Abstract
Gene editing methods are an attractive therapeutic option for Duchenne muscular dystrophy, and they have an immediate application in the generation of research models. To generate myoblast cultures that could be useful in in vitro drug screening, we have optimised a CRISPR/Cas9 gene edition protocol. We have successfully used it in wild type immortalised myoblasts to delete exon 52 of the dystrophin gene, modelling a common Duchenne muscular dystrophy mutation; and in patient’s immortalised cultures we have deleted an inhibitory microRNA target region of the utrophin UTR, leading to utrophin upregulation. We have characterised these cultures by demonstrating, respectively, inhibition of dystrophin expression and overexpression of utrophin, and evaluating the expression of myogenic factors (Myf5 and MyH3) and components of the dystrophin associated glycoprotein complex (α-sarcoglycan and β-dystroglycan). To demonstrate their use in the assessment of DMD treatments, we have performed exon skipping on the DMDΔ52-Model and have used the unedited DMD cultures/ DMD-UTRN-Model combo to assess utrophin overexpression after drug treatment. While the practical use of DMDΔ52-Model is limited to the validation to our gene editing protocol, DMD-UTRN-Model presents a possible therapeutic gene edition target as well as a useful positive control in the screening of utrophin overexpression drugs.
Similar content being viewed by others
Introduction
Duchenne muscular dystrophy (DMD) is a fatal X-linked recessive disease affecting 1 out of 5.000 newborn males. It is commonly caused by deletions disrupting the open reading frame of the DMD gene causing a lack of dystrophin protein1. Lack of dystrophin in DMD patients’ muscles leads to progressive muscle wasting and degeneration. Patients carrying out of frame mutations present a severe DMD phenotype, while those carrying in-frame mutations, such as in Becker muscular dystrophy (BMD)2, may produce a partially functional dystrophin and present milder phenotypes. Dystrophin plays a major role in membrane stabilization during muscle contraction, linking the actin cytoskeleton to the sarcolemma3 and also contributes to extracellular signalling4.
Although no definitive cure for DMD is available, a handful of drugs have been recently approved by different regulatory agencies: ataluren induces readthrough of premature stop codons during mRNA translation, generating a full length dystrophin protein5; while antisense oligonucleotide drugs (eteplirsen, golodirsen, viltolarsen and casimersen), produce a shorter but functional protein by restoring the DMD reading frame modulating splicing via exon skipping6,7. All approved drugs are mutation specific and designed to rescue specific patient mutations only present, respectively, in 13% (ataluren), 13% (eteplirsen) and 8% of DMD patients8 (golodirsen, viltolarsen and casimersen). It is therefore important to assess exon-skipping strategies targeting other DMD exons9 and therapies that may benefit all DMD and BMD patients, independent of their mutations. One such potential therapy is gene transfer: several trials are ongoing testing different drugs (SGT-001, SRP-9001 or PF-06939926) that include mini or micro-dystrophins in adeno-associated viruses driven by different promoters. Early positive safety and tolerability data in clinical trials10 suggests the potential of this therapy to provide clinically meaningful functional improvement in DMD patients11. Likewise, different stem cell-based strategies aim to replenish the muscle stem cell pool with dystrophin-competent cells as a potential therapy for DMD: while a clinical trial using HLA-matched donor mesoangioblasts failed to show any functional improvements12, recent preclinical studies are focused on autologous transplantation of corrected stem cells13,14. An additional limitation of many of these therapies (both approved and in development) is their extremely high costs, which will limit the access of many patients to these drugs.
As a complement to these therapies aiming to restore dystrophin expression, many compounds targeting secondary effects of dystrophin deficiency or looking for alternatives to dystrophin are also under evaluation.
Utrophin (UTRN) is an autosomal paralog of dystrophin, expressed in skeletal muscle cells during embryonic development, but restricted to neuromuscular and myotendinous junctions in the mature muscle fibre15. Overexpression of utrophin in skeletal muscle in DMD animal models can partially compensate the lack of dystrophin and improve DMD phenotype16,17,18. Importantly, ectopic and high levels of utrophin in myoblasts are not associated with toxicity, making utrophin upregulation an interesting therapeutic strategy applicable to all patients, regardless of their specific mutation19,20. Ezutromid/SMT-C1100 was the first utrophin modulator evaluated in clinical assays but was recently abandoned due to lack of evidence of utrophin restoration, nor clinical improvement demonstrated in patients21,22. Alternatively, other studies proposed new strategies to upregulate UTRN by blocking the inhibitory target region of microRNAs repressing UTRN expression23,24,25. Recently, utrophin upregulation has been efficiently achieved using gene therapy in multiple animal models with non-immunogenic side effects26. Several new compounds that aim to overexpress utrophin are currently being developed27,28,29, and this preclinical development could benefit from a gold standard or an adequate positive control to use in these assays.
In vitro cellular models are particularly useful to assess the efficiency of novel therapies for DMD. However, only a few human immortalized muscle cell lines derived from DMD patients are currently available30. Due to the wide spectrum of DMD mutations and the difficulties to obtain DMD patient muscle biopsies, DMD-myoblasts models would provide a powerful resource for in vitro drug screening and study disease rescue mechanisms. CRISPR/Cas9 currently represents a very efficient and versatile genome-engineering tool, introducing small and large DNA modifications in different cell types and organisms31. In the presence of two single guide (sg) RNAs targeting two different loci on the same chromosome, Cas9 can induce two DNA double strand breaks (DDSBs), leading in some cases to deletion of the excised DNA segment through repair by the non-homologous end joining (NHEJ) pathway32. Like antisense oligo-mediated exon skipping therapies at RNA level, CRISPR/Cas9 can therefore be used to remove mutations by deleting mutated exons and restore the open reading frame of the DMD gene33,34,35. The advantage of this approach is that the genetic modification, once introduced, is stable over cell cycles. CRISPR/Cas9 has been successfully employed to correct mutations and/or restore the open reading frame recovering dystrophin expression both in vitro and in vivo36,37. However, some hurdles have been reported, such as difficulties transfecting myoblast or a recent study in the golden retriever muscular dystrophy dog (GRMD), where no dystrophin restoration at protein level was evident after gene editing using this technology38. More importantly, as well as these preclinical problems others such as possible off-target problems or immunogenicity linked with the use of Cas939 may delay their clinical application. However, while gene editing as therapeutic option still needs further development, CRISPR/Cas9 methodology has been applied to provide a large number of new animal models to further understand DMD pathology and perform preclinical studies40.
In our quest to optimise the preclinical development of new therapies for DMD, we have developed a genome editing strategy applicable in control and patient myoblasts. Here, we report two new cell cultured models that can be successfully used for preclinical assessment of new DMD therapies: a culture that replicates a patient’s deletion (DMDΔ52-Model) and another that overexpresses utrophin (DMD-UTRN-Model).
Results
Generation of cell culture models by gene editing
We completed two different gene editing projects: objective 1 aimed to delete exon 52 of the DMD gene (a common mutation in DMD patients) in control immortalised human myoblasts to generate a disease model (DMDΔ52-Model); objective 2 (DMD-UTRN-Model) was to delete in the UTRN gene of DMD immortalized human myoblasts an inhibitory microRNA target region to generate a utrophin ectopic expression rescue model.
Each edition required to design two sgRNAs flanking the region to be deleted to generate two DDSBs leading to the removal of that region (Fig. 1A,D). All the 25 different combinations of the sgRNAs designed, cutting before (× 5) and after (× 5) the target region, were tested in HEK293 cultures first (Supplementary figure 1A and B). The most efficient combination of sgRNAs in HEK293 cells for each objective was selected to be used in the transfection of human immortalized myoblasts (Supplementary figure 1C and D).
Editing approach and genotyping DMD and UTRN deletion breakpoints in edited myoblast clones. (A, D) Schematic representation of our strategy for editing the DMD (A) and the UTRN loci (D). A pair of flanking sgRNAs were co-transfected in order to delete DMD exon 52 (A) or the inhibitory microRNA target region contained in the 5′UTR of UTRN (D). (B,C) PCR genotyping of DMD edited clones (B) and Sanger sequencing of clone 7 or DMD∆52-Model (C). (E,F) PCR genotyping of UTRN edited clones (E) and Sanger sequencing of clone 8 or DMD-UTRN-Model (F). Larger products in agarose gels (B,E) indicate non-edited clones, and shorter ones correspond with the expected deletion. (C,F) Sequences of the smaller bands confirmed the expected gene editing for objective 1: DMD∆52-Model (C) and objective 2: DMD-UTRN-Model (F).
To accomplish objective 1, two GFP-plasmids, each encoding Cas9 nuclease and either sgRNA 2 or sgRNA 6, were transfected into human immortalized control myoblasts. For objective 2, the selected GFP-plasmids, encoded Cas9 nuclease and either sgRNA 22 or sgRNA 26 and were transfected into human immortalized DMD myoblasts. After FACS sorting of individual GFP-positive cells, clones were expanded for DNA extraction (Supplementary figure 2). Clones were analysed to confirm the presence of the desired deletions by genomic PCR performed with specific primers for each targeted gene (Fig. 1B,E), amplicons corresponding in size with the expected deletions were analysed by Sanger sequencing, and the expected deletions were confirmed in all the positive clones (Fig. 1C,F).
For objective 1 two positive clones were obtained, clone number 2 and 7 but only clone 7 was used for further analysis and called DMDΔ52-Model (Fig. 1B,C). For objective 2, two clones were edited in only one allele, corresponding to numbers 3 and 4 and other two were completely edited, numbers 2 and 8. In this case, clone number 8 was selected to be used for further analysis and was called DMD-UTRN-Model (Fig. 1E,F).
To evaluate any potential off-target effects, each selected sgRNA was analysed in silico using the bioinformatics web-tool CRISPOR41. We selected the six most likely off-target sites for each sgRNA and analysed each one of them in edited clones through PCR followed by Sanger sequencing. We found no off-target effects in any of the 12 sites studied for each clone sites (Supplementary figure 3).
Analysis of dystrophin and utrophin expression in edited clones
We compared dystrophin expression in myotubes of the DMD∆52-Model to that of controls and DMD cultures and confirmed that it was abolished by immunocytochemistry (Fig. 2A), western blot (Fig. 2B), myoblot (Fig. 2C) and droplet digital PCR (ddPCR) (Fig. 2D). Dystrophin levels in this model, where exon 52 had been removed by CRISPR/Cas9 editing, were statistically no different than those seen in a culture from a DMD patient.
Characterization of DMD∆52-Model cultures. Dystrophin expression in DMD∆52-Model cultures compared to control myotubes and DMD myotubes were studied by immunocytochemistry (A), western blotting (full-length blots are presented in Supplementary Figure 5) (B) and myoblots. (C) Myoblot experiments, where n = 24 wells per cell type were compared, were performed twice. (D) Dystrophin expression in DMD∆52-Model cultures was compared to control myotubes by ddPCR. For ddPCR experiments three technical replicates per sample and condition were run in parallel and a no template control (NTC) was included as negative control. α-sarcoglycan (E) and β-dystroglycan (F) expression was studied in control myotubes compared to DMD∆52-Model myotubes by myoblot, where n = 10 and n = 20 wells per cell type were compared respectively. (G) Differentiated myotubes of DMD∆52-Model and control cultures were inmunostained with MF20 and Hoechst antibodies. Fusion index was calculated as the ratio between the number of nuclei in differentiated myotubes (defined as > 2 nuclei and MF20-positive cells) compared to the total number of nuclei. For quantification, five fields per cell line were randomly chosen and more than 200 nuclei were counted. Analysis was performed using ImageJ software. (H) Differentiation markers, Myf5 and MyH3, were studied by ddPCR at different fusion times in DMD∆52-Model cultures compared to control myotubes. For ddPCR experiments three technical replicates per sample and condition were run in parallel and a no template control (NTC) was included as negative control. (*p value < 0.05, **p value < 0.01, ****p value < 0.0001). (p values were determined with Mann–Whitney U test and error bars represent mean ± SEM).
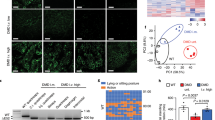
Immunocytochemistry showed the increase of utrophin expression between unedited DMD and DMD-UTRN-Model myotubes (Fig. 3A) and this increase was corroborated by western blot (a 195% increase, Fig. 3B), myoblot analysis (close to 50% increase, Fig. 3C) and ddPCR (a 148% increase, Fig. 3D). We also quantified dystrophin and utrophin expression in the edited cultures by myoblot during their differentiation process and compared these with control and DMD myotubes (Supplementary figure 4, bar chart).
Characterization of DMD-UTRN-Model cultures. Utrophin expression in DMD myotubes compared to DMD-UTRN-Model studied by immunocytochemistry (A), western blotting (full-length blots are presented in Supplementary Figure 5) (B) and myoblots (C). Myoblot experiments, where n = 48 wells per cell type were compared, were performed twice. (D) Utrophin expression in DMD-UTRN-Model cultures was compared to DMD myotubes by ddPCR. For ddPCR experiments three technical replicates per sample and condition were run in parallel and a no template control (NTC) was included as negative control. α-sarcoglycan (E) and β-dystroglycan (F) expression was studied in DMD myotubes compared to DMD-UTRN-Model myotubes by myoblot, where n = 10 and n = 20 wells per cell type were compared respectively. (G) Differentiated myotubes of DMD-UTRN-Model and DMD cultures were inmunostained for MF20 and Hoechst. Fusion index was calculated as the ratio between the number of nuclei in differentiated myotubes (defined as > 2 nuclei and MF20-positive cells) compared to the total number of nuclei. For quantification, five fields per cell line were randomly chosen and more than 200 nuclei were counted. Analysis was performed using ImageJ software. (H) Differentiation markers, Myf5 and MyH3, were studied by ddPCR at different fusion times in DMD-UTRN-Model cultures compared to DMD myotubes. For ddPCR experiments three technical replicates per sample and condition were run in parallel and a no template control (NTC) was included as negative control. (*p value < 0.05, **p value < 0.01, ****p value < 0.0001). (p values were determined with Mann–Whitney U test and error bars represent mean ± SEM).
To characterise further the edited models, we studied the expression of two members of dystrophin/utrophin glycoprotein complex: α-sarcoglycan and β-dystroglycan. Myoblot analysis showed that in the DMD∆52-Model, expression of both α-sarcoglycan and β-dystroglycan was significantly lower compared to control myotubes, suggesting a possible disruption of the dystrophin associated protein complex (Fig. 2E,F). On the other hand, there were no significant differences between α-sarcoglycan and β-dystroglycan expression between the DMD-UTRN-Model and DMD myotubes although both proteins seem to be slightly increased in the DMD-UTRN-Model (Fig. 3E,F).
Analysis of differentiation markers expression in edited clones
As we suspected that the editing and cloning process could have affected the differentiation of the edited models, the fusion index (%) of edited and non-edited myotubes after MF20 and Hoechst inmunocytochemistry was calculated (Figs. 2G and 3G) and the expression of different myogenic regulatory factors like the myogenic factor 5 (Myf5) and the myosin heavy chain isoform 3 (MyH3) were analysed at different time points during myotube formation in DMD∆52-Model and DMD-UTRN-Model as well as in their corresponding unedited cultures by ddPCR (Figs. 2H and 3H). Fusion index was clearly lower in DMD∆52-Model compared to control myoblasts (Fig. 2G) while no significant differences were found between DMD-UTRN-Model and DMD cultures (Fig. 3G). As expected, in both models during the differentiation process Myf5 expression decreased while MyH3 increased and followed the same pattern in their original cells. However, we observed that the MyH3 marker is significantly lower in the edited models at days 5 and 7 after initiating the differentiation process (Figs. 2H and 3H).
The MF20 differentiation marker was also analysed by myoblot in edited cultures and we could observe a decrease in both edited clones, no matter the deletion, compared with their corresponding controls (Supplementary figure 4, red lines).
Evaluation of therapies in newly generated model cell lines
To assess if the DMD∆52-Model could be useful to test potential mutation specific therapies for DMD, we evaluated the exon skipping efficiency of an antisense oligonucleotide in this culture. We treated DMD∆52-Model cultures with an antisense oligonucleotide drug that can skip exon 5142 and restore the open reading frame of DMD. After treatment with this drug, we confirmed that exon skipping had taken place at RNA level (Fig. 4A), and a limited restoration of dystrophin expression by myoblot analysis (Fig. 4B).
Evaluation of potential therapies in the generated cell culture models. (A) DMD∆52-Model cell line was treated with an antisense to skip exon 51 and the effect was evaluated by RT-PCR and nested PCR analysis. Gel picture shows a pattern corresponding with the correct skipping, which was confirmed by Sanger sequencing. The same experiment evaluated by myoblot using n = 20 replicate wells per condition (B) showed the restoration of dystrophin expression in the treated cultures compared to non-treated (*p values < 0.05. p values were determined with Mann–Whitney U test). (C) The DMD-UTRN-Model was used as a positive control in an experiment in which unedited DMD cultures were treated with different ezutromid concentrations to up-regulate utrophin expression. Myoblot analysis using n = 8 replicate wells per condition of the treated cultures shows that ezutromid had no significant effect in DMD cultures while utrophin expression is significantly increased in DMD-UTRN-Model compared to unedited DMD cultures. (**p values < 0.01. P values were determined with Mann–Whitney U test and error bars represent mean ± SEM).
To test our DMD-UTRN-Model as a possible utrophin overexpression control, we cultured it alongside the original unedited DMD cultures, which we treated with several concentrations of ezutromid and we evaluated the expression of utrophin in all cultures. We observed that utrophin was hardly modified in DMD cultures treated with ezutromid while a robust overexpression was confirmed in the DMD-UTRN-Model compared to the unedited DMD cultures (Fig. 4C). We also confirmed that this overexpression was stable during a time course experiment (Supplementary figure 4D).
Discussion
CRISPR/Cas9 may in the future be a potential treatment for Duchenne muscular dystrophy and several studies have shown efficacy in mice models43,44,45, an most recently in dogs, which is currently the most advanced example of its application to DMD35. However, this methodology is already very useful for researchers looking for cell culture models: in the case of many neuromuscular disorders muscle biopsies are not routinely collected during diagnosis and seldom cultured. This means there are few good culture models of the disease and even fewer of specific mutations. Creating such cultures modifying existing ones is a practical way of addressing this problem. If this is combined with the use of immortalised cultures as templates30, it increases the possibility of performing more experiments with a given culture. We have used this approach in this manuscript.
Although we are concerned about the efficiency limitations of our gene editing protocol, specially due to transfection difficulties in myoblasts reported also by other laboratories33,46; we have successfully applied it to edit 2 different regions in two different cell backgrounds (Control and DMD), and we consider that those described and fully characterised in this manuscript could be relevant research models that we would be happy to share. The first of these models is an immortalised DMD disease cell culture model, (DMD∆52-Model) that lacks exon 52 of the DMD gene, which disrupts the ORF and dystrophin expression. This model could be useful to evaluate mutation-independent drug treatments, and also exon skipping drugs that aim to skip exons 51 or 5347,48 as skipping either exon in this case would restore the ORF and dystrophin expression. We have demonstrated that DMD∆52-Model lacks dystrophin expression and that this can be reverted through treatment with an exon 51 skipping drug. However, the main interest of this model was demonstrating the efficacy of the technique, as immortalised cultures from patients with the same deletion are currently available. We have replicated this protocol to generate other DMD-like cultures (details will be included in a manuscript being currently drafted).
An immortalised cell culture model constitutively expressing utrophin, DMD-UTRN-Model, is both a proof of principle of a possible therapeutic option to overexpress utrophin as a substitute for dystrophin, and a valuable research tool. After the recent failure of ezutromid in clinical trials, the search of drugs that could overexpress utrophin, including drug re-purposing, is ongoing49,50. However, there are no reliable positive controls that could be used to compare such treatments. We propose that our cell model could serve that purpose, offering researchers useful custom controls for their studies. We have tested this hypothesis and compared the stable utrophin overexpression quantified in the DMD-UTRN-Model with the utrophin that is expressed after treatment of the unedited DMD patient cultures with ezutromid, the lead market candidate in this field until very recently, with positive results. Previous studies in muscle sections show that DMD patients already overexpress utrophin, in many cases 4 to 5-fold the levels seen in control muscle sections51. Our choice to target this particular UTR region, increases basal overexpression in DMD cultures, and the amount of overexpression varies significantly when evaluated by western blot (close to 2 times) or our preferred method, myoblots (close to 1.5 times). We like to consider that myoblot evaluation reflects more closely the actual protein expression, as it is not subjected to many of the inherent problems of western blotting when evaluating very large proteins and we are able to include many more replicates52. This is why we cannot comment yet on the differences in expression between our study and other published studies that also aimed to overexpress utrophin by gene editing, but which 1) targeted different promoter regions (UTRN A or UTRN B) of utrophin and 2) evaluated their results by western blot analysis46. We would be interested on studying this matter further to analyse the differences in utrophin expression when targeting different regions.
We created this model exclusively for in vitro screening and not as a model for cell therapy. Before a similar approach to ours could be considered a viable one; a more extensive characterisation would be required, as preliminary study of the generated models revealed that some myogenic regulatory factors were affected after gene editing no matter the deletion, for instance, MyH3 expression was significantly decreased in both edited cultures. These findings could be related with changes in the secretory phenotype after single cell sorting in edited models, as it has been shown that myoblasts microenvironment in vitro can affect to cell proliferation and differentiation. In particular, some studies reported autocrine factors like transforming growth factor β (TGF-β) that can inhibit myogenic differentiation53,54. Nevertheless, further research is needed to confirm this hypothesis and this is outside the scope of this project.
As a conclusion, after optimization of a gene editing protocol for its application to edit myoblasts (a rather difficult target), we have created two new cell culture models that we have used as tools in our search for new therapies for DMD. We expect our protocol to be useful to other muscle researchers and we are looking forward expanding the use of the DMD-UTRN-Model in the screening of new treatments for DMD.
Methods
CRISPR/Cas9 tools
Specific sgRNA guides were designed using the online bioinformatics tool http://crispr.mit.edu55. Ten different guides (five before and five after the target region) were designed targeting exon 52 flanking regions in DMD gene and another ten targeting a repressor binding site in the UTR 3′ region of UTRN gene and selected according to their score number (Table 1). They were cloned into a plasmid containing Cas9 from S. pyogenes with 2A-EGFP pSpCas9 (BB)-2A-GFP (PX458) (Addgene plasmid # 48138, deposited by Feng Zhang). All sgRNAs were cloned using BbsI sites.
Cell cultures
Human embryonic kidney 293 (HEK 293) cells, used in the preliminary selection of the best sgRNA combinations for our experiments, were purchased from the European Collection of Authenticated Cell cultures (ECCAC) via Sigma-Aldrich, Spain, and maintained following the manufacturer’s protocols.
Immortalized myoblasts derived from muscle biopsies from healthy controls and DMD patients were provided by the CNMD Biobank, London, UK and the Institut de Myologie Paris, France. Myoblast were cultured using skeletal muscle medium (SMM) (Promocell, Germany) and seeded on Matrigel coated plates. After reaching 80% confluency, cells were transduced with MyoD adenoviral particles (Applied Biological Materials Inc, Canada) and switched to differentiation medium (DMEM plus 2% horse serum and penicillin– streptomycin) to facilitate myotube formation.
Cell culture transfection with gene edition tools
All different sgRNAs combinations were transfected into HEK 293 cells using lipofectamine 2000® (Thermo Fisher Scientific), according to the manufacturer’s protocol. Myoblasts seeded in 6 well plates at 70–80% confluence were transfected with 1.5ug of each plasmid with the most efficient guide RNA combination using ViaFect™ (Promega) transfection reagent (1:5 ratio).
FACS selection of GFP-positive myoblasts
48 h after transfection, myoblasts were trypsinised and collected for fluorescence activated cell sorting (FACS) at the Cell Analytics Facility (BD FACS Jazz) Achucarro Basque Center for Neuroscience (Leioa, Spain). GFP-positive cells were seeded individually in 96 well plates for clonal selection. The first colonies were visible around 7 days post-sorting. Clones were expanded from single cell to near-confluence and expanded into larger well plates to be harvested 15–30 days post-sorting. Myoblasts often developed elongated and stressed shapes during this clonal expansion after single cell sorting. Harvested cultures were aliquoted: some aliquots were frozen for archival; others were pelleted for DNA analysis, while replicates were cultured further for characterization by immunocytochemistry, western blot and myoblots (see schematic workflow in Supplementary figure 2).
Analysis of gene editing
DNA was extracted from cell pellets using a QIAamp® DNA Mini Kit, Qiagen. PCR amplification targeting the edited regions was carried out using Taq DNA Polymerase (Recombinant), Invitrogen, under the following conditions: preheating 3′ 94 °C, 25 cycles of 94° for 3′, 94° 20″, 63° 20″, 72° 1′ and 72° 5′ and DMD-Seq-D52-DOWN-F2 and DMD-Seq-D52-DOWN-R2 primers (see Supplementary Table 1). PCR products were resolved in 2% TAE-agarose gels and purified with QIAquick® Gel extraction Kit, Qiagen. PCR amplicons corresponding to the expected length were analysed by Sanger sequencing at the sequencing platform of Biocruces Bizkaia Health Research Institute using DMD-Seq-D52-DOWN-F2 and DMD-Seq-D52-DOWN-R2 primers (see Supplementary Table 1).
Off-target analysis of mutations in clonal lines
Potential off-target region loci of each sgRNA used were predicted using CRISPOR bioinformatics tool http://crispor.tefor.net/. The six most probable off-target sequences per guide (Tables 2 and 3) were analysed in the edited clones using genomic PCR and Sanger sequencing. Primer sets flanking off-target sites and the corresponding internal primers used for Sanger sequencing are listed in Supplementary Table 2.
Primary antibodies
Anti-dystrophin: Dys1 (Leica Biosystems), Mandys1, Mandys106 (The MDA Monoclonal Antibody Resource) and anti-dystrophin antibody (Abcam 15277).
Anti-utrophin: Mancho7 (The MDA Monoclonal Antibody Resource).
Anti-myosin heavy chain (MF20: Developmental Studies Hybridoma Bank).
Anti α-sarcoglycan: NCL-L-a_SARC (Leica Biosystems).
Anti β-dystroglycan: NCL-b-DG (Leica Biosystems).
Immunostaining assays
Original and edited clones for objectives 1 (DMD∆52-Model) and 2 (DMD-UTRN-Model) were cultured and immunostained for dystrophin or utrophin expression. Edited clones were seeded into chamber slides and treated with a MyoD virus, (Applied Biological Materials Inc, Canada) to facilitate differentiation into myotubes56. After seven days differentiating, samples were fixed with 4% PFA. Cultures were permeabilised with Triton 0.5% and then blocked for half an hour with BSA 2%. Afterwards, immunostaining was performed overnight at 4 °C with the required antibodies. Primary antibodies used for dystrophin staining were a mix of Dys1, Mandys1 and Mandys106 at 1:100 dilution and for utrophin staining was Mancho 7 diluted at 1:50. The following day, after being washed with PBS Tween 0.1%, cells were stained with Alexa Fluor 488 goat anti-mouse (Invitrogen) for 1 h at room temperature. Hoechst 1/2000 was used for nuclei staining and chamber slides were mounted with PermaFluor™ Aqueous Mounting Medium (Thermoscientific). Images were captured with a LEICA DMI 6000B microscope at the Microscopy Platform at Biocruces Bizkaia Health Research Institute.
In-cell western assay (myoblot)
Myoblots were performed as described before52. In short, clones were seeded in 96-well plates and incubated for 24 h in SMMC, after which they were treated with MyoD virus and incubated in differentiation media for 7 days. Then, plates were fixed with ice-cold methanol, permeabilised and blocked before incubation with the required primary antibodies overnight: anti-dystrophin mix (Dys1, Mandys1 and Mandys106 at 1:100), anti-utrophin (Mancho 7 antibody at 1:50), anti α-sarcoglycan (NCL-L-a_SARC at 1:10), anti β-dystroglycan (NCL-b-DG at 1:20) and anti-myosin heavy chain antibody (MF20 at 1:100) that was used to evaluate differentiation. Next day, plates were incubated with the secondary antibodies. Biotin-mediated amplification (Abcam 6788 goat antimouse IgG biotin 1:2000) was used to increase dystrophin signal. Secondary antibodies, IRDye 800cw streptavidin 1:2000 and IRDye 800CW goat anti-mouse 1:500, were prepared together with CellTag 700 Stain (LI-COR® Biosciences) at 1:1000 dilution and incubated for 1 h at RT and protected from light. After incubation, plates were analysed using the Odyssey® CLx Imager (LI-COR® Biosciences).
Treatment with antisense exon skipping drugs
Cultures in 96 wells and P6 wells were treated with a 2′MOE-phosphorotioate antisense oligonucleotide (AO) aiming to skip DMD exon 51 (5′-[ T*C*A*A*G*G*A*A*G*A*T*G*G*C*A*T*T*T*C*T]-3′, Eurogentec, Belgium) by transfection with Lipofectamine as described in47,48 and analysed by either myoblot (96 well plates) or RT-PCR (pellets extracted from 6 well plates).
RT-PCR
RNA was extracted from cell pellets (RNeasy mini kit, Qiagen) according to the manufacturer’s protocol. Reverse transcription of the samples was performed using (SuperScrip™ IV Reverse Transcriptase, Invitrogen) according to the manufacturer’s protocol. cDNA samples were either used for digital droplet PCR analysis or amplified by nested PCR using specific primers sets (Supplementary Table 1) and Taq DNA Polymerase (Recombinant), Invitrogen, as described in48 for exon skipping analysis. Amplified samples were resolved in TAE-agarose and PCR amplicons of interest were first analysed with Gel Doc TM EZ Imager, BIORAD and then purified with (QIAquick® Gel extraction Kit, QIAGEN) for sequencing analysis. Before DNA extractions bands were semi-quantified using Image J.
Treatment with utrophin overexpression drugs
Ezutromid was diluted first in DMSO and finally in differentiation medium to different concentrations and added to myoblasts in 96 well plates 7 days after differentiation. Twenty-four hours after treatment, medium was removed, and plates were fixed with ice-cold methanol for myoblot analysis.
Western blot
Cell cultures were seeded into 6 well plates and trypsinized after 7 days of differentiation. Then, cell pellets were solubilized in lysis/loading buffer and denatured at 95 °C for 5 min. Samples were loaded onto a NuPAGE® Novex® 3–8% Tris–Acetate Gel3–8% (Thermo Fisher Scientific) and run in Novex Tris–Acetate SDS Running Buffer (Thermo Fisher Scientific) for 60 min at 70 V + 120 min at 150 V at 4 °C. Protein wet transfer was performed overnight at 4 °C using an Immobilon®-FL PVDF membrane (Merck™). Next day, membranes were stained with Revert ™ 700 Total Protein Stain (Li-Cor) for total protein measurement, blocked with Intercept (PBS) Blocking Buffer (Li-Cor) for 2 h and incubated overnight at 4 °C with the primary antibodies (1/200 anti-dystrophin antibody Abcam15277 or 1/50 anti-utrophin antibody Mancho 7). After washing steps with PBS-Tween 0.1%, membranes were incubated with secondary antibodies for 1 h (1/5000 IRDye 800CW goat anti-rabbit 926-32211 or IRDye 800CW goat anti-mouse 926-32210, Li-Cor) at room temperature, washed again with PBS-Tween 0.1% and scanned using an Odyssey Clx imaging system. Quantification of bands was performed using Image Studio ™ software.
Droplet digital PCR (ddPCR)
Gene expression was detected and quantified using a QX200™ Droplet Digital™ PCR system (Bio-Rad). The reaction was performed using 2 μl of cDNA in a 20 μl reaction volume containing: 1 μl of Utrophin Taqman probe (ID: Hs01125975, FAM labelled), 1 μl of MYF5 ddPCR™ GEX Assay probe (ID: dHsaCPE5026295, HEX labelled) or MYH3 Taqman probe (ID: Hs01074230, VIC labelled), 10 μl of ddPCR™ Supermix for Probes (no dUTP) (Bio-Rad) and 6 μl of DNase/RNase-free H2O.
To generate the droplets, 20 μL of the previous ddPCR reaction and 70 μL of Droplet Generation Oil for Probes (Bio-Rad) were added to the 8-channel droplet generation cartridge according to manufacturer instructions and this cartridge was placed in the QX200 droplet generator (Bio-Rad). Then, 40 μL of the resulting droplet emulsion was transferred to a semi-skirted 96 well PCR plate (Eppendorf), sealed with foil and amplified on a thermal cycler using the following amplification conditions: enzyme activation 10′, 40 cycles of 94 °C for 30″ and 55 °C for 1′, and heat deactivation 10′ 98 °C.
Plates containing the amplified droplets were loaded into the QX200 droplet reader and results were analysed using QuantaSoft software™ (Bio-Rad).
Statistical analysis
Mann–Whitney U test was used throughout this study to calculate P values for determination of statistical significance (*p value < 0.05, **p value < 0.01, ****p value < 0.0001). Statistical analysis was performed using GraphPad Prism software.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. The raw data that support the findings of this study are available from the corresponding author, V A-G, upon reasonable request.
References
Hoffman, E. P., Brown, R. H. Jr. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928. https://doi.org/10.1016/0092-8674(87)90579-4 (1987).
Anthony, K. et al. Dystrophin quantification and clinical correlations in Becker muscular dystrophy: Implications for clinical trials. Brain 134, 3547–3559. https://doi.org/10.1093/brain/awr291 (2011).
Muntoni, F., Torelli, S. & Ferlini, A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2, 731–740. https://doi.org/10.1016/s1474-4422(03)00585-4 (2003).
Lai, Y. et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 119, 624–635. https://doi.org/10.1172/JCI36612 (2009).
Finkel, R. S. Read-through strategies for suppression of nonsense mutations in Duchenne/ Becker muscular dystrophy: aminoglycosides and ataluren (PTC124). J. Child. Neurol. 25, 1158–1164. https://doi.org/10.1177/0883073810371129 (2010).
Kinali, M. et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 8, 918–928. https://doi.org/10.1016/S1474-4422(09)70211-X (2009).
Muntoni, F. et al. Golodirsen induces exon skipping leading to sarcolemmal dystrophin expression in duchenne muscular dystrophy patients with mutations amenable to exon 53 skipping (S22.001). Neurology 90 (2018).
Aartsma-Rus, A., Van Deutekom, J. C., Fokkema, I. F., Van Ommen, G. J. & Den Dunnen, J. T. Entries in the Leiden Duchenne muscular dystrophy mutation database: An overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144. https://doi.org/10.1002/mus.20586 (2006).
Arechavala-Gomeza, V., Anthony, K., Morgan, J. & Muntoni, F. Antisense oligonucleotide-mediated exon skipping for Duchenne muscular dystrophy: Progress and challenges. Curr. Gene Ther. 12, 152–160. https://doi.org/10.2174/156652312800840621 (2012).
Mendell, J. R. et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: A nonrandomized controlled trial. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1484 (2020).
Duan, D. Micro-dystrophin gene therapy goes systemic in duchenne muscular dystrophy patients. Hum. Gene Ther. 29, 733–736. https://doi.org/10.1089/hum.2018.012 (2018).
Cossu, G. et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 7, 1513–1528. https://doi.org/10.15252/emmm.201505636 (2015).
Sun, C., Serra, C., Lee, G. & Wagner, K. R. Stem cell-based therapies for Duchenne muscular dystrophy. Exp. Neurol. 323, 113086. https://doi.org/10.1016/j.expneurol.2019.113086 (2020).
Meng, J. et al. Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne Muscular Dystrophy. Sci. Rep. 6, 19750. https://doi.org/10.1038/srep19750 (2016).
Tinsley, J. M. et al. Primary structure of dystrophin-related protein. Nature 360, 591–593. https://doi.org/10.1038/360591a0 (1992).
Tinsley, J. et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 4, 1441–1444 (1998).
Cerletti, M. et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 10, 750–757. https://doi.org/10.1038/sj.gt.3301941 (2003).
Tinsley, J. M. et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384, 349–353. https://doi.org/10.1038/384349a0 (1996).
Fisher, R. et al. Non-toxic ubiquitous over-expression of utrophin in the mdx mouse. Neuromuscul. Disord. 11, 713–721 (2001).
Fairclough, R. J., Wood, M. J. & Davies, K. E. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat. Rev. Genet. 14, 373–378. https://doi.org/10.1038/nrg3460 (2013).
Tinsley, J., Robinson, N. & Davies, K. E. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to healthy male adult volunteers. J. Clin. Pharmacol. 55, 698–707. https://doi.org/10.1002/jcph.468 (2015).
Ricotti, V. et al. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to pediatric patients with duchenne muscular dystrophy. PLoS ONE 11, e0152840. https://doi.org/10.1371/journal.pone.0152840 (2016).
Mishra, M. K., Loro, E., Sengupta, K., Wilton, S. D. & Khurana, T. S. Functional improvement of dystrophic muscle by repression of utrophin: let-7c interaction. PLoS ONE 12, e0182676. https://doi.org/10.1371/journal.pone.0182676 (2017).
Sengupta, K. et al. Genome editing-mediated utrophin upregulation in Duchenne muscular dystrophy stem cells. mol Ther Nucleic Acids 22, 500–509. https://doi.org/10.1016/j.omtn.2020.08.031 (2020).
Sengupta, K., Loro, E. & Khurana, T. S. PMO-based let-7c site blocking oligonucleotide (SBO) mediated utrophin upregulation in mdx mice, a therapeutic approach for Duchenne muscular dystrophy (DMD). Sci. Rep. 10, 21492. https://doi.org/10.1038/s41598-020-76338-1 (2020).
Song, Y. et al. Non-immunogenic utrophin gene therapy for the treatment of muscular dystrophy animal models. Nat Med 25, 1505–1511. https://doi.org/10.1038/s41591-019-0594-0 (2019).
Ito, M., Ehara, Y., Li, J., Inada, K. & Ohno, K. Protein-anchoring therapy of Biglycan for Mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther 28, 428–436. https://doi.org/10.1089/hum.2015.088 (2017).
Pisani, C. et al. Utrophin up-regulation by artificial transcription factors induces muscle rescue and impacts the neuromuscular junction in mdx mice. Biochim Biophys Acta Mol Basis Dis 1172–1182, 2018. https://doi.org/10.1016/j.bbadis.2018.01.030 (1864).
Soblechero-Martin, P., Lopez-Martinez, A., de la Puente-Ovejero, L., Vallejo-Illarramendi, A. & Arechavala-Gomeza, V. Utrophin modulator drugs as potential therapies for Duchenne and Becker muscular dystrophies. Neuropathol Appl Neurobiol https://doi.org/10.1111/nan.12735 (2021).
Mamchaoui, K. et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle 1, 34. https://doi.org/10.1186/2044-5040-1-34 (2011).
Wright, A. V., Nunez, J. K. & Doudna, J. A. Biology and applications of CRISPR systems: Harnessing nature’s toolbox for genome engineering. Cell 164, 29–44. https://doi.org/10.1016/j.cell.2015.12.035 (2016).
He, Z. et al. Highly efficient targeted chromosome deletions using CRISPR/Cas9. Biotechnol. Bioeng. 112, 1060–1064. https://doi.org/10.1002/bit.25490 (2015).
Ousterout, D. G. et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun 6, 6244. https://doi.org/10.1038/ncomms7244 (2015).
Young, C. S. et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18, 533–540. https://doi.org/10.1016/j.stem.2016.01.021 (2016).
Amoasii, L. et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91. https://doi.org/10.1126/science.aau1549 (2018).
Min, Y. L. et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci. Adv. 5, eaav4324. https://doi.org/10.1126/sciadv.aav4324 (2019).
Duchene, B. L. et al. CRISPR-induced deletion with SaCas9 restores dystrophin expression in dystrophic models in vitro and in vivo. Mol Ther 26, 2604–2616. https://doi.org/10.1016/j.ymthe.2018.08.010 (2018).
Mata Lopez, S. et al. Challenges associated with homologous directed repair using CRISPR-Cas9 and TALEN to edit the DMD genetic mutation in canine Duchenne muscular dystrophy. PLoS ONE 15, e0228072. https://doi.org/10.1371/journal.pone.0228072 (2020).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med 25, 249–254. https://doi.org/10.1038/s41591-018-0326-x (2019).
Lim, K. R. Q., Nguyen, Q., Dzierlega, K., Huang, Y. & Yokota, T. CRISPR-generated animal models of duchenne muscular dystrophy. Genes https://doi.org/10.3390/genes11030342 (2020).
Haeussler, M. et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148–148. https://doi.org/10.1186/s13059-016-1012-2 (2016).
van Deutekom, J. C. et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 357, 2677–2686. https://doi.org/10.1056/NEJMoa073108 (2007).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407. https://doi.org/10.1126/science.aad5143 (2016).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411. https://doi.org/10.1126/science.aad5177 (2016).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403. https://doi.org/10.1126/science.aad5725 (2016).
Wojtal, D. et al. Spell checking nature: Versatility of CRISPR/Cas9 for developing treatments for inherited disorders. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2015.11.012 (2015).
Popplewell, L. J. et al. Comparative analysis of antisense oligonucleotide sequences targeting exon 53 of the human DMD gene: Implications for future clinical trials. Neuromuscul. Disord. 20, 102–110. https://doi.org/10.1016/j.nmd.2009.10.013 (2010).
Arechavala-Gomeza, V. et al. Comparative analysis of antisense oligonucleotide sequences for targeted skipping of exon 51 during dystrophin pre-mRNA splicing in human muscle. Hum. Gene Ther. 18, 798–810. https://doi.org/10.1089/hum.2006.061 (2007).
Guiraud, S. & Davies, K. E. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr. Opin. Pharmacol. 34, 36–48. https://doi.org/10.1016/j.coph.2017.04.002 (2017).
Loro, E. et al. High-throughput identification of post-transcriptional utrophin up-regulators for Duchenne muscle dystrophy (DMD) therapy. Sci. Rep. 10, 2132. https://doi.org/10.1038/s41598-020-58737-6 (2020).
Arechavala-Gomeza, V. et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol. Appl. Neurobiol. 36, 265–274. https://doi.org/10.1111/j.1365-2990.2009.01056.x (2010).
Ruiz-Del-Yerro, E., Garcia-Jimenez, I., Mamchaoui, K. & Arechavala-Gomeza, V. Myoblots: dystrophin quantification by in-cell western assay for a streamlined development of Duchenne muscular dystrophy (DMD) treatments. Neuropathol. Appl. Neurobiol. 44, 463–473. https://doi.org/10.1111/nan.12448 (2018).
Jiwlawat, N., Lynch, E., Jeffrey, J., Van Dyke, J. M. & Suzuki, M. Current progress and challenges for skeletal muscle differentiation from human pluripotent stem cells using transgene-free approaches. Stem Cells Int. 2018, 6241681. https://doi.org/10.1155/2018/6241681 (2018).
Melone, M. A., Peluso, G., Petillo, O., Galderisi, U. & Cotrufo, R. Defective growth in vitro of Duchenne Muscular Dystrophy myoblasts: The molecular and biochemical basis. J. Cell Biochem. 76, 118–132. https://doi.org/10.1002/(sici)1097-4644(20000101)76:1%3c118::aid-jcb12%3e3.3.co;2-6 (1999).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308. https://doi.org/10.1038/nprot.2013.143 (2013).
Roest, P. A. et al. Application of in vitro Myo-differentiation of non-muscle cells to enhance gene expression and facilitate analysis of muscle proteins. Neuromuscul Disord 6, 195–202. https://doi.org/10.1016/0960-8966(96)00006-5 (1996).
Acknowledgements
We acknowledge the use of cell cultures provided by the Queen Square Centre for Neuromuscular Disorders BioBank (CNMD Biobank, London, UK) and the Institut de Myologie (Paris, France, immortalised cultures). We gratefully acknowledge the use of the antibodies provided by Professor Glenn Morris from the Muscular Dystrophy Association (MDA) Monoclonal Antibody Resource, which distributes antibodies for research in neuromuscular diseases worldwide from Oswestry, United Kingdom. The MF20 antibody developed by D.A. Fischman, Weill Cornell Medical College, was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. Cell sorting experiments were performed at the Cell Analytics Facility at Achucarro- Basque Centre of Neuroscience (Leioa, Spain). We would like to thank Ms. Karmele Alapont-Celaya for her assistance in the completion of some laboratory tasks during this project. Drs. Gustavo Pérez-Nanclares and Ana Belén de la Hoz at the Genetics-Genomics Facility, Biocruces Bizkaia Health Research Institute, are also gratefully acknowledged for their help with sequencing reactions. Special thanks are due to Drs. Maria Dolores-García Vázquez at the Cell Culture Facility and Dr Javier Díez-García at the Microscopy Facility, both at Biocruces Bizkaia Health Research Institute, for their assistance at their corresponding platforms.
Funding
This work was supported by funding from Health Institute Carlos III (ISCIII, Spain) and the European Regional Development Fund, (ERDF/FEDER), ‘A way of making Europe’: Grant PI15/00333; Basque Government (grants 2016111029, 2018222035 and 2020333012) and Duchenne Parent Project Spain (grant 05/2016). P. S-M holds a Rio Hortega Fellowship from ISCIII (CM19/00104). V.A-G holds a Miguel Servet Fellowship from the ISCIII (CPII17/00004), part-funded by ERDF/FEDER. V.A-G also acknowledges funding from Ikerbasque (Basque Foundation for Science). A.L-M acknowledges funding by Biocruces Bizkaia Health Research Institute (BC/I/DIV/19/001). F. G is supported by a Ramon y Cajal Grant in Biomedicine (RYC-2014-16751) from the Ministry of Economy and Competitivity (MINECO), Spain. This work also acknowledges funding from the Research Unit of the Bilbao-Basurto Integrated Health Organisation (OSIBB).
Author information
Authors and Affiliations
Contributions
P.S.-M., formal analysis, investigation, data curation, writing or original draft, review and editing, visualization, supervision. E.A.-A., investigation, data curation, writing -review and editing. A.A.-M., investigation, writing—review and editing. L.d.-l.-P.-O., investigation, writing—review and editing. I.G.-J., methodology, investigation, review and editing, supervision. G.G.-I., investigation, writing—review and editing. I.L.-A., investigation, writing—review and editing. A.L.-M., investigation, writing—review and editing. J.P.-G., investigation, writing—review and editing. E.R.-D.-Y., methodology, investigation, writing-review and editing, supervision. F.G conceptualization, formal analysis, writing-review and editing. V.A.-G.: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing or original draft, review and editing, visualization, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soblechero-Martín, P., Albiasu-Arteta, E., Anton-Martinez, A. et al. Duchenne muscular dystrophy cell culture models created by CRISPR/Cas9 gene editing and their application in drug screening . Sci Rep 11, 18188 (2021). https://doi.org/10.1038/s41598-021-97730-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97730-5
This article is cited by
-
Genome editing
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.