Abstract
Coleoptera are currently considered a fundamental tool to help solve criminal investigations, allowing forensic entomologists to estimate post-mortem intervals and obtain other ecology-related information. Thanatophilus rugosus (Linnaeus, 1758) is an important necrophagous beetle distributed through most of the Palaearctic region, where it is readily found on human bodies and animal carcasses. In this study, the new thermal summation models for all the developmental stages of Thanatophilus rugosus are provided. Beetles were reared at six different constant and ecologically relevant temperatures (12, 14, 16, 18, 20, and 22 °C), and their developmental times were measured. Thermal summation constants were calculated for each developmental stage (egg, three larval instars, post-feeding stage, and pupa).
Similar content being viewed by others
Introduction
Insect specimens found on a carrion during criminal investigations are a valuable piece of evidence when investigating time intervals relevant for the forensic sciences, e.g., Postmortem interval (PMI) and Pre-appearance interval (PAI)1,2. Using developmental information of Silphinae in medico-legal cases has nowadays proven to have many advantages when calculating the post-mortem interval3.
Thanatophilus Leach, 1815 (Silphidae: Silphinae) is distributed in Europe, Asia, North America, and Africa4. Forensic entomologists took interest in the genus only recently as they recognized its value for the field5,6. Many aspects of its ecology and morphology have already been studied such as the seasonal and daily rhythms7,8, preference to a particular soil type9, chemical ecology10, larval morphology and instar identification11. Recently, developmental models have been proposed for some of the Thanatophilus species e.g., T. micans (Fabricius, 1794)5, T. capensis (Wiedemann, 1821)3 and T. sinuatus (Fabricius, 1775)12. However, many more have yet to be studied, as this genus is one of the most diverse in the subfamily Silphinae with 24 described members to date4.
Along with other species colonizing carrion, Thanatophilus rugosus (Linneaus, 1758) is frequently found on carcasses during early spring in central Europe13,14 and throughout most of the year also in Southern Europe15. Despite the species being extensively collected on carcasses during criminal investigations across Europe, no developmental studies have been published so far.
In this article we present thermal summation models (TSM) based on the full developmental cycle of T. rugosus. Furthermore, we analyze whether the sex of an individual influences the developmental length, and whether the time proportions among certain instars are constant and independent. Additionally, survival rates among temperatures and developmental stages are evaluated.
Results
Development of T. rugosus
Overall, 713 individuals of T. rugosus were analyzed at six constant temperatures (12, 14, 16, 18, 20 and 22 °C). In all tested temperatures, individuals were able to complete the life cycle from egg to adulthood. In total, 526 out of 713 specimens reached adulthood.
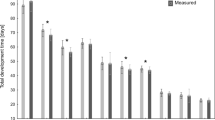
The observed developmental lengths differed among temperatures. The duration of the development at the lowest temperature (12 °C) was 88.36 days (SD = 8.70; N = 88) while at the highest temperature (22 °C) was only 25.66 days (SD = 1.48; N = 70) (Table 2). The greatest proportion of the T. rugosus development cycle was spent in the Post–feeding (24%) and the pupal (33%) stages, while the shortest time was spent in the L1 and L2 stages (7–8%) in all studied temperatures.
Survival rate and mortality
In total, the survival rate was above 70% in all the tested temperatures. Among all of the developmental stages, first larval instar (L1) and egg stage (Egg) had the highest in mortality rates, specifically 5% (N = 52) of deaths for L1 and 6.1% (N = 37) of deaths for Egg. The lowest mortality rate values were observed at the third larval instar (L3), post-feeding stage (PF) and pupal stage (Pupae), specifically 1.3% (N = 8) of deaths, 1.3% (N = 8) and 1.2% (N = 11) respectively. Second larval instar (L2) mortality rate was 3.8%. Survival rates values for each developmental stage are given in Table 1. Regarding the tested temperatures, the highest mortality rate was found at the highest temperature of 22 °C, where 25.5% of individuals did not complete their development. Increased mortality was found at 14 °C (19.2%) and 18 °C (19.5%) as well. Cannibalistic behavior in L3 larvae was observed towards the younger developmental stages of L2. Larvae cannibalized by the L3 were recognized due to the feeding marks on the ventral side, with their carcasses mostly comprising of the remnants of dorsal plates.
Thermal summation model
Thermal summation models were established for all developmental stages (Fig. 1, Table 2). The accumulated degree days (ADD) and lower developmental threshold (LDT) values were calculated with expected errors (Table 3). To complete the whole development from the egg to the emergence of imago the individual needs to accumulate 362.75 ADD with a LDT set at 8.52 °C. Values coefficient of determination (R2), for all inspected models were above 0.85, indicating good fit on the data (Table 2).
Ikemoto and Takai30 thermal summation model for all developmental stages of T. rugosus: (a) egg, (b) 1st larval instar, (c) 2nd larval instar, (d) 3rd larval instar, (e) post-feeding, (f) pupae. The points indicate the data used for the regression analysis.
Effect of sex on developmental length
The effect of sex on the developmental length was calculated using a group of beetles that were able to complete development until the adult stage, from across all of the studied temperatures. The sex ratio (the number of males related to the number of females) was on average 1.067 (i.e. in favor of males); however, the probability of males and females in the sample did not differ significantly from equality (Exact binomial test, N = 253, 95% CI [0.44, 0.52], p value = 0.4842).
The developmental length was similar for both sexes throughout all of the developmental stages and even as a total (see Fig. 2). Comparison of the null and sex model by AIC values showed that the null model had a significantly lower AIC value (null AIC = 15,460.29, sex AIC = 15,464.73), therefore the information about the sex did not significantly improve the fit of the model.
Developmental length differences between sexes. Horizontal lines within the boxes indicate median values; upper and lower boxes indicate the 75th and 25th percentiles, respectively. Whiskers indicate the values within the 1.5 interquartile ranges. Small dots are outliers. Females indicated in clear black boxes and Males in faded gray color boxes.
Developmental rate isomorphy—DRI
Dirichlet regression was applied to the individual development data for the Egg, L1, L2, L3, PF and the Pupae of T. rugosus reared under all studied temperatures. When comparing all three models (mod0, mod1 and mod2), the mod2 with quadratic effect of temperature had the lowest value of AIC (AIC = − 13,909.4), followed by the mod1 with temperature as the explanatory variable (AIC = − 13,839.0). On the opposite side was the mod0 implying DRI, which was accompanied with the highest value of AIC (AIC = − 13,638.2), suggesting the worst fit to the data. Therefore, we can conclude that the proportion of time spent at the individual developmental stages is not constant but has a quadratic effect with temperature.
Discussion
This manuscript proposes the first thermal summation model for all the developmental stages of T. rugosus. This work complements the recent research on the Central European carrion beetles of forensic importance (see11,12,16,17,18,19). Moreover, the results provided are also applicable in countries outside Europe where T. rugosus occurs20.
Overall, the survival rate was above 70% (N = 526) in all the studied temperatures. These results suggest that the tested temperatures were not stressful or unfavorable. On the contrary, the survival rates indicate that all the tested temperatures were optimal for the beetle’s successful development, ensuring a more extensive data set for the TSM. We found that T. rugosus is likely to survive and perform slightly better at the lower intermediate temperatures (16–18 °C) rather than the high ones (> 20 °C). This could be associated to its natural seasonal activity in Europe7,13,15.
Developmental data at two constant temperatures were previously published by Ref.21, for species referred to as Silpha rugosa Linnaeus, 1758, which is an invalid taxonomic name for T. rugosus. However, T. rugosus has never been reported from Algeria where the study took place, nor northern Africa in general20, and its occurrence here is unlikely. We believe that the developmental information presented by Guerroudj and Berchi is actually referring to T. ruficornis (Kuster, 1851). T. ruficornis does occur in Algeria and can be misidentified as T. rugosus, due to the similar shape and texture of elytra22. Both species can be further separated morphologically, e.g., by the color of the antennomeres (combination of black and red in T. ruficornis, uniformly black in T. rugosus) and by the differences in shape of the posterior margin in the female’s abdominal tergum VIII (lateral emargination being deeper than the middle one in T. ruficornis, while equally emarginated in T. rugosus22,23). This means that the species was most likely misidentified and the developmental data in fact referred to T. ruficornis instead of T. rugosus. This would also explain the difference in our developmental data and those reported by21. They recorded that the development at 23 °C took 32 days, whereas our data suggests that the development at a lower temperature (22 °C) took on average 25.66 days, which represents a significant difference. This shows that correct identification of the insect species investigated in criminal sciences is critical for the precise calculation of the PMI because for even a closely related species, the physiological requirements may vary greatly.
The information provided by our TSM models and their parameters allow us to compare the thermal requirements of T. rugosus with other studied species from the genus and put them into perspective with their underlying ecology. It seems that even species that occur together do not share a similar lower developmental threshold. The LDT of T. rugosus (LDTT.rugosus = 8.528) differs considerably from its closest European sister species, T. sinuatus (LDTT.sinuatus = 9.85)12. However, total ADD are very similar for both species (ADDT.rugosus = 362.7 and ADDT.sinuatus = 360.4)12. When compared to the Afrotropical species, the thermal constants vary significantly. For T. capensis, the LDT and ADD values (9.04; 384.1)3 were relatively close to the ones of both T. rugosus and T. sinuatus12. Interestingly, T. micans represents a peculiar case, where its TSM values of LDT are the highest (LDTT.micans = 13.26) and ADD is the lowest (ADDT.micans = 197.97)3, when compared to the Thanatophilus species aforementioned (T. rugosus, T. sinuatus and T. capensis)3,12.
Honěk24 mentions that the species thermal constants show a negative relationship between LDT and ADD, therefore the species with the higher LDT requires a lower amount of ADD than the species with lower LDT. LDT and ADD values can be linked to environmental adaptations such as seasonality and temperature depending on the species distribution24,25. In practice, it is expected that the lower LDT values can be found in a species occurring in temperate regions such as T. rugosus, T. sinuatus and T. capensis. On the other hand, higher values of LDT can be expected for the species distributed throughout the tropical areas such as T. micans. The different values of LDT and ADD between T. rugosus and T. sinautus demonstrate their differences in seasonal activity, that regulate competition between these two closely related sympatric species25.
The LDT and ADD values also differ among other silphid species outside the genus Thanatophilus. In Necrodes littoralis (Linnaeus 1758) the LDT value (LDTN.littoralis = 8.49) is closer to the one of T. rugosus, however the ADD is much higher (ADDN.littoralis = 468.89), probably due to the larger size of the species. On contrary, in Necrophila brunnicollis (Kraatz 1877) both parameters vary significantly (LDTN.brunnicollis = 14.71) (ADDN.brunnicollis = 243.74) when compared to T. rugosus. In this case we could attribute the discrepancy to the adaptation to different climatic conditions as N. brunnicollis is a tropical species while T. rugosus is a temperate species.
Regarding the developmental times, differences were found between T. rugosus and other Thanatophilus species (Table 4). T. rugosus seems to develop faster at all temperatures compared to T. sinuatus as well as Afrotropical species T. micans and T. capensis. In addition, T. rugosus needs less time to develop also compared to other silphid species, like Necrophila brunnicollis (Kraatz 1877)26 and for Necrodes littoralis (Linnaeus 1758)18. In addition, our results confirm that the proportion of time spent in a particular developmental stage is dependent on the temperature. A faster development rate and the violation of the DRI could be suggested for species distributed in the temperate region as its developmental rate and growth can be adjusted based on the seasonal limitations and photoperiods27. Our findings are thus in accordance with the results already published in other forensically important species of Silphidae12,26.
Here we present the first thermal summation models for all developmental stages of T. rugosus reared at six constant temperatures (12, 14, 16, 18, 20 and 22 °C) under controlled laboratory conditions. The models allow T. rugosus to become another important tool for forensic entomology. The added benefit of including this species is its utility during the cold season when many other forensically important species are absent. Moreover, the data provided here can be used to validate PMI calculations based on other forensically relevant insects.
Methods
Laboratory colony establishment
Adult beetles of T. rugosus were collected using pitfall traps baited with pork muscle tissue (Sus scrofa Linnaeus, 1758) (Mammalia: Suidae) and beef muscle tissue (Bos taurus Linnaeus, 1758) (Mammalia: Bovidae). The sampling was conducted in the Czech Republic in 2020. Specimens were sampled at three localities: Stará Lysá (50° 13′ 08.0′′ N, 14° 48′ 25.5′′ E), Slapy (49° 47′ 31.9′′ N, 14° 23′ 52.7′′ E) and Prague—Lysolaje (50° 07′ 33.0′′ N 14° 21′ 44.0′′ E). Due to the seasonal and ecological preferences of the species, individuals were collected between April and May by placing the traps in open areas, in habitats such as harvested agricultural fields or grasslands15,28.
The methodology used for larval breeding in this study follows Montoya-Molina et al.12 and was originally based on Ridgeway et al.3, with modifications described in detail there. The larvae of the first and second instar from the same clutch of eggs were kept together under the condition that they were at the same developmental stage. If some larvae molted before the rest of the group, they were moved to separate Petri dish. Developmental milestones (egg, 1st instar larva, 2nd instar larva, 3rd instar larva, Post-feeding stage) and pupae) were identified based on morphological features described by Novák et al.16. The whole breeding process was monitored daily.
Colonies were kept inside climatic chambers (custom made by CIRIS s.r.o.) at six constant temperatures (12, 14, 16, 18, 20 and 22 °C) and 16 h of light and 8 h of dark photoperiod regime, maintained by a fluorescent light (Osram L 8W/640) to simulate the light conditions during the species breeding season. The temperatures used were based on previous rearing of this beetle by Jakubec et al.11.
Mortality rate analysis
Survival data were evaluated by a nonparametrical log rank test using the function survdiff from the R package survival29. The effect of temperature on mortality was investigated at all experimental temperatures (12, 14, 16, 18, 20 and 22 °C). We measured the mortality of immature individuals starting with the egg and the data were right hand censored at the moment of adult beetle emergence. Bonferonni correction was used for post–hoc comparisons of the treatments.
Thermal summation model
To model the relationships between the developmental time and the temperature for all the developmental stages, a linear regression model defined by Ikemoto and Takai30 was applied. Both estimated parameters of the TSM (LDT and ADD) are defined as a slope and intercept of the linear regression respectively. Therefore, both LDT and ADD can be easily calculated along with their respective standard errors30.
Effect of sex on development length
Across all temperatures, only the individuals that have completed the development until the adult stage were selected for the study, as only these specimens could be sexed.
To assess the potential differences in developmental time between males and females, two linear mixed effect regression models were fitted (null and “sex” model). Response variables of both models were the developmental lengths of the six stages (Egg, L1, L2, L3, PF and Pupae) and their total. Null and the alternative model also shared two fixed effect explanatory variables: temperature (12, 14, 16, 18, 20 and 22 °C) and developmental stage. Because the measurements of the development times were done repeatedly on the same individual, the identity of that specimen had to be incorporated into the analysis, therefore we used it as a random effect in both models. The only difference between the alternative and the null models was fitting the latter with sex as a fixed effect explanatory variable. The fit of these two models was compared via AIC criteria to find out if the information about sex does improve it significantly.
Developmental rate isomorphy (DRI) and sex ratio
The presence of developmental rate isomorphy (DRI) was tested using Dirichlet regression as suggested by Boukal et al.27. The isomorphy hypothesis suggests that the development rates do not depend on the temperature. For the analysis, a proposed null model (mod0) was tested without the explanatory variables. The mod0 implies that the proportions of time spent in individual instars are constant and independent of other factors (true presence of the DRI in the species). Two alternative models were fitted, one with temperature as the explanatory variable (mod1) and the other with expected quadratic effect of temperature (mod2). Both alternative models suggest that the DRI is not present in the species. Relative quality of each model was evaluated by Akaike information criteria (AIC), to allow comparison. The one with the lowest value was considered as the most appropriate description of the underlying relationship.
A Binomial test was conducted to determine whether the observed sex ratio for the species is significantly different from the expected one (1:1).
Data management and analysis
All data management and analyses were carried out using the R statistical program31. Additionally, we used a “lme4” package for fitting mixed effect models and visual outputs were processed via packages “ggplot2” and “sjPlot”32,33,34.
References
Matuszewski, S. Post-mortem interval estimation based on insect evidence: Current challenges. Insects 12, 314 (2021).
Matuszewski, S. & Mądra-Bielewicz, A. Validation of temperature methods for the estimation of pre-appearance interval in carrion insects. Forensic Sci. Med. Pathol. 12, 50–57 (2016).
Ridgeway, J. A., Midgley, J. M., Collett, I. J. & Villet, M. H. Advantages of using development models of the carrion beetles Thanatophilus micans (Fabricius) and T. mutilatus (Castelneau) (Coleoptera: Silphidae) for estimating minimum post mortem intervals, verified with case data. Int. J. Legal Med. 128, 207–220 (2014).
Newton, A. F. Staphyliniformia world catalog database (Nov 2018). Staphyliniformia World Catalog Database (2021). http://archive.fieldmuseum.org/peet_staph (Accessed 5 April 2021).
Midgley, J. M. & Villet, M. H. Development of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) at constant temperatures. Int. J. Legal Med. 123, 285–292 (2009).
Midgley, J. M., Richards, C. S. & Villet, M. H. The utility of coleoptera in forensic investigations. In Current Concepts in Forensic Entomology (eds Amendt, J. et al.) 57–68 (Springer, 2010).
Kočárek, P. Diel activity patterns of carrion-visiting Coleoptera studied by time-sorting pitfall traps. Biologia (Bratisl.) 57, 199–211 (2002).
Dekeirsschieter, J., Frederickx, C., Verheggen, F. J., Boxho, P. & Haubruge, E. Forensic entomology investigations from doctor marcel leclercq (1924–2008): A review of cases from 1969 to 2005. J. Med. Entomol. 50, 935–954 (2013).
Jakubec, P. & Růžička, J. Is the type of soil an important factor determining the local abundance of carrion beetles (Coleoptera: Silphidae)? Eur. J. Entomol. 112, 747–754 (2015).
Dekeirsschieter, J. et al. Electrophysiological and behavioral responses of Thanatophilus sinuatus fabricius (Coleoptera: Silphidae) to selected cadaveric volatile organic compounds. J. Forensic Sci. 58, 917–923 (2013).
Jakubec, P., Novák, M., Qubaiová, J., Šuláková, H. & Růžička, J. Description of immature stages of Thanatophilus sinuatus (Coleoptera: Silphidae). Int. J. Legal Med. 133, 1549–1565 (2019).
Montoya-Molina, S. et al. Developmental models of the forensically important carrion beetle, Thanatophilus sinuatus (Coleoptera: Silphidae). J. Med. Entomol. 58, 1041–1047 (2021).
Frątczak-Łagiewska, K. & Matuszewski, S. Resource partitioning between closely related carrion beetles: Thanatophilus sinuatus (F.) and Thanatophilus rugosus (L.) (Coleoptera: Silphidae). Entomol. Gen. 37, 143–156 (2018).
Esh, M. & Oxbrough, A. Macrohabitat associations and phenology of carrion beetles (Coleoptera: Silphidae, Leiodidae: Cholevinae). J. Insect Conserv. https://doi.org/10.1007/s10841-020-00278-4 (2020).
Bonacci, T., Greco, S. & Brandmayr, T. Z. Insect fauna and degradation activity of Thanatophilus species on carrion in Southern Italy (Coleoptera: Silphidae). Entomol. Gen. 33, 63–70 (2011).
Novák, M., Jakubec, P., Qubaiová, J., Šuláková, H. & Růžička, J. Revisited larval morphology of Thanatophilus rugosus (Coleoptera: Silphidae). Int. J. Legal Med. 132, 939–954 (2018).
Charabidze, D., Vincent, B., Pasquerault, T. & Hedouin, V. The biology and ecology of Necrodes littoralis, a species of forensic interest in Europe. Int. J. Legal Med. 130, 273–280 (2016).
Gruszka, J. & Matuszewski, S. Estimation of physiological age at emergence based on traits of the forensically useful adult carrion beetle Necrodes littoralis L. (Silphidae). Forensic Sci. Int. 314, 110407 (2020).
Frątczak-Łagiewska, K., Grzywacz, A. & Matuszewski, S. Development and validation of forensically useful growth models for Central European population of Creophilus maxillosus L. (Coleoptera: Staphylinidae). Int. J. Legal Med. 134, 1531–1545 (2020).
Růžička, J. Silphidae. In Catalogue of Palaearctic Coleoptera Volume 2/1. Hydrophiloidea—Staphylinoidea, Revised and Updated Edition (eds Löbl, I. & Löbl, D.) 291–304 (Brill, 2015).
Guerroudj, F. Z. & Berchi, S. Effect of temperature on the development of carrion beetle Silpha rugosa (Linnaeus, 1758) (Coleoptera: Silphidae) in Algeria. J. Entomol. Zool. Stud. 4, 920–922 (2016).
Schawaller, W. Taxonomie und Faunistik der Gattung Thanatophilus (Coleoptera: Silphidae). Stuttgarter Beiträge zur Naturkunde Ser. A 351, 1–21 (1981).
Piloña, F. P., Valcárcel, J. P. & Rey-Daluz, F. Catálogo de los Silphidae y Ayrtidae de la Península Ibérica e Islas Baleares. Bol. la Soc. Entomol. Aragon. 30, 1–32 (2002).
Honěk, A. Geographical variation in thermal requirements for development. Eur. J. Entomol. 93, 303–312 (1996).
Trudgill, D. L., Honek, A., Li, D. & Van Straalen, N. M. Thermal time—Concepts and utility. Ann. Appl. Biol. 146, 1–14 (2005).
Jakubec, P., Qubaiová, J., Novák, M. & Růžička, J. Developmental biology of forensically important beetle, Necrophila (Calosilpha) brunnicollis (Coleoptera: Silphidae). J. Med. Entomol. 58, 64–70 (2020).
Boukal, D. S. et al. Analyses of developmental rate isomorphy in ectotherms: introducing the Dirichlet regression. PLoS ONE 10, e0129341 (2015).
Kočárek, P. Diurnal activity rhythms and niche differentiation in a carrion beetle assemblage ( Coleoptera : Silphidae ) in Opava, the Czech Republic. Biol. Rhythm Res. 32, 431–438 (2001).
Therneau, T. M. A Package for Survival Analysis in R (2021).
Ikemoto, T. & Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Entomol. 29, 671–682 (2000).
R Core Team. R: A Language and Environment for Statistical Computing [Program] (2020). https://www.r-project.org/.
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2009).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science (2020). Accessed 1 Sept 2020. https://CRAN.R-project.org/package=sjPlot.
Acknowledgements
We are grateful to Karolina Mahlerová (CULS Prague, Czech Republic) for her support during the field work and collecting of the samples and Chris Harding (Prague) for language corrections. The project was supported by the Internal Grant Agency of the Faculty of Environmental Sciences, CULS Prague (42110/1312/3159).
Author information
Authors and Affiliations
Contributions
S.M.M. and P.J. designed the study, collected material in the field, bred the beetles in the laboratory, analyzed the data and wrote the manuscript. J.Q. and M.N. collected material in the field, bred the beetles and reviewed the manuscript. J.R. and H.Š. collected material in the field and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montoya-Molina, S., Jakubec, P., Qubaiová, J. et al. Developmental models of the carrion beetle Thanatophilus rugosus (Linnaeus, 1758) (Coleoptera: Silphidae). Sci Rep 11, 19377 (2021). https://doi.org/10.1038/s41598-021-98833-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98833-9
This article is cited by
-
Integrative taxonomy and species distribution models of the genus Diamesus Hope, 1840 (Coleoptera: Staphylinidae: Silphinae)
Scientific Reports (2023)
-
Temperature models of development for Necrodes littoralis L. (Coleoptera: Silphidae), a carrion beetle of forensic importance in the Palearctic region
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.