Abstract
Growth hormone (GH) is one of the critical factors in maintaining glucose metabolism. B-cell translocation gene 2 (BTG2) and yin yang 1 (YY1) are key regulators of diverse metabolic processes. In this study, we investigated the link between GH and BTG2–YY1 signaling pathway in glucose metabolism. GH treatment elevated the expression of hepatic Btg2 and Yy1 in primary mouse hepatocytes and mouse livers. Glucose production in primary mouse hepatocytes and serum blood glucose levels were increased during GH exposure. Overexpression of hepatic Btg2 and Yy1 induced key gluconeogenic enzymes phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6 phosphatase (G6PC) as well as glucose production in primary mouse hepatocytes, whereas this phenomenon was markedly diminished by knockdown of Btg2 and Yy1. Here, we identified the YY1-binding site on the Pck1 and G6pc gene promoters using reporter assays and point mutation analysis. The regulation of hepatic gluconeogenic genes induced by GH treatment was clearly linked with YY1 recruitment on gluconeogenic gene promoters. Overall, this study demonstrates that BTG2 and YY1 are novel regulators of GH-dependent regulation of hepatic gluconeogenic genes and glucose production. BTG2 and YY1 may be crucial therapeutic targets to intervene in metabolic dysfunction in response to the GH-dependent signaling pathway.
Similar content being viewed by others
Introduction
Growth hormone (GH) is produced by somatotropic cells of the anterior pituitary gland. GH binding to GH receptor results in activation of Janus kinase 2 (Jak2) molecules and signal transducer and activator of transcription (STAT) family of transcription factors, which regulate expression of multiple target genes in response to GH signaling1. GH plays an important role in the regulation of aging, cancer, glucose metabolism, and lipid metabolism1,2. Both GH deficiency and GH excess are associated with disruptions in carbohydrate metabolism. Moreover, GH is closely linked to decreased oxidation and uptake of glucose in skeletal muscle and increased hepatic gluconeogenesis leading to insulin antagonist effects2,3. It is well-documented that GH is increased during fasting and suppressed during feeding state4,5. A recent report suggests that alterations in glucose metabolism and diabetes are common complications of acromegaly caused by excess production of GH6.
Glucose is used as a major energy source in organisms, from bacteria to humans. The liver stores the excess glucose as glycogen and supplies glucose during fasting. Hepatocytes produce glucose through gluconeogenesis under prolonged fasting condition7. Hepatic gluconeogenesis is generally associated with the transcriptional regulation of rate-limiting key enzymes, such as phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6 phosphatase (G6PC). These hepatic gluconeogenic genes are regulated by numbers of transcription factors, nuclear receptors, and coregulators, including cAMP-response element-binding protein (CREB), forkhead box protein O1 (FOXO1), CCAAT/enhancing-binding protein alpha (C/EBPα), estrogen-related receptor gamma (ERRγ), peroxisome proliferators-activated receptor γ coativator-1α (PGC-1α), CREB-regulated transcription coactivator 2 (CRTC2)8,9,10,11,12,13,14. Especially, cAMP-PKA-CREB signaling pathway stimulates the expression of hepatic gluconeogenic genes and PGC-1α8. Hepatic deletion of FOXO1 markedly decreases both glycogenolysis and gluconeogenesis in mice9. C/EBPα is known to increase the gluconeogenesis7,10, whereas hepatocyte-specific deletion of C/EBPα is reported to significantly decrease gluconeogenesis11.
B-cell translocation gene 2 (BTG2) is an anti-proliferative gene and it is downregulated in many human cancers15,16. Previous reports showed that BTG2 is induced by growth factors in several cell types17,18. BTG2 is highly expressed in the liver and it can also be detected in various other tissues. Our previous study demonstrated that BTG2 acts as a crucial coactivator of CREB to regulate hepatic gluconeogenesis in hepatocytes19 and as a positive regulator of hepatic gluconeogenesis via the induction of Nur77 in diabetic mouse model20. Yin Yang 1 (YY1) is a member of the polycomb protein family and functions as a transcription factor. It is predominantly expressed in diverse tissues and involved in the regulation of multiple target genes via chromatin modification21,22,23. Lu et al. demonstrated that YY1 promotes gluconeogenesis through glucocorticoid receptor in the livers of mice24. It has also been shown that YY1 represses insulin/insulin-like growth factor (IGF)-signaling activation and skeletal muscle-specific YY1 knockout mice improved glucose tolerance and insulin-signaling activation25. However, the critical role of BTG2 and YY1 in regulating the GH-dependent hepatic glucose metabolism remains unexplored.
In this study, we demonstrated that GH treatment significantly increased hepatic gluconeogenesis via the induction of BTG2 and YY1 gene expression. Moreover, disruption of Btg2 and Yy1 markedly attenuated GH-mediated induction of hepatic gluconeogenesis. Our findings suggest that BTG2 and YY1 are key regulators of GH-induced hepatic gluconeogenesis. Therefore, BTG2 and YY1 may be novel potential therapeutic targets to combat metabolic dysfunction in response to the GH-dependent signaling pathway.
Results
Growth hormone increases hepatic BTG2 and YY1 gene expression
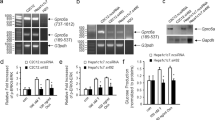
Our previous report demonstrated that GH increased hepatic gluconeogenesis, but this phenomenon was abolished by metformin-ataxia telangiectasia mutated (ATM)-AMP-activated protein kinase (AMPK)-small heterodimer partner (SHP) signaling pathway26. Here, we examined more deeply the role of GH on hepatic gluconeogenesis in mouse livers and primary mouse hepatocytes. GH treatment significantly increased the levels of Pck1 and G6pc along with the increased expression of Btg2 and Yy1 (Fig. 1a) in primary mouse hepatocytes. As expected, glucose production was efficiently elevated by GH treatment (Fig. 1b). Consistent with primary mouse hepatocytes data, GH challenge increased the mRNA levels of Btg2, Yy1, Pck1, and G6pc in mouse livers (Fig. 1c). Similarly, GH exposure significantly increased blood glucose levels relative to that of the control groups (Fig. 1d). Overall, these findings strongly suggest that GH plays an important role in hepatic gluconeogenesis and it can also induce Btg2 and Yy1 gene expression.
Growth hormone elevates hepatic gluconeogenesis in mouse primary hepatocytes and mouse liver. (a) Mouse primary hepatocytes (MPH) were treated with growth hormone (GH, 500 ng/ml) for 3 h. Gene expressions were analyzed by qPCR using the indicated primers. (b) Glucose output assay in MPH exposed to GH for 3 h. (c) Male C57BL/6 wild-type (WT) mice were injected intraperitoneally with GH (2 μg/g) for 7 days. (d) Blood glucose concentrations were measured from the indicated groups. n = 5 mice per group. *P < 0.05 vs. untreated control cells and mice.
Growth hormone-mediated induction of hepatic gluconeogenesis is BTG2 dependent
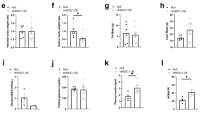
Next, we investigated the critical role of BTG2 as a key modulator of gluconeogenesis using an adenoviral delivery system for the overexpression of Btg2 (Ad-Btg2) or the control green fluorescent protein (Ad-GFP) in primary mouse hepatocytes. As shown in Fig. 2a, the Btg2 mRNA was robustly increased by Ad-Btg2. Overexpression of Btg2 significantly increased the expression of Yy1, Pck1, and G6pc compared to Ad-GFP control groups, but not the expression of specificity protein 1 (Sp1), a zinc finger transcription factor known to regulate many cellular processes. Interestingly, glucose production was also increased by Ad-Btg2 compared to Ad-GFP control groups (Fig. 2b). Next, we further verified whether BTG2 modulates GH-mediated induction of gluconeogenic gene expression and glucose production in primary mouse hepatocytes. The GH-induced protein and mRNA levels of BTG2, YY1, PCK1, and G6PC were dramatically downregulated by endogenous knockdown of Btg2 (Fig. 2c,d). Consistently, the increase of hepatic glucose production induced by GH treatment was markedly impaired when Btg2 was silenced (Fig. 2e). Taken together, these findings imply the role of BTG2 in mediating GH-induced hepatic gluconeogenesis.
Induction of hepatic gluconeogenesis by GH is BTG2 dependent. (a) MPHs were infected with Ad-GFP and Ad-Btg2 at a multiplicity of infection (MOI) of 60 for 36 h. qPCR analysis was performed to examine the expression of indicated genes using specific primers. Sp1 is a negative control. (b) Glucose production assay in MPHs infected with Ad-GFP and Ad-Btg2 for 36 h. (c) Whole cell extracts from MPHs of the indicated groups were subjected to immunoblot analysis with the indicated antibodies. (d) MPHs were infected with lentiviral-shBtg2 (shBtg2) at 60 MOI for 36 h, followed by GH treatment for 3 h. Total RNAs were utilized for qPCR analysis with gene-specific primers. (e) Glucose production assay in MPHs infected with shBtg2 for 36 h, and then treated with GH for 3 h. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. untreated control, Ad-GFP, or GH-treated cells.
Growth hormone- and BTG2-induced hepatic gluconeogenesis depends on YY1
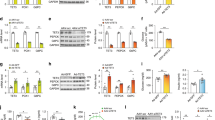
To investigate the vital role of YY1 on hepatic gluconeogenesis by GH in primary mouse hepatocytes and the liver of mice. Yy1 expression was successfully overexpressed by adenoviral delivery system. Overexpression of Ad-Yy1 significantly augmented Pck1 and G6pc mRNA expression compared to Ad-GFP control groups in primary mouse hepatocytes and mouse livers, but not Btg2 expression (Fig. 3a,b). Notably, glucose production was efficiently elevated by GH treatment (Fig. 3c). We further explored whether YY1 is involved in hepatic gluconeogenic gene regulation and glucose production. The protein and mRNA levels of YY1 were successfully silenced in GH-treated primary hepatocytes using lentiviral delivery system for shRNA Yy1 (shYy1). The increase of gluconeogenic genes by GH treatment was markedly reduced in Yy1 silenced group (Fig. 3d,e). Interestingly, GH-mediated induction of hepatic glucose production was also prominently decreased in Yy1 knockdown group (Fig. 3f).
GH- and Btg2-stimulated hepatic gluconeogenesis is mediated by YY1. (a) MPHs were infected with Ad-GFP and Ad-Yy1 at 60 MOI for 36 h. Gene expressions were analyzed by qPCR using gene-specific primers. (b) WT mice were injected with Ad-GFP and Ad-Yy1 via tail-vein for 7 days. Indicated gene expressions were analyzed by qPCR with specific primers. (c) Glucose production assay in MPH infected with Ad-GFP and Ad-Yy1 for 36 h. (d) MPHs were infected with lentiviral-shYy1 (shYy1) at 60 MOI for 36 h and followed by GH for 3 h. Total protein was isolated and analyzed using Western blot analysis with various antibodies. (e) MPHs were infected with lentiviral-shYy1 (shYy1) at 60 MOI for 36 h and then exposed with GH for 3 h. Total RNAs were utilized for qPCR analysis with indicated primers. (f) Glucose production assay in MPHs infected with shYy1 for 36 h, and then treated with GH for 3 h. (g) MPH were infected with Ad-GFP, Ad-Btg2, and shYy1 at 60 MOI for 36 h. Whole cell extracts were analyzed using Western blot analysis with various antibodies. (h) MPHs were infected with Ad-GFP, Ad-Btg2, and shYy1 at 60 MOI for 36 h. Quantitative PCR analysis was performed using gene-specific primers. (i) Glucose production assay was performed in MPHs infected with Ad-GFP, Ad-Yy1, and shYy1 for 36 h. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. untreated control, Ad-GFP, GH-treated cells, or Ad-Btg2-infected cells.
To determine whether BTG2-mediated induction of gluconeogenic key enzymes and glucose production can be modulated by YY1, we evaluated the effect of Yy1 on the regulation of hepatic gluconeogenesis using Ad-Btg2 and shYy1 in primary mouse hepatocytes. Ad-Btg2 significantly increased the expression of YY1, PCK1, and G6PC genes, while this phenomenon was markedly diminished by silencing of Yy1 in primary hepatocytes, but not the expression of BTG2 (Fig. 3g,h). Similarly, Ad-Btg2-mediated increase in glucose production was markedly negated by silencing of Yy1 (Fig. 3i). Overall, these results suggest that GH- and BTG2-stimulated hepatic gluconeogenesis is mediated by YY1.
YY1 is a novel regulator of hepatic gluconeogenic gene transcription
To determine whether the transcriptional activity of Yy1 in response to GH treatment regulates hepatic gluconeogenic gene expression at the transcriptional level, we examined transient transfection assays using luciferase reporter constructs containing the Pck1 and G6pc gene promoters in AML-12 cells. As shown in Fig. 4a, GH treatment significantly increased the activity of Pck1 and G6pc gene promoters in hepatocytes. In addition, transiently expressed Btg2 and Yy1 significantly elevated the promoter activities of Pck1 and G6pc as compared to the control groups. To identify the putative transcription activation site by GH on hepatic gluconeogenic gene promoter, serial deletion constructs of the Pck1 and G6pc gene promoters were used for transient transfection and luciferase reporter gene assay. The activity of Pck1 promoter induced by GH treatment was retained with deletion up to − 800 bp and this activation was completely lost around the − 490 bp construct indicating the presence of GH activation site between − 800 and − 490 bp of Pck1 promoter (Fig. 4b). Moreover, G6pc promoter activity in response to GH exposure was retained with a deletion up to − 500 bp, and it was lost when used the minimal promoter (− 200 bp) construct (Fig. 4c). These observations indicate that the YY1-binding element required for the GH response is located within the region between − 800 and − 490 bp on the Pck1 gene promoter and − 500 and − 200 bp on the G6pc gene promoter.
YY1 is a key regulator of hepatic gluconeogenic gene transcription. (a) AML-12 cells were transiently transfected with Btg2, Yy1, and the indicated reporter genes for 36 h. After transfection, the cells were treated with GH for 3 h. (b, c) AML-12 cells were transfected with wild-type and serial deletion forms of the Pck1 (b) and G6pc (c) reporter constructs for 36 h and then treated with GH for 3 h. (d, e) AML-12 cells were cotransfected with wild-type (wt) and mutant (mt) forms of the Pck1 (d) and G6pc (e) gene promoter, Btg2, and Yy1 for 36 h. After transfection, the cells were treated with GH for 3 h. Luciferase activity was normalized to β-galactosidase activity in each well. *P < 0.05, **P < 0.01 vs. untreated control cells. (f, g) Chromatin immunoprecipitation (ChIP) assay for the recruitment of YY1 on the Pck1 (f) and G6pc (g) gene promoter. MPHs were infected with shBtg2 for 36 h, and then treated with GH for 3 h. Cell lysates were immunoprecipitated with an anti-YY1 antibody. Purified DNA samples were used to perform PCR using primers binding to the specific proximal (Pro) and nonspecific distal (Dis) regions on the Pck1 (f) and G6pc (g) gene promoter. 10% of the soluble chromatin was used as an input. *P < 0.05 vs. untreated control or GH-treated cells.
Our in-silico analysis predicted that there is YY1-binding site on Pck1 and G6pc promoters. To further evaluate the functional significance of the YY1-binding region on the Pck1 and G6pc gene promoter, site-directed mutagenesis was carried out on the Pck1 and G6pc gene promoters. Wild-type (Pck1 wt) and the mutant reporter plasmid (Pck1 mt) were transiently transfected with Btg2 and Yy1 in hepatocytes. GH treatment as well as overexpression of Btg2 and Yy1 significantly increased Yy1-dependent activity of Pck1 gene promoter, and this phenomenon was prominently abolished in Yy1-mutant Pck1 promoter (Fig. 4d). Similarly, GH treatment or transiently expressed Btg2 and Yy1 efficiently enhanced G6pc gene promoter, and this increase was significantly hampered in the YY1-binding mutated (mt) G6pc promoter (Fig. 4e). Overall, these results suggest that the YY1-binding site can be sufficient to mediate the activation of Pck1 and G6pc gene promoters in response to GH treatment. Finally, we conducted chromatin immunoprecipitation (ChIP) assays in primary mouse hepatocytes to identify the YY1-binding site on the Pck1 and G6pc gene promoter. GH exposure strongly enhanced YY1 occupancy on the proximal region (Pro, − 800/− 600) of the Pck1 promoter but not in the non-specific distal region (Dis, − 2000/− 1800) of the Pck1 promoter (Fig. 4f). Moreover, we identified GH-induced YY1 recruitment on the proximal region (Pro, − 300/− 100) of the G6pc promoter but not in the distal region (Dis, − 2000/− 1800) of the G6pc promoter (Fig. 4g). Overall, these findings strongly suggest that YY1 is recruited to both Pck1 and G6pc gene promoters to mediate GH-induced Pck1 and G6pc gene transcription.
Discussion
In this study, we have demonstrated that GH promotes hepatic gluconeogenesis via the induction of the BTG2–YY1 signaling pathway, and it was markedly abolished by shBtg2 and shYy1 gene silencing. Consistent with gene expression data showing upregulation of hepatic gluconeogenic gene expression and glucose production was also significantly increased by the BTG2–YY1 signaling pathway in primary hepatocytes. Based on these findings, we suggest that the BTG2–YY1 signaling network exerts as a critical factor for the regulation of hepatic gluconeogenesis during the GH-dependent pathway.
Cui et al. have demonstrated that upregulation of multiple target genes (BTG2, c-FOS, and SOCS3) by GH treatment are mediated by specific mechanisms involving C/EBPβ and basic leucine zipper (bZIP) family transcription factors in adipocytes27. However, the potential link between GH and BTG2 in the regulation of hepatic gluconeogenesis has not been studied yet. Consistent with previous report, we found that GH exposure significantly increased the expression of BTG2 and YY1 genes, along with the increased key hepatic gluconeogenic gene (Pck1 and G6pc) expression as well as glucose levels in mouse livers and primary hepatocytes (Fig. 1). Therefore, we speculate that BTG2 might be an important factor to regulate hepatic gluconeogenic gene expression and glucose production in response to GH signaling.
Our previous study has revealed that the induction of BTG2 by gluconeogenic signals (fasting state, forskolin, and glucagon treatment) positively regulates hepcidin gene expression and hepatic hepcidin production involved in iron metabolism by stimulating YY1 expression23. BTG2 also elevated hepatic gluconeogenesis via the induction of Nur77 in the livers of diabetic mice20. However, several other studies have demonstrated that hepatic gluconeogenesis is regulated by hepatocyte nuclear factor (HNF)-4α, HNF-6, and STAT5 during GH exposure26,28,29. Particularly, we investigated whether BTG2–YY1 signaling pathway may affect hepatic gluconeogenic gene expression and glucose production in response to GH treatment in primary mouse hepatocytes and mouse livers. As anticipated, GH significantly induced hepatic gluconeogenic gene expression, glucose production, and blood glucose levels (Fig. 1), whereas this stimulatory effect of GH was strikingly reduced in Btg2 or Yy1 silenced group (Figs. 2, 3). Our current study suggests that GH is a crucial regulator of hepatic gluconeogenesis by upregulating the BTG2–YY1 signaling network. Therefore, the identified BTG2–YY1 signaling pathway seems to be critical for hepatic GH-dependent gluconeogenic signaling.
YY1 is well-characterized to regulate hepcidin gene expression through BTG2–YY1 signaling network in mouse livers and primary hepatocytes23. In addition, BTG2 participates in the regulation of hepatic glucose metabolism through Nur77 and CREB induction both in vivo and in vitro19,20. Based on these findings, we proposed a novel molecular mechanism that BTG2–YY1 axis mediated hepatic gluconeogenic gene expression. As shown in Fig. 4, recruitment of BTG2 and YY1 on key gluconeogenic enzyme promoters were confirmed in hepatocytes. In response to GH treatment, endogenous YY1 was directly recruited to the YY1-binding site (proximal) region of both Pck1 and G6pc gene promoters. Overall, our findings suggest a novel link between hepatic gluconeogenic gene transcription and BTG2–YY1 signaling pathway. However, we cannot rule out the possibility that one or the other unexplored mechanisms by unknown transcription factors or coregulators to regulates target gene transcription.
In conclusion, this present study demonstrates that GH augments hepatic gluconeogenesis by upregulating the BTG2–YY1 signaling pathway. Moreover, it suggests that BTG2–YY1 signaling pathway mediates GH-induced hepatic gluconeogenesis. Therefore, as described in Fig. 5, we propose a novel molecular mechanism involved in hepatic glucose metabolism by the BTG2–YY1 signaling network that may provide a better understanding to develop a novel therapeutic agent for metabolic dysfunction like diabetes.
Materials and methods
Animal experiments
8-week-old male C57BL/6 mice (Samtako, Osan, Republic of Korea) were used for the following experiments. For the growth hormone (GH) stimulation experiments, wild-type (WT) mice were injected intraperitoneally with GH (ProSpec, Tany Technogen, Ltd., Rehovot, Israel) (2 μg/g of body weight) for 7 days, as previously described26. At the end of the specified experiments, we euthanized the mice with CO2 and harvested liver tissues and blood samples. All animal experiments and protocols were approved and performed by the Institutional Animal Care and Use Committee of the Kyungpook National University according to the rules and procedures of the National Institutes of Health. The study was carried out following ARRIVE guidelines.
Blood glucose level
Mouse blood was drawn from the tail vein and plasma glucose concentration was measured with ACCU-CHEK Active Glucometer (Roche Diagnostics, Mannheim, Germany) as reported20.
Culture of primary mouse hepatocytes
Mouse primary hepatocytes (MPH) were isolated from the livers of 8-week-old male C57BL/6 mice (Samtako) by a two-step portal vein collagenase (Collagenase IA, Sigma-Aldrich, St. Louis, MO, USA) perfusion method and purified by centrifugation. Freshly prepared hepatocytes were cultured in M199 medium (Cellgro, Herndon, VA, USA), as described previously20,23. After trypan blue staining for determination of viability and attachment, cells were infected with the indicated adenoviruses, lentiviruses, and/or treated with GH (500 ng/ml).
Cell culture and transient transfection assays
AML-12 cells (immortalized mouse hepatocyte) were cultured in DMEM/F-12 medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), insulin-transferrin-selenium (ITS, Gibco-BRL), dexamethasone (40 ng/ml, Sigma-Aldrich), and antibiotics (Gibco-BRL). The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. Transient transfection assays were performed with AML12 cells as reported previously20,23. Briefly, transient transfection was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The total amount of DNA was adjusted to 0.8 μg per well by the addition of each appropriate amount of empty vector and β-galactosidase plasmids as the internal control. Luciferase activity was measured and normalized to β-galactosidase activity. All data are representative of at least three independent experiments.
Construction of plasmids and DNA
The reporter plasmids encoding G6pc- and Pck1-Luc were kindly provided by Dr. Hueng-Sik Choi (Chonnam National University, Gwangju, Republic of Korea), as previously described20. Expression vectors of Btg2 and Yy1 were used as described previously23. The point mutation form of G6pc and Pck1 promoter were generated using the site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) using the specific primers: G6pc forward, 5′-AGTGTGCTTTAGTTAATAAT-3′, and reverse, 5′-ATTATTAACTAAAGCACACT-3′ and Pck1 forward, 5′-CATAGTTTTTTTTCAGGCAG-3′, and reverse, 5′-CTGCCTGAAAA-AAAACTATG-3′. All constructs or plasmids were confirmed by DNA sequencing.
Glucose output assay
Glucose production from primary mouse hepatocytes was measured according to the manufacturer’s protocol using a colorimetric glucose oxidase assay kit (Sigma-Aldrich). Briefly, after plating, the cells were washed three times with phosphate-buffered saline (PBS), and then the medium was replaced with glucose production buffer (glucose-free DMEM, pH 7.4, containing 20 mM sodium lactate, 1 mM sodium pyruvate, and 15 mM HEPES without phenol red). The glucose production assays in the medium were conducted in triplicate as previously reported19,20.
Recombinant adenoviruses
Adenoviruses encoding full-length of Btg2 (Ad-Btg2), green fluorescent protein (GFP), and lentiviral delivery system of Btg2-targeted shRNA (shBtg2) were described previously23. Ad-Yy1 and recombinant lentiviral silencing system (shYy1) have been mentioned previously30. Adenoviruses were transduced at a multiplicity of infection (MOI) of 60 for 36 h in primary mouse hepatocytes, which was conducted according to the manufacturer’s instructions. Ad-Yy1 (a single dose of 1 × 109 plaque-forming units) were intravenously injected to WT mice for 7 days, as previously described30.
RNA isolation and quantitative real-time PCR (qPCR) analysis
Total RNA was isolated from primary mouse hepatocytes and mouse livers using the TRIzol reagent (Invitrogen), and cDNA was synthesized using the Maxima® First Strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania), as previously mentioned26,30. Quantitative real-time PCR (qPCR) was performed using the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and the StepOne™ Real-time PCR system (Applied Biosystem). We determine the expressions of Btg2, Yy1, Pck1, Sp1, and G6pc genes using qPCR, as previously described20,23,31. The expression of each target gene was normalized to ribosomal L32 expression.
Western blot analysis
Protein lysates were isolated from liver tissues and primary mouse hepatocytes using a radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (Elpis-Biotech, Dajeon, Republic of Korea). Proteins from whole-cell extracts were separated using 10% SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA), as described previously23,26. The blotted membranes were probed with BTG2 (1:1000), YY1 (1:1000), PCK1 (1:1000), β-actin (1:3000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and/or G6PC (1:1000, Abcam, Cambridge, UK) antibodies. After incubation with the indicated antibodies, immunoreactive proteins were developed with an ECL Western Blot Detection Reagent (Amersham Biosciences).
Chromatin immunoprecipitation assay (ChIP)
The ChIP assay was performed according to the manufacturer’s protocol, as described previously23. Briefly, primary mouse hepatocytes were fixed with 1% formaldehyde and sonicated. The soluble chromatin was subjected to immunoprecipitation using anti-YY1 (Santa Cruz Biotechnology) followed by incubation with protein A/G PLUS agarose bead (Santa Cruz Biotechnology) and salmon sperm DNA (Upstate Biotechnology, Lake Placid, NY, USA). After elution, DNA samples were recovered by phenol/chloroform extraction, and qPCR was performed using corresponding primers encompassing the proximal region of G6pc/Pck1 promoter (G6pc forward 5′-CTCTGGCCTGGCTTCAAGGA-3′, reverse 5′-ACTTTTGTCTAAAATCTATT-3′, Pck1 forward 5′-CTCTGGCCTGGCTTCAAGGA-3′, reverse 5′-ACTTTTGTCTAAAATCTATT-3′) and the non-specific distal region of G6pc/Pck1 promoter (G6pc forward 5′-TCAATAATAACTGAGTTGAG-3′, reverse 5′-CGTCTGATATATCTCAAGTC-3′, Pck1 forward 5′-TGCCATGGCTCACAGTGCCT-3′, reverse 5′-GTTACGAAATGACCTGGAGG-3′).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, CA, USA). Statistical significance of the differences between the two groups was determined using Student’s t-test, and multiple comparisons were analyzed using one-way analysis of variance (ANOVA). All data are expressed as mean ± standard error of mean. A P value less than 0.05 was considered statistically significant.
References
Rotwein, P. & Chia, D. J. Gene regulation by growth hormone. Pediatr. Nephrol. 25, 651–658 (2010).
Vijayakumar, A., Novosyadlyy, R., Wu, Y., Yakar, S. & LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 20, 1–7 (2010).
Bonfig, W. & Holl, R. W. Mini review/commentary: Growth hormone treatment in children with type 1 diabetes. Int. J. Mol. Sci. 20(3), 772. https://doi.org/10.3390/ijms20030772 (2019).
Ho, K. Y. et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Invest. 81, 968–975 (1988).
Sakharova, A. A. et al. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 93, 2755–2759 (2008).
Hannon, A. M., Thompson, C. J. & Sherlock, M. Diabetes in patients with acromegaly. Curr. Diab. Rep. 17, 8 (2017).
Rui, L. Energy metabolism in the liver. Compr. Physiol. 4, 177–197 (2014).
Herzig, S. et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001).
Matsumoto, M., Pocai, A., Rossetti, L., Depinho, R. A. & Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 6, 208–216 (2007).
Inoue, Y., Inoue, J., Lambert, G., Yim, S. H. & Gonzalez, F. J. Disruption of hepatic C/EBPalpha results in impaired glucose tolerance and age-dependent hepatosteatosis. J. Biol. Chem. 279, 44740–44748 (2004).
Wang, N. D. et al. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269, 1108–1112 (1995).
Kim, D. K. et al. Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J. Biol. Chem. 287, 21628–21639 (2012).
Yoon, J. C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001).
Le Lay, J. et al. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 10, 55–62 (2009).
Matsuda, S., Rouault, J., Magaud, J. & Berthet, C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 497, 67–72 (2001).
Ficazzola, M. A. et al. Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis 22, 1271–1279 (2001).
Bradbury, A., Possenti, R., Shooter, E. M. & Tirone, F. Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proc. Natl. Acad. Sci. USA 88, 3353–3357 (1991).
Fletcher, B. S. et al. Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J. Biol. Chem. 266, 14511–14518 (1991).
Hwang, S. L. et al. B-cell translocation gene-2 increases hepatic gluconeogenesis via induction of CREB. Biochem. Biophys. Res. Commun. 427, 801–805 (2012).
Kim, Y. D. et al. B-cell translocation gene 2 regulates hepatic glucose homeostasis via induction of orphan nuclear receptor Nur77 in diabetic mouse model. Diabetes 63, 1870–1880 (2014).
Thomas, M. J. & Seto, E. Unlocking the mechanisms of transcription factor YY1: Are chromatin modifying enzymes the key?. Gene 236, 197–208 (1999).
Gordon, S., Akopyan, G., Garban, H. & Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 25, 1125–1142 (2006).
Lee, S. E., Hwang, S. L., Jang, W. G., Chang, H. W. & Kim, Y. D. B-cell translocation gene 2 promotes hepatic hepcidin production via induction of Yin Yang 1. Biochem. Biophys. Res. Commun. 460, 996–1001 (2015).
Lu, Y. et al. Yin Yang 1 promotes hepatic gluconeogenesis through upregulation of glucocorticoid receptor. Diabetes 62, 1064–1073 (2013).
Blattler, S. M. et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 15, 505–517 (2012).
Kim, Y. D. et al. Orphan nuclear receptor small heterodimer partner negatively regulates growth hormone-mediated induction of hepatic gluconeogenesis through inhibition of signal transducer and activator of transcription 5 (STAT5) transactivation. J. Biol. Chem. 287, 37098–37108 (2012).
Cui, T. X. et al. C/EBPbeta mediates growth hormone-regulated expression of multiple target genes. Mol. Endocrinol. 25, 681–693 (2011).
Lahuna, O. et al. Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol. Endocrinol. 14, 285–294 (2000).
Lee, Y. S. et al. Orphan nuclear receptor SHP interacts with and represses hepatocyte nuclear factor-6 (HNF-6) transactivation. Biochem. J. 413, 559–569 (2008).
Lee, S. E. et al. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. J. Pineal. Res. 68, e12638 (2020).
Choi, J. A. et al. Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 35, 83–93 (2016).
Acknowledgements
We would like to thank Drs. Don-Kyu Kim (Chonnam National University, Gwangju, Republic of Korea) and Hye-Young Seo (Keimyung University School of Medicine, Daegu, Republic of Korea) for excellent technical suggestions and helpful discussions. This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government Ministry of Science and ICT (MSIT) (NRF-2021R1A2C1008768 to Y.D.K.).
Author information
Authors and Affiliations
Contributions
J.R.J. and S.A. contributed to the design of study, performance of experiments, interpretation of data, and writing the draft; and S.G. and B.N. contributed to the analysis and interpretation of data, and critical review the manuscript; Y.D.K. contributed to the conception and design of the experiments, drafting of the manuscript, and critical review the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jo, JR., An, S., Ghosh, S. et al. Growth hormone promotes hepatic gluconeogenesis by enhancing BTG2–YY1 signaling pathway. Sci Rep 11, 18999 (2021). https://doi.org/10.1038/s41598-021-98537-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98537-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.