic reactor was exposed to a four LEDs system with a maximum wavelength of 384 nm (Supplementary Scheme S1a). The intensity of each LED varied between 120 and 127 W m−2, which was measured at 3 cm from the reactor wall by a UV–Vis spectroradiometer apparatus (USB2000+, OceanOptics, USA).
Additional tests under continuous mode were performed using an acrylic reactor system with a maximum volume of 40 mL constituted by a chamber containing the immobilised TiO2-700 film (0.013 g). The reactor was sealed and purged with an argon flow (10 mL min−1) for ca. 2 h. The irradiation device, constituted by one LED source (λmax = 384 nm; 127 W m−2), was used at 3 cm from the immobilised catalyst (Supplementary Scheme S1b). The reactor system was continuously fed with 5 mM of Fe(NO3)3·9H2O solution previously saturated with argon. The residence time was kept at 20 min, taking into account the results obtained in batch mode. The accumulated dissolved oxygen was measured using an O2-sensor FOXY probe.
Blank experiments were performed in all experiments, namely in the absence of catalysts, without adding sacrificial agents under illumination and dark conditions. All the reactions were carried out in triplicate.
References
Thiemens, M. H. Oxygen origins. Nat. Chem. 4, 66–66 (2012).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Tang, J., Durrant, J. R. & Klug, D. R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 130, 13885–13891 (2008).
Chen, X., Li, N., Kong, Z., Ong, W.-J. & Zhao, X. Photocatalytic fixation of nitrogen to ammonia: State-of-the-art advancements and future prospects. Mater. Horiz. 5, 9–27 (2018).
Kegel, J., Povey, I. M. & Pemble, M. E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 54, 409–428 (2018).
Liu, H. et al. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater. 30, 1800295 (2018).
Ma, H. et al. Where do photogenerated holes at the g-C3N4/water interface go for water splitting: H2O or OH−? Nanoscale 10, 15624–15631 (2018).
Moreira, N. F. F. et al. Metal-free g-C3N4 photocatalysis of organic micropollutants in urban wastewater under visible light. Appl. Catal. B 248, 184–192 (2019).
Gao, Y. et al. Recent advances in visible-light-driven conversion of CO2 by photocatalysts into fuels or value-added chemicals. Carbon Res. Conv. 3, 46–59 (2020).
Lin, S., Huang, H., Ma, T. & Zhang, Y. Photocatalytic oxygen evolution from water splitting. Adv. Sci. 8, 2002458 (2021).
Curcio, A. et al. Unlocking the potential of mechanochemical coupling: Boosting the oxygen evolution reaction by mating proton acceptors with electron donors. Adv. Funct. Mater. 31, 2008077 (2021).
Li, D. et al. Unraveling the kinetics of photocatalytic water oxidation on WO3. J. Phys. Chem. Lett. 11, 412–418 (2020).
Mi, Y. & Weng, Y. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 5, 11482 (2015).
Silva, C. G. et al. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B 178, 82–90 (2015).
Schneider, J. & Bahnemann, D. W. Undesired role of sacrificial reagents in photocatalysis. J. Phys. Chem. Lett. 4, 3479–3483 (2013).
Silva, C. G., Juárez, R., Marino, T., Molinari, R. & García, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 133, 595–602 (2011).
Ohno, T., Haga, D., Fujihara, K., Kaizaki, K. & Matsumura, M. Unique effects of iron(III) ions on photocatalytic and photoelectrochemical properties of titanium dioxide. J. Phys. Chem. B 101, 6415–6419 (1997).
Ohmori, T., Takahashi, H., Mametsuka, H. & Suzuki, E. Photocatalytic oxygen evolution on α-Fe2O3 films using Fe3+ ion as a sacrificial oxidizing agent. Phys. Chem. Chem. Phys. 2, 3519–3522 (2000).
Pham, H. H. & Wang, L.-W. Oxygen vacancy and hole conduction in amorphous TiO2. Phys. Chem. Chem. Phys. 17, 541–550 (2015).
Mathew, S. et al. UV-visible photoluminescence of TiO2 nanoparticles prepared by hydrothermal method. J. Fluoresc. 22, 1563–1569 (2012).
Kim, M. G. et al. Effects of calcination temperature on the phase composition, photocatalytic degradation, and virucidal activities of TiO2 nanoparticles. ACS Omega 6, 10668–10678 (2021).
Hao, L., Huang, H., Zhang, Y. & Ma, T. Oxygen vacant semiconductor photocatalysts. Adv. Funct. Mater. 31, 2100919 (2021).
Huang, X. et al. Impact of oxygen vacancies on TiO2 charge carrier transfer for photoelectrochemical water splitting. Dalton Trans. 49, 2184–2189 (2020).
Pan, X., Yang, M.-Q., Fu, X., Zhang, N. & Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 5, 3601–3614 (2013).
Zhang, Y. et al. Direct observation of oxygen vacancy self-healing on TiO2 photocatalysts for solar water splitting. Angew. Chem. Int. Ed. 58, 14229–14233 (2019).
Nishimoto, Y., Hasegawa, Y., Adachi, K. & Yamazaki, S. Factors affecting oxygen evolution through water oxidation on polycrystalline titanium dioxide. RSC Adv. 6, 46994–47000 (2016).
Jeon, T. H. et al. Ag(I) ions working as a hole-transfer mediator in photoelectrocatalytic water oxidation on WO3 film. Nat. Commun. 11, 967 (2020).
Kominami, H., Murakami, S.-Y., Kato, J.-I., Kera, Y. & Ohtani, B. Correlation between some physical properties of titanium dioxide particles and their photocatalytic activity for some probe reactions in aqueous systems. J. Phys. Chem. B 106, 10501–10507 (2002).
Maeda, K. Effects of the physicochemical properties of rutile titania powder on photocatalytic water oxidation. ACS Catal. 4, 1632–1636 (2014).
Sun, Q. & Xu, Y. Evaluating intrinsic photocatalytic activities of anatase and rutile TiO2 for organic degradation in water. J. Phys. Chem. C 114, 18911–18918 (2010).
Luttrell, T. et al. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 4, 4043 (2014).
Zhang, J., Xu, Q., Feng, Z., Li, M. & Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 47, 1766–1769 (2008).
Amano, F., Nakata, M. & Ishinaga, E. Photocatalytic activity of rutile titania for hydrogen evolution. Chem. Lett. 43, 509–511 (2014).
Li, Y.-F. & Selloni, A. Pathway of photocatalytic oxygen evolution on aqueous TiO2 anatase and insights into the different activities of anatase and rutile. ACS Catal. 6, 4769–4774 (2016).
Li, Y. et al. Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures. Nat. Commun. 10, 4421 (2019).
Han, F., Xu, J., Zhou, J., Tang, J. & Tang, W. Oxygen vacancy-engineered Fe2O3 nanoarrays as free-standing electrodes for flexible asymmetric supercapacitors. Nanoscale 11, 12477–12483 (2019).
Sampaio, M. J., Silva, C. G., Marques, R. R. N., Silva, A. M. T. & Faria, J. L. Carbon nanotube–TiO2 thin films for photocatalytic applications. Catal. Today 161, 91–96 (2011).
Acknowledgements
This work was financially supported by Project POCI-01-0145-FEDER-030674 (MicroPhotOGen, PTDC/NAN-MAT/30674/2017) funded by ERDF through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI)—and by national funds through FCT—Fundação para a Ciência e a Tecnologia. We would like to thank the scientific collaboration financially supported by: Base-UIDB/50020/2020 and Programmatic-UIDP/50020/2020 Funding of Associate Laboratory LSRE-LCM—funded by national funds through FCT/MCTES (PIDDAC), and when appropriate co-financed by FEDER under PT2020 Partnership Agreement: CQVR: UIDB/QUI/00616/2020. We are also indebted to MSc Rui Rocha and the CEMUP team (Portugal) for technical assistance and advice with SEM measurements.
Author information
Authors and Affiliations
Contributions
M.J.S.: Conceptualization, Investigation, Funding acquisition, Methodology, Project administration, Supervision, Writing—review & editing original manuscript. Z.Y.: Formal analysis, Resources, Review & Editing. J.C.L.: Investigation, Formal analysis, Resources. P.B.T.: Formal analysis, Resources. C.G.S.: Methodology, Review & editing. L.L.: Formal analysis, Resources, Review & editing. J.L.F.: Conceptualization, Review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sampaio, M.J., Yu, Z., Lopes, J.C. et al. Light-driven oxygen evolution from water oxidation with immobilised TiO2 engineered for high performance. Sci Rep 11, 21306 (2021). https://doi.org/10.1038/s41598-021-99841-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99841-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.