Abstract
Poly(ethylene terephthalate) (PET) is a widely used plastic in bottles and fibers; its waste products pollute the environment owing to its remarkable durability. Recently, Ideonella sakaiensis 201-F6 was isolated as a unique bacterium that can degrade and assimilate PET, thus paving the way for the bioremediation and bioconversion of PET waste. We found that this strain harbors a poly(hydroxyalkanoate) (PHA) synthesis gene cluster, which is highly homologous with that of Cupriavidus necator, an efficient PHA producer. Cells grown on PET accumulated intracellular PHA at high levels. Collectively, our findings in this study demonstrate that I. sakaiensis can mediate the direct conversion of non-biodegradable PET into environment-friendly plastic, providing a new approach for PET recycling.
Similar content being viewed by others
Introduction
Petroleum-based plastics are used extensively worldwide. However, owing to their inertness, plastics entering the environment are tough to degrade, and their accumulation has serious negative impacts1. Recent reports on microbial degradation of plastics including polyethylene2, polystyrene3, and poly(ethylene terephthalate) (PET)4 highlight these problems, but the number of such reports is limited. Particularly, mechanisms underlying PET biodegradation are best known by analyzing Ideonella sakaiensis strain 201-F64. This bacterium uses two unique enzymes, PET hydrolase (PETase) and mono(2-hydroxyethyl) terephthalic acid (MHET) hydrolase (MHETase), to hydrolyze PET into terephthalic acid (TPA) and ethylene glycol (EG) monomers, which are then assimilated. Biodegradable plastics can be developed as more environmentally compatible and sustainable alternatives to common plastics. Poly(hydroxyalkanoate) (PHA), a polyester synthesized by various microorganisms for intracellular carbon and energy storage, is a promising biodegradable plastic, given its excellent biodegradability5. A representative PHA-producing strain, Cupriavidus necator H16 can produce and accumulate massive quantities of the PHA poly(3-hydroxybutyrate) (PHB), of up to ≈80 w/w% of dry cell weight (DCW, ≈4 g/L culture), using plant oils as the sole carbon source for growth6. A critical challenge in microbial fermentation-mediated biodegradable plastic production is lowering the carbon source cost, as it influences the product price5. PET is cheap and abundant but deleterious to the environment; thus, the digestion of PET is expected to be a useful carbon source for PHA production. Kenny et al.7 isolated Pseudomonas species, including the GO16 strain, which can accumulate PHAs with monomers comprising more than six carbons (medium chain-length PHAs) from TPA. These strains, when cultured using TPA derived from PET pyrolysis products, accumulated PHA at ≈25 w/w% of DCW (≈1 g/L culture), thereby achieving a PHA production of 0.25 g/L. Tiso et al.8 modified the GO16 strain for EG and TPA assimilation. They cultured the strain with TPA and EG, which were obtained by complete PET hydrolysis using a thermostable polyester hydrolase, to obtain PHAs at 7 w/w% of DCW (2.3 g/L culture) with a PHA production of 0.15 g/L. Here, we investigated the use of I. sakaiensis for directly converting PET into PHA, which does not require prior PET hydrolysis.
Results and discussion
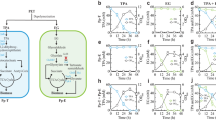
We found a gene cluster on the Ideonella sakaiensis 201-F6 genome essential for converting acetyl coenzyme A (acetyl-CoA) into PHAs (Fig. 1). The cluster comprises phaC, phaA, and phaB encoding PHA synthase, β-ketothiolase, and acetoacetyl-CoA reductase, respectively. The amino acid sequences of these proteins are highly similar to those of the proteins produced by C. necator, indicating that this bacterium is a potential PHB producer. I. sakaiensis was cultured on PET granules in a minimal medium (yeast extract-sodium carbonate-vitamins-oyster shell [YSVO] medium) for 3, 6, and 9 days, and stained with Nile red, a lipophilic fluorescent dye. Microscopy revealed that fluorescence intensity in the intracellular regions increased over time with the highest abundance and intensity on day 6 (Fig. 2a,c). This was likely to have affected the bacterial cell area, which increased 1.3-fold between days 3 and 6 (Fig. 2a,b). Transmission electron microscopy (TEM) of the 6-day-cultured cells showed multiple large granules in the cytoplasm (Fig. 2d), which were consistent with those observed using fluorescence microscopy (Fig. 2a). The chloroform-soluble fraction was extracted from the lyophilized I. sakaiensis cells cultured on PET and analyzed via proton nuclear magnetic resonance (1H NMR) spectroscopy and size exclusion chromatography (SEC) to determine the chemical structure and molecular weight, respectively. The 1H NMR spectrum showed typical signals of poly(3-hydroxybutyrate) (PHB), as noted previously9 (Fig. 3a). SEC revealed a number-average-molecular weight (Mn) of 3.2 × 105 and a weight-average-molecular weight (Mw) of 8.0 × 105 (Fig. 3b), which are similar to that of PHB produced by C. necator (Mn, 4.0 × 105; Mw, 1.0 × 106)6. To quantify the PHA content, I. sakaiensis was cultured in PET granules-added YSVO medium while monitoring the DCW. We have previously shown that I. sakaiensis degrades PET films, exhibited by a significant reduction in their weight4 and that it causes an increase in the proportion of the functional (hydroxyl and carboxyl) groups through surface hydrolysis10. However, the amount of PET hydrolysates in the culture supernatant was small (0.003% of MHET units of the degraded PET), indicating their rapid uptake into the cell. Therefore, we used the weight loss of PET as another measure of the degradation. I. sakaiensis exhibited minimal growth on the culture medium without PET, with the highest DCW of 0.040 g/L, but much higher growth on the medium with PET with DCW of up to 1.6 ± 0.1 g/L, concurrent with a PET weight loss of 7.6 ± 0.6 g/L (Fig. 4a), indicating that PET was a major carbon source for its growth; oyster shell, consisting mostly of calcium carbonate and various trace amounts of minerals11, has little to no factors that contribute to cell growth. The PHB content was quantified by performing gas chromatography (GC) of the lyophilized cells after methanolysis. Methyl 3-hydroxybutyrate (3HB) (~ 100 mol%) along with trace amounts of methyl 3-hydroxyvalerate (3HV) were detected among the potential methylated-PHA monomers (Table 1), indicating that the synthesized PHA was nearly a PHB homopolymer. The highest amount of accumulated PHA was observed on day 6, accounting for 48 ± 5 w/w% of the DCW (Fig. 4b, Table 1). The highest PHA production rate was 0.75 ± 0.09 g/L on day 6. Subsequently, the carbon yield of PHA from PET on day 6 was calculated as 9.0 ± 1.5 % (Table 1), indicating that a majority of the carbon in PET was converted into other compounds including carbon dioxide through respiration. We further examined cultivation of I. sakaiensis in YSVO medium supplemented with the PET monomer(s); 0.5 w/v% disodium terephthalate (TPA-2Na), 0.5 w/v% EG, or a mixture of both. The DCWs were significantly higher when compared to the culture without carbon source: 0.21 ± 0.03 g/L on TPA-2Na, 0.15 ± 0.02 g/L on EG, and 0.33 ± 0.02 g/L on TPA-2Na/EG for 2 days, and small amounts of PHA (< 5.0 wt%) were detected (Fig. 5 and Table 1). These facts indicated that I. sakaiensis was able to incorporate extracellular TPA and EG and then metabolized them to acetyl-CoA and other intermediates. However, considering much high cell growth and PHA accumulation on PET, the cellular ability to assimilate the monomer(s) appeared to be poor. One of explanations for the observations would be better preference of the uptake system of I. sakaiensis to PET hydrolytic oligomer products rather than TPA and EG.
Conservation of poly(hydroxyalkanoate) (PHA) synthesis gene clusters on the genomes of I. sakaiensis 201-F6 (GenBank Accession no. NZ_BBYR01000000) and C. necator H16 (NZ_CP039287). Open reading frame numbers without locus tag prefixes (ISF6 for I. sakaiensis; H16 for C. necator) are indicated in the genes. Gene products and amino acid sequence identities are indicated between the genes.
Microscopy of I. sakaiensis grown on poly(ethylene terephthalate) (PET). (a) Bright field and fluorescence microscopy of Nile red-stained I. sakaiensis cells after growth on PET for 0 (pre-cultured cells in 802 nutrient-rich medium for 1 day), 3, 6, and 9 days. Box-and-whisker plots show the distribution of cell areas (b) and average fluorescence intensity of Nile red in cells (c); median values, 25th to 75th percentiles, and range are shown. n, number of cells or fluorescent dot assemblies. ND, not detected. Different letters above the plots indicate significant differences among groups (Tukey's multiple comparisons test, P < 0.0001). (d) Transmission electron microscopy image of cells grown on PET granules for 6 days. Scale bar, 600 nm.
Analyses of chloroform-soluble fraction of I. sakaiensis cells grown on poly(ethylene terephthalate) (PET) granules for 6 days. (a) Proton nuclear magnetic resonance (1H NMR) spectrum. Inlet shows the chemical structure of poly(3-hydroxybutyrate) (PHB). (b) Size exclusion chromatography (SEC). Mn, number average molecular weight; Mw, weight average molecular weight; PDI, polydispersity index. Molecular weight values were calculated by comparison with poly(methylmethacrylate) (PMMA) calibration standards.
Time course of poly(hydroxyalkanoate) (PHA) production by I. sakaiensis grown on poly(ethylene terephthalate) (PET). (a) Growth of I. sakaiensis on PET. The cells were cultured with and without PET granules (10 g) in YSVO medium for 3, 6, and 9 days at 30 °C. Dry cell weight (DCW; blue closed squares, with PET; blue open squares, without PET) and weight loss of PET granules (red closed triangles) were measured. (b) Time course of PHA production. PHA production with and without PET shown as blue closed squares and open squares, respectively. PHA content per DCW shown as red closed circles. Data are also shown in Table 1. Error bars in represent the standard error among three independent biological replicates.
Growth of I. sakaiensis on poly(ethylene terephthalate) (PET) monomers. The cells were cultured with or without 0.5 w/v% disodium terephthalate (TPA-2Na), 0.5 w/v% ethylene glycol (EG), and a mixture of the two compounds (TPA-2Na/EG) in YSVO medium at 30 °C, and then their dry cell weight was measured. Error bars in represent the standard error among three independent biological replicates.
In conclusion, we demonstrated that I. sakaiensis could not only metabolize PET, but also produce PHA, and that these two pathways were functionally linked. This information can contribute to the development of new PHA production pathways, which can help reduce plastic pollution and increase the production of inexpensive biodegradable plastics and PET recycling. Compared with previous studies on PHA production from the digestion of PET by Pseudomonas GO167 and the modified strain8, I. sakaiensis produced 3.0- and 5.0-fold more PHA, respectively, from PET. However, scaling up the production levels to an industrial scale remains challenging. The metabolic and fermentation engineering strategies previously applied for C. necator, which enable the production of PHA copolymer (> 100 g/L) by high-density fed-batch cultivation under nitrogen limitation12, offers a potentially useful approach.
Methods
Culture
Ideonella sakaiensis was cultured as described previously4,13 with several modifications. Briefly, I. sakaiensis was pre-cultured in NITE Biological Resource Center (NBRC) 802 medium (1.0 w/v% polypeptone, 0.2 w/v% yeast extract, and 0.1 w/v% MgSO4) at 30 °C and harvested by centrifugation (5000×g, 5 min, 4 °C). The cell pellet was resuspended in YSV medium (0.01 w/v% yeast extract, 0.02 w/v% sodium hydrogen carbonate, 0.1 w/v% ammonium sulfate, 0.01 w/v% calcium carbonate, 0.1 v/v% vitamin mixture [0.25 w/v% thiamine-HCl, 0.005 w/v% biotin, and 0.05 w/v% vitamin B12], and 1 v/v% trace elements [0.1 w/v% FeSO4·7H2O, 0.1 w/v% MgSO4·7H2O, 0.01 w/v% CuSO4·5H2O, 0.01 w/v% MnSO4·5H2O, and 0.01 w/v% ZnSO4·7H2O] in 10 mM phosphate buffer; pH 7.4). The suspension was inoculated into a Petri dish (90 mm in diameter) containing 30 mL YSV medium supplemented with ≈ 300 mg of oyster shells as a pH adjusting agent (YSVO medium) in the presence or absence of 10 g (≈ 560 pieces) of PET granules shaped like an elliptic cylinder [2 (minor axis) × 3 (major axis) × 3 (height) mm]; 5.8% crystallinity determined by differential scanning calorimetry (Bell Polyester Products, Inc., Yamaguchi, Japan), 0.5 w/v% of disodium terephthalate (TPA-2Na), EG, or a mixture of 0.5 w/v% TPA-2Na and 0.5 w/v% EG as the carbon source to adjust the absorbance measured at 660 nm to 0.002. The culture dish was placed in a reciprocal shaker set at 50 strokes/min at 30 °C. After filtering the culture fluid through a 5 µm pore size poly-vinylidene difluoride filter (Merck Millipore, Billerica, MA) to remove the small broken pieces of oyster shells, cells were harvested, lyophilized using an FZ-2.5 freeze-dry system (Labconco, Kansas City, MO), and weighed. As a portion of the cells was trapped by the filter, the DCW was corrected using the amounts of protein before and after filtration. Briefly, cells were mixed with an equal volume of 10 w/v% sodium dodecyl sulfate (SDS) and heated at 90 °C for 10 min to lyse the cells and solubilize the cellular protein, and the protein concentration was determined with a DC protein assay kit (BioRad, Hercules, CA) with comparison to a calibration curve using bovine serum albumin. The PET granules were washed with 1 w/v% SDS, distilled water, and then ethanol. After drying in air and a desiccator, the granules were weighed to determine the weight reduction.
Fluorescence microscopy
PHA that accumulated in the cells was stained with Nile red (Fujifilm Wako Pure Chemical, Osaka, Japan)14. A glass slide was coated with 0.01 w/v% poly-l-lysine (Sigma-Aldrich, St. Lois, MO) for cell attachment. The culture suspension (100 µL) was placed on the poly-l-lysine-coated glass slide for 10 min and then aspirated. The cells attached to the glass slide were incubated with a drop of 10 µg/mL Nile red in D-PBS(−) (Nacalai Tesque, Kyoto, Japan) for 30 min, washed with D-PBS(−), mounted with Prolong Diamond antifade medium (Thermo Fisher Scientific, Waltham, MA) for immobilization, and covered with a coverslip. Cells were observed using a fluorescence microscope (BZ-X800, Keyence, Osaka, Japan). The frame of each individual cell was manually identified, and the cell area was calculated using the BZ-X800 Analyzer software (Keyence). PHA formation was visualized using a tetramethylrhodamine isothiocyanate filter set (λem ≈ 554 nm, λex ≈ 570 nm). The fluorescence intensity of the Nile red-stained dot assembly was calculated on average using the BZ-X800 Analyzer software.
TEM
The I. sakaiensis cells grown on PET granules for 6 days were harvested by centrifugation, fixed with phosphate-buffered 2% glutaraldehyde, and post-fixed with 2% osmium tetraoxide for 3 h on ice. Next, the samples were dehydrated using an ethanol gradient and embedded in epoxy resin. Ultrathin sections of the specimen were stained with uranyl acetate and lead, and subjected to TEM using the H-7600 transmission electron microscope (Hitachi, Tokyo, Japan).
Polymer extraction and analyses
Samples were prepared as described previously15 with several modifications. The lyophilized cells of I. sakaiensis were suspended in chloroform with vigorous stirring for 1 day, and the extract was filtered through a polytetrafluoroethylene filter (pore size, 0.2 µm). The filtrate was mixed with twofold volume of 35% methanol:35% ethanol:30% water (v/v/v), and the precipitate was dried in vacuo and then dissolved in chloroform. To determine the molecular weight of the polymer, the chloroform-soluble fraction was analyzed using a size exclusion chromatography system equipped with a RI-2031Plus refractive detector (Jasco, Tokyo, Japan) and two in-line TSKgel GMHHR-M columns with a TSK-guard column HHR-H (Tosoh, Tokyo, Japan) at 40 °C. Chloroform was used as the mobile phase and passed through the column at a flow rate of 1 mL/min. The molecular weight was estimated by comparison with poly(methylmethacrylate) (PMMA) standards (PMMA calibration kit M-M-10, Agilent Technologies, Santa Clara, CA). To analyze the polymer chemical structure, the chloroform-soluble fraction was further purified by precipitation in cold methanol. The precipitate was dissolved in deuterated chloroform (CDCl3) and analyzed via proton nuclear magnetic resonance (1H NMR) (JNM-ECX400P, Jeol, Tokyo, Japan).
PHA quantification and composition analysis
The cellular PHA content and composition were determined by GC after direct methanolysis of the dried cells in the presence of 15% sulfuric acid at 100 °C for 140 min, as described previously16. The GC analysis was performed using GC-2014 (Shimadzu, Kyoto, Japan) equipped with an InertCap 1 capillary column (30 m × 0.23 mm; GL Sciences, Tokyo, Japan) and a flame ionization detector. PHA composition was further determined by mass spectrometry.
References
Taniguchi, I. et al. Biodegradation of PET: Current status and application aspects. ACS Catal. 9, 4089–4105. https://doi.org/10.1021/acscatal.8b05171 (2019).
Yang, J., Yang, Y., Wu, W. M., Zhao, J. & Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 48, 13776–13784. https://doi.org/10.1021/es504038a (2014).
Yang, Y. et al. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 2. Role of gut microorganisms. Environ. Sci. Technol. 49, 12087–12093. https://doi.org/10.1021/acs.est.5b02663 (2015).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199. https://doi.org/10.1126/science.aad6359 (2016).
Akaraonye, E., Keshavarz, T. & Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 85, 732–743. https://doi.org/10.1002/jctb.2392 (2010).
Fukui, T. & Doi, Y. Efficient production of polyhydroxyalkanoates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl. Microbiol. Biotechnol. 49, 333–336. https://doi.org/10.1007/s002530051178 (1998).
Kenny, S. T. et al. Up-cycling of PET (polyethylene terephthalate) to the biodegradable plastic PHA (polyhydroxyalkanoate). Environ. Sci. Technol. 42, 7696–7701. https://doi.org/10.1021/es801010e (2008).
Tiso, T. et al. Bio-upcycling of polyethylene terephthalate. bioRxiv https://doi.org/10.1101/2020.03.16.993592 (2020).
Jan, S. et al. 1H NMR spectroscopic determination of poly 3-hydroxybutyrate extracted from microbial biomass. Enzyme Microb. Technol. 18, 195–201. https://doi.org/10.1016/0141-0229(95)00096-8 (1996).
Yoshida, S. et al. Response to comment on “A bacterium that degrades and assimilates poly(ethylene terephthalate)”. Science 353, 759. https://doi.org/10.1126/science.aaf8625 (2016).
Wang, H. Y., Kuo, W. T., Lin, C. C. & Po-Yo, C. Study of the material properties of fly ash added to oyster cement mortar. Constr. Build. Mater. 41, 532–537. https://doi.org/10.1016/j.conbuildmat.2012.11.021 (2013).
Sato, S., Fujiki, T. & Matsumoto, K. Construction of a stable plasmid vector for industrial production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by a recombinant Cupriavidus necator H16 strain. J. Biosci. Bioeng. 116, 677–681. https://doi.org/10.1016/j.jbiosc.2013.05.026 (2013).
Yoshida, S., Hiraga, K., Taniguchi, I. & Oda, K. Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. Methods Enzymol. 648, 187–205. https://doi.org/10.1016/bs.mie.2020.12.007 (2021).
Degelau, A., Scheper, T., Bailey, J. E. & Guske, C. Fluorometric measurement of poly-b hydroxybutyrate in Alcaligenes eutrophus by flow cytometry and spectrofluorometry. Appl. Microbiol. Biotechnol. 42, 653–657. https://doi.org/10.1007/BF00171939 (1995).
Kenny, S. T. et al. Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 95, 623–633. https://doi.org/10.1007/s00253-012-4058-4 (2012).
Kato, M., Bao, H. J., Kang, C. K., Fukui, T. & Doi, Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium chain length 3-hydroxyalkanaic acids by Pseudomonas sp. 61–3 from sugars. Appl. Microbiol. Biotechnol. 45, 363–370. https://doi.org/10.1007/s002530050697 (1996).
Acknowledgements
We are grateful to Prof. Kohei Oda for the kind gift of I. sakaiensis. We thank Dr. Tsuyoshi Ando, Yumiko Kawakami, and Mari Nakagawa for research assistance. This work was funded by JSPS KAKENHI (18K19177 and 21H02104 to S.Y.), the Leading Initiative for Excellent Young Researchers (LEADER) program by MEXT (16810109 to S.Y.), Fusion Oriented REsearch for disruptive Science and Technology (FOREST) program by JST (1126326 to S.Y.), and the Fuji Seal Foundation (to R.S.).
Author information
Authors and Affiliations
Contributions
S.Y. designed the study. R.F. conducted the growth experiments and analyzed the data. R.S. conducted fluorescence microscopy and analyzed the data. H.A. and R.F. conducted the 1H NMR and SEC experiments and analyzed the data. T.F. conducted the GC experiments and analyzed the data. S.Y., R.F, and T.F. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujiwara, R., Sanuki, R., Ajiro, H. et al. Direct fermentative conversion of poly(ethylene terephthalate) into poly(hydroxyalkanoate) by Ideonella sakaiensis. Sci Rep 11, 19991 (2021). https://doi.org/10.1038/s41598-021-99528-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99528-x
This article is cited by
-
A mycofactocin-associated dehydrogenase is essential for ethylene glycol metabolism by Rhodococcus jostii RHA1
Applied Microbiology and Biotechnology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.