Abstract
The vascular complications have been a major cause of morbidity and mortality among all subtypes of BCR-ABL1 negative myeloproliferative neoplasms (MPN), but the ethnicity-specific data was limited. We therefore conducted a multi-center retrospective, longitudinal cohort study to evaluate the incidence, characteristics and risk factors of thromboembolic events of MPN patients. Of 256 patients, 27.3% experienced thromboembolic events, majority of which occurred before or within 12 months of MPN diagnosis. The multivariable Cox proportional analyses identified leukocytosis (HR 2.67, 95% CI 1.36–5.24, q = 0.004) and history of thrombosis (HR 9.68, 95% CI 2.00–46.88, q = 0.005) as the risk factors for thromboembolism. In subgroup analysis of polycythemia vera and hemoglobin concentration (HR 1.97, 95% CI 1.28–3.04, q = 0.002) appeared to be a significant risk factor of thrombosis, along with age and thrombosis history. In essential thrombocythemia, severity of the established IPSET score was closely correlated with the frequency of thromboembolic events. In primary myelofibrosis, history of thrombosis was associated with thrombosis events (HR 13.85, 95% CI 1.2–159.5, q = 0.035). Overall survival was worse in patients who experienced thromboembolic events. Our study highlighted the importance of recognizing high risk patients and implementing personalized intervention.
Similar content being viewed by others
Introduction
BCR-ABL1 negative myeloproliferative neoplasms (MPN) represent a heterogeneous group of clonal hematopoietic cells, comprising polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF)1. Despite the relatively indolent clinical course, many patients with PV or ET experience arterial or venous thrombosis attributed to high blood viscosity2. In patients with PMF, excessive inflammatory cytokines release results in fibrotic changes in bone marrow, vascular complications and constitutional symptoms3. All in all, thromboembolic events are a major cause of morbidity and mortality among all subtypes of MPN4,5.
The discovery of the JAK2V617F mutation has allowed for development of novel therapeutic agents and has encouraged the efforts towards molecular diagnostics for MPN6,7,8. Despite the better understanding of the disease however, data regarding the disease course of MPN in Asian populations remain scarce. Recently three South Korean epidemiological studies showed that the incidence and prevalence of MPN is on the rise at a rate of 3.8 times in last ten years9,10,11. With such increment, MPN impose a cumulative threat to public health by inducing substantial economic and social burdens. Given that, understanding the incidence and risk factors for major complications of MPN, namely thromboembolic events, is important. Previous studies have reported the prevalence of thrombosis among MPN patients was 20% to 30% or more, which can occur throughout the disease course 12,13. More specifically, a recent meta-analysis of 29 cohort studies including populations of Europe, North America, Asia, and Australia reported that the pooled prevalence of arterial or venous thrombosis among MPN patients at diagnosis was 20% (95% CI, 16.6–23.8%)12. In German study, 33.6% suffered from vascular events throughout the disease course13. Asian population has traditionally been associated with lower incidence of idiopathic thromboembolism and certain types of cancer-associated thromboembolisms compared to other ethnicities14,15,16. Recognizing the paucity of data on thromboembolic events in Asian MPN patients, we conducted this study to find out the incidence, characteristics, and risk factors of thromboembolic events in Asian MPN patients.
Materials and methods
Study design and subjects
This was a multi-center retrospective, longitudinal cohort study of BCR-ABL1 negative MPN patients over 18 years old. The study period was set between January 2008 and December 2018. From 2008 to 2015, the diagnosis of MPN was made according to the 2008 World Health Organization (WHO) classification. From 2016 to 2018, the diagnosis of MPN was made according to the revised 2016 WHO classification. At first 406 patients were identified, but after excluding patients without bone marrow examination results (n = 22), those without JAK2 V617F mutational status and/or blood counts at diagnosis (n = 100), and those diagnosed before 2008 (n = 28), 256 patients were finally included for analysis. Other driver mutational status, such as JAK2 exon 12, CALR and MPL, was checked if clinically indicated. Their medical records were reviewed and analyzed for demographics, disease characteristics, treatment including cytoreductive, antiplatelet and anticoagulation therapy, and clinical course. The study was conducted according to Declaration of Helsinki and was approved by the institutional review board (IRB) of each hospital (Seoul National University Hospital IRB, IRB approval number J-1809-006-968; Seoul National University Bundang Hospital IRB, IRB approval number B-1809-492-404; Seoul National University Boramae Medical Center IRB, IRB approval number 20-2018-50). All patient data were anonymized and de-identified prior to analysis, and thus the requirement for patient consent was waived by the IRB of all hospitals (same as above).
Definitions
Cardiovascular disease (CVD) risk factors were obesity with BMI over 25, smoking, hypertension, diabetes, and dyslipidemia. Thrombosis was categorized into arterial or venous thromboembolisms. Arterial thrombosis included acute coronary syndrome (ACS), stroke and peripheral artery disease (PAD). Venous thromboembolism included deep vein thrombosis (DVT), pulmonary embolism (PE) and splanchnic vein thrombosis (SVT). Other minor occlusive events, including stable angina and superficial thrombophlebitis were not included.
The variables clinically relevant to thrombosis were defined per NCCN Guidelines®17. Clinically relevant risk factors included age at diagnosis, sex, previous thrombotic event, hepatomegaly, splenomegaly, WBC > 15 × 109/L, hemoglobin count, platelet count, JAK2 V617F mutation status, and two or more of the cardiovascular risk factors mentioned above.
The presence of thromboembolic event was confirmed with an imaging modality, and the time of event was recorded as the date of imaging, when thromboembolic event was subjectively confirmed.
Cytogenetic studies were performed onsite, whose satisfactory performance was monitored by a national external quality assurance scheme. Bone marrow cells were cultured for 24 h then karyotype was analyzed using the standard G-banding technique. The karyotypes were constructed and chromosomal abnormalities were reported in accordance with the 2016 International System for Human Cytogenetic Nomenclature.
Statistical analysis
The primary objective of this study was to investigate the incidence of thromboembolic events in homogeneous East Asian MPN patients per MPN subtype. MPN was divided into PV, ET, PMF and MPN-unclassifiable (MPN-U). The secondary objectives included the characteristics of thromboembolism, the effect of thromboembolism on survival, and indirect comparison with other ethnicities.
The incidence of a thromboembolic event was calculated at any time (within 12 months or at the time of MPN diagnosis, and during the follow-up). Categorical variables were summarized with the frequencies in number and rates in percentages. Continuous variables were represented with the median values and ranges. Differences were assessed using Mann–Whitney test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables. The multiple imputation approach was applied to the missing values of BMI in 82 patients. Of multiple sets of data imputed, the set that had lowest standard error was chosen. Then obesity and the number of cardiovascular risk factors were recalculated upon the newly imputed data. Statistical analyses with the multiple regression model were reconstructed from the newly imputed data. While all patients had information on JAK2V617F mutations, 196 patients lacked MPL data and 192 patients lacked CALR data. Because more than one third of the patients lacked these data, MPL and CALR variables were not considered in this study. Multivariable Cox proportional-hazard models were constructed to find risk factors for thromboembolic events and correlation among covariates. False Discovery Rate approach with Benjamini–Hochberg procedures was used, and q-value < 0.05 was considered statistically significant. Since cardiovascular risk factors have been well defined in previous study5, instead of examining each of the cardiovascular risk factors as separate variables in the regression model, ‘two or more cardiovascular risks’ was considered to be an important risk factor. Since some variables are well-known risk factors with established threshold for the thrombotic events, they were dichotomized for multiple regression model. For example, age over 60 years old is a well-known risk factor for PV2. BMI was specifically defined as categorical variable because obesity criteria for Korean population was defined as BMI over 25. Leukocytosis is another widely studied risk factor. ECLAP study had shown the association between leukocytosis over 15 × 109/L with the increase of venous thrombosis in PV patients18. Thus, these variables were converted into categorical variables. Other continuous variables such as hemoglobin and platelet counts were handled as quantitative values in the multiple regression model.
Overall survival (OS) was defined as the time from MPN diagnosis to death of any cause. The OS curves were estimated using the Kaplan–Meier method. If patients survived without death, the survival was censored at the latest date of follow-up when no death was confirmed. p-values of < 0.05 were considered statistically significant for Kaplan–Meier curves. These data were analyzed using the Statistical Package for the Social Sciences software (IBM SPSS Statistics, Version 22.0, New York, NY, USA).
Cumulative incidence of thromboembolic events of the MPN disease and subgroups was estimated taking into account the first event and considering death as a competitor and compared by Gray’s test. For this part of the analyses, the statistical software R (www.r-project.org) was used.
Results
Patient characteristics and treatment
The baseline characteristics are shown in Table 1. Among 256 MPN patients, the most common diagnosis was ET (42.2%, 108/256), followed by PMF (27.7%, 71/256) and PV (24.6%, 63/256). The median age at diagnosis for the entire cohort was 62 years (range, 19–88) and JAK2V617F mutation was positive in 73.0% (187/256). MPL mutation was tested in 60 patients and was positive in 2 ET patients and 1 MPN-unclassifiable (MPN-U) patient. CALR mutation was available in 64 patients, and was found positive in 5 ET, 10 PMF, and 1 MPN-U patient.
Antithrombotic therapy was done in 84% (215/256) of MPN patients. Aspirin was most often used but various antithrombotic agents including clopidogrel, triflusal, prasugrel, dabigatran, and ticagrelor. In each subgroup, 94% (59/63) of PV, 85% (92/108) of ET, 56% of PMF (40/71), and 86% (12/14) of MPN-U were treated with antiplatelets. In PV patients, 79.4% (50/63) received phlebotomy. Hydroxyurea was prescribed to 83% (52/63), 77% (83/108), 47% (33/71), and 86% (12/14) of PV, ET, PMF, and MPN-U, respectively. Anagrelide was prescribed to 15.9% (10/63), 69.4% (75/108), 25.4% (18/71), and 35.7% (5/14) of PV, ET, PMF, and MPN-U patients, respectively. Ruxolitinib was prescribed to 41% (29/71) of the PMF patients.
At the time of diagnosis, 164 patients had one of the CVD risk factors (Table 1): 59/174 (33.9%) patients had BMI over 25, 33/256 (12.9%) patients were active smokers, 89/256 (34.8%) patients had hypertension, 32/256 (12.5%) patients had diabetes, 15/256 (5.9%) patients had underlying dyslipidemia.
Incidence of thromboembolic events
As shown in Fig. 1, 27.3% (70/256) experienced thromboembolic events. In subgroup analysis, MPN-U patient had the highest rate of thrombosis (35.7%, 5/14), followed by PV (34.9%, 22/63), ET (25%, n = 27/108) and PMF (23%, n = 16/71). For all MPN patients, the cumulative incidence of thromboembolic events was calculated as 6.3 (95% CI 3.3–9.3) at 1 year, 15.3 (95% CI 10.9–19.7) at 5 years, and 28.3 (95% CI 22.8–33.8) at 10 years. In subgroup analysis, the cumulative incidence rate of PV was 11.1 (95% CI 3.3–18.9) at 1 year, 20.3 (95% CI 10.4–30.2) at 5 years, 32.1 (95% CI 20.6–43.6) at 10 years. For ET, the cumulative incidence rate was 3.7 (95% CI 0.1–7.3) at 1 year, 15.2 (95% CI 8.4–22) at 5 years, 26.3 (95% CI 18–34.6) at 10 years. For PMF, the cumulative incidence rate was 5.7 (95% CI 0.3–11.1) at 1 year, 9.2 (95% CI 2.5–15.9) at 5 years.
Site and characteristics of thromboembolic events
The sites and timing of thromboembolic events are shown in Fig. 1. Majority of the patients (72.8%, 51/70) experienced thromboembolic events within 12 months before or at the time of MPN diagnosis. Three patients experienced recurrent thrombosis, and all were JAK2 positive ET patients. First patient had underlying protein C deficiency and suffered from alternating bleeding and thrombotic events. Second patient had concomitant chronic renal failure unrelated to ET, and despite continuous low dose aspirin treatment, the patient experienced multiple arterial thrombosis and stroke events 7 years after ET diagnosis. The last patient had not underlying disease but suffered from coronary artery disease and then peripheral artery disease despite the use of clopidogrel and cilostazol.
Stroke (16.8%, 43/256 was the most common cause of thrombosis, followed by ACS (5.9%, 15/256), and SVT (3.1%, 8/256). DVT or PE occurred in only PV and ET. SVT was particularly prevalent in the PMF (6.6%, 4/71) and the MPN-U (7.1%, 1/14) patients.
No patients experienced sinus vein thromboses or arm vein thrombosis. As for arterial events, 1 patient experienced tibial artery occlusion and 4 experienced splenic infarction.
Risk factors of thromboembolic events
For all MPN patients, multivariable analysis identified leukocytosis (HR 2.67, 95% CI 1.36–5.24, p = 0.004), and history of thrombosis (HR 5.24, 95% CI 2.73–10.08, p < 0.001) as risk factors for thromboembolism as shown in Table 2.
The risk stratification of PV and ET is based on the age (older than 60 years) and history of previous thrombosis19,20. When PV patients were classified accordingly, thrombosis occurred in 31.7% of high-risk group, in contrast to 3.2% in low-risk group, as shown in Fig. 2a. In the subgroup analysis of PV patients, the history of thrombosis (HR 9.68, 95% CI 2.00–46.88, p = 0.005), and hemoglobin count (HR 1.97, 95% CI 1.28–3.04, p = 0.002) were recognized as risk factors for thrombosis in multivariable analyses, as shown in Table 2.
When ET were classified according to traditional risk stratification, thrombosis occurred in 23.1% of high-risk group, in comparison to 1.9% in low-risk group as shown in Fig. 2a. When the ET patients were analyzed by International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET)19, thrombosis rate increased from 1.9% in low risk to 20.4% in high risk as shown in Fig. 2b. It is also notable that thrombosis prior to ET diagnosis only occurred in the high-risk groups. The multivariable Cox proportional model for ET patients showed that the history of thrombosis was associated with increased thrombosis events, as in Table 2.
For PMF patients, history of thrombosis was associated with increased thrombosis events in multivariable analysis, as shown in Table 2.
Survival
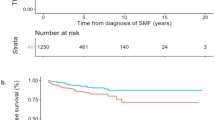
The follow-up period for each MPN subtypes were 49 months, 64 months, 35 months, and 33 months for PV, ET, PMF, and MPN-U, respectively. The number of the patients followed up at 1 year, 5 years and 10 years were 136, 77, and 21, respectively. During the median follow-up of 46 months (range 12–161), 5 patients experienced secondary transformation: 2 to acute myeloid leukemia (1 ET, time to progression 43 months; 1 PMF, time to progression 7 months) and 3 to secondary myelofibrosis (1 PV, time to progression 127 months; 2 ET, median time to progression 63 months). The estimated overall survival for the entire cohort was 96.2%. While PV patients were associated with best survival and PMF patients with worst survival, the difference did not reach statistical difference. As shown in Fig. 3, patients with thromboembolic events had shorter overall survival compared to those who did not (p = 0.009).
Discussion
The importance of our study lies in that (1) we present rare data on the incidence and characteristics of East Asian patients, which seem to be similar to Western data albeit the traditional belief that Asians are less prone to thromboembolism; (2) we report risk factors for thromboembolism; and that (3) management of thromboembolism can lead not only to better quality of life but also better survival.
As shown in Table 3, our study provides evidence that Korean patients had similar frequency of thrombosis compared to that of Western patients, ranging 16 ~ 41%19,23,24,28,31,32,33. More specifically, recent meta-analysis of 29 cohort studies including populations of Europe, North America, Asia, and Australia reported that the pooled prevalence of arterial or venous thrombosis among MPN patients at diagnosis was 20% (95% CI, 16.6–23.8%)12. In our study, 27.3% of the patients experienced overall thrombosis, within close range of other ethnic populations. Also in subgroup analysis, despite the diverging rates of the thromboembolic events among multiple ethnic groups, our data provided the evidence that the Asians were not of lower risk of vascular complications. It is also notable that over one third of MPN-U patients experienced thromboembolism among all subgroups, albeit the small number of populations and the scarcity of the preceding data. When compared by the site of the thrombosis, stroke and ACS were the most prevalent but thromboses in uncommon sites like SVT were also reported, shown in Table 4. Markedly high prevalence of stroke and its decrease during the follow-up period indicated the role of the treatment with antiplatelet agents in reducing vascular complications in Asian MPN patients. Considerable number of patients who were diagnosed with MPNs within a month of thrombotic events also suggested the need for the early diagnosis and treatment to prevent vascular complications, as observed in the ECLAP study and the German MPN registry13,18.
Risk factors for thromboembolism varied across subtypes of MPN, emphasizing the importance of individualized treatment depending on the subtype, presentation and comorbidities. PV was associated with the highest incidence of thromboembolism among MPN subgroups, comparable to previous observations that reported 26–39%19,22,23. In addition, that hemoglobin concentration was markedly associated with thrombosis events implied that effective control of hyperviscosity with phlebotomy is required to control the disease and reduce the vascular complications23. In ET, the severity of IPSET score was predictive of thromboembolic events. Therefore, ET patients should be treated more comprehensively including management of cardiovascular comorbidities to prevent thromboembolism in ET patients. In PMF, age over 60 years, JAK2V617F mutational status, and previous thrombosis had been identified as risk factors of thrombosis26,27. In our study, however, history of thrombotic event was the only predictive variable on multivariable analysis that included age, sex, organomegaly, JAK2V617F mutation status, blood counts, and cardiovascular risk factors as covariates (p = 0.035). The shorter overall survival of the patients with thromboembolic events underscored the importance of recognizing high risk patients and implementing personalized intervention.
One of the most obvious pitfall of the study is its retrospective nature. Also, there is a possibility of referral bias, since all participating hospitals are academic centers. However, considering the longevity of MPN patients, prospective data is very difficult to accrue. The small number of MPN-U patients may have overestimated the prevalence of thromboembolism which was comparable to that of the PV, and the rate of splanchnic vein thrombosis (7.1%, 1/14). However, preliminary data showed that MPN-U patients with normal blood count may present with rare unexplained thrombosis, especially in splanchnic vein28,29. Lastly, to compensate for the changes in the MPN diagnostic criteria after 2015, we selected patients with legitimate bone marrow examinations results and initial laboratory findings. Thus, it is our assumption that patients were little affected by the changes in the diagnostic criteria. Previous studies have compared the difference in thrombosis events of patients who were diagnosed with 2008 versus 2016 criteria34,35. The major updates in the 2016 WHO classification for Philadelphia-negative MPN aimed at distinguishing between masked PV and JAK2-mutated ET, and between prefibrotic and overtly fibrotic PMF. In PV, the reduced hemoglobin level threshold unveiled that up to 72% of the patients newly diagnosed with prodromal/masked PV had history of thromboembolism. On the other hand, pre-PMF is known to have comparable cumulative incidence of major thrombosis to that of ET. Unfortunately, since there are only 28 PV, 66 ET, 23 PMF, and 6 MPN-U patients were diagnosed before 2015, subgroup comparison analysis was not carried out. Further studies with more patient data on the masked PV and pre-PMF would strengthen our knowledge in understanding the vascular complications of MPN patients. All in all, a descriptive epidemiology study can assist public health planning, policy making, fair allocation of limited healthcare resources, and understanding of disease secular trends. In this regard, regardless of study limitations, it is our belief that our study holds its values.
Conclusion
Our study provides better understanding of the epidemiology, characteristics and risk factors of thromboembolic events in East Asian patients with MPN, who have been underrepresented in previous studies. Meticulous risk factor evaluation is crucial for prevention of thrombosis and better survival.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tefferi, A. & Pardanani, A. Myeloproliferative neoplasms: A contemporary review. JAMA Oncol. 1, 97–105. https://doi.org/10.1001/jamaoncol.2015.89 (2015).
Tefferi, A. & Barbui, T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 92, 94–108. https://doi.org/10.1002/ajh.24607 (2017).
Takenaka, K., Shimoda, K. & Akashi, K. Recent advances in the diagnosis and management of primary myelofibrosis. Korean J. Intern. Med. 33, 679–690. https://doi.org/10.3904/kjim.2018.033 (2018).
Hultcrantz, M. et al. Risk and cause of death in patients diagnosed with myeloproliferative neoplasms in Sweden between 1973 and 2005: A population-based study. J. Clin. Oncol. 33, 2288–2295. https://doi.org/10.1200/JCO.2014.57.6652 (2015).
Cervantes, F., Passamonti, F. & Barosi, G. Life expectancy and prognostic factors in the classic BCR/ABL-negative myeloproliferative disorders. Leukemia 22, 905–914. https://doi.org/10.1038/leu.2008.72 (2008).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790. https://doi.org/10.1056/NEJMoa051113 (2005).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807. https://doi.org/10.1056/NEJMoa1110557 (2012).
Vannucchi, A. M. et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 372, 426–435. https://doi.org/10.1056/NEJMoa1409002 (2015).
Lim, Y., Lee, J. O. & Bang, S. M. Incidence, survival and prevalence statistics of classical myeloproliferative neoplasm in Korea. J. Korean Med. Sci. 31, 1579–1585. https://doi.org/10.3346/jkms.2016.31.10.1579 (2016).
Byun, J. M. et al. Real world epidemiology of myeloproliferative neoplasms: A population based study in Korea 2004–2013. Ann. Hematol. 96, 373–381. https://doi.org/10.1007/s00277-016-2902-9 (2017).
Hong, J. et al. Risk of disease transformation and second primary solid tumors in patients with myeloproliferative neoplasms. Blood Adv. 3, 3700–3708. https://doi.org/10.1182/bloodadvances.2019000655 (2019).
Rungjirajittranon, T. et al. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 19, 184. https://doi.org/10.1186/s12885-019-5387-9 (2019).
Kaifie, A. et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): Analysis from the German SAL-MPN-registry. J. Hematol. Oncol. 9, 18. https://doi.org/10.1186/s13045-016-0242-9 (2016).
Hong, J. et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS ONE 13, e0191897. https://doi.org/10.1371/journal.pone.0191897 (2018).
Oh, S. Y. et al. Venous thromboembolism in patients with pancreatic adenocarcinoma: Lower incidence in Asian ethnicity. Thromb. Res. 122, 485–490. https://doi.org/10.1016/j.thromres.2007.12.015 (2008).
Koh, Y. et al. Low incidence of clinically apparent thromboembolism in Korean patients with multiple myeloma treated with thalidomide. Ann. Hematol. 89, 201–206. https://doi.org/10.1007/s00277-009-0807-6 (2010).
https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
Landilfi, R. et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109, 2446–2452. https://doi.org/10.1182/blood-2006-08-042515 (2007).
Szuber, N. et al. 3,023 Mayo clinic patients with myeloproliferative neoplasms: Risk-stratified comparison of survival and outcomes data among disease subgroups. Blood 132, 3035–3035. https://doi.org/10.1182/blood-2018-99-109740 (2018).
Carobbio, A. et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 117, 5857–5859. https://doi.org/10.1182/blood-2011-02-339002 (2011).
Barbui, T. et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 120, 5128–5133; quiz 5252. https://doi.org/10.1182/blood-2012-07-444067 (2012).
Marchioli, R. et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 23, 2224–2232. https://doi.org/10.1200/jco.2005.07.062 (2005).
Kaifie, A. et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): Analysis from the German SAL-MPN-registry. J. Hematol. Oncol. 9, 18. https://doi.org/10.1186/s13045-016-0242-9 (2016).
Enblom, A. et al. High rate of abnormal blood values and vascular complications before diagnosis of myeloproliferative neoplasms. Eur. J. Intern. Med. 26, 344–347. https://doi.org/10.1016/j.ejim.2015.03.009 (2015).
Landolfi, R. et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N. Engl. J. Med. 8(350), 114–124. https://doi.org/10.1056/NEJMoa035572 (2004).
Barbui, T. et al. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood 4(115), 778–782. https://doi.org/10.1182/blood-2009-08-238956 (2010).
Elliott, M. A. et al. Thrombosis in myelofibrosis: Prior thrombosis is the only predictive factor and most venous events are provoked. Haematologica 10(95), 1788–1791. https://doi.org/10.3324/haematol.2010.025064 (2010).
De Stefano, V. et al. Splanchnic vein thrombosis in myeloproliferative neoplasms: Risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 6, e493. https://doi.org/10.1038/bcj.2016.103 (2016).
Kiladjian, J.-J. et al. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: A report on 241 cases. Blood 111, 4922–4929. https://doi.org/10.1182/blood-2007-11-125328 (2008).
Di Veroli, A. et al. Incidence of early thrombosis in myeloproliferative neoplasms (MPN): A prospective analysis from the gruppo laziale of Ph-negative MPN. Blood 128, 1951–1951. https://doi.org/10.1182/blood.V128.22.1951.1951 (2016).
Soyer, N. et al. Multicenter retrospective analysis of turkish patients with chronic myeloproliferative neoplasms. Turk. J. Haematol. 34, 27–33. https://doi.org/10.4274/tjh.2016.0005 (2017).
Abdulkarim, K., Samuelsson, J., Johansson, P. & Andréasson, B. Risk factors for vascular complications and treatment patterns at diagnosis of 2389 PV and ET patients: Real-world data from the Swedish MPN Registry. Eur. J. Haematol. 98, 577–583. https://doi.org/10.1111/ejh.12873 (2017).
Seguro, F. S. et al. Risk factors and incidence of thrombosis in a Brazilian cohort of patients with Philadelphia-negative myeloproliferative neoplasms. J. Thromb. Thrombolysis 49, 667–672. https://doi.org/10.1007/s11239-019-02029-y (2020).
Barbui, T. et al. Diagnostic impact of the 2016 revised WHO criteria for polycythemia vera. Am. J. Hematol. 92, 417–419. https://doi.org/10.1002/ajh.24684 (2017).
Finazzi, G. et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia 26, 716–719. https://doi.org/10.1038/leu.2011.258 (2012).
Funding
This work was partially supported by the National Research Foundation of Korea (NRF-2020R1F1A1076106).
Author information
Authors and Affiliations
Contributions
J.B. and J.H. conceptualized the study. J.B. set methodology. J.K. and J.B. performed formal analysis and investigation. All authors performed data curation. J.B. and J.H. supervised the study. J.K. and J.B. wrote original manuscript. J.B. had funding. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J., Byun, J.M., Hong, J. et al. Incidence, characteristics and risk factors of thromboembolic events in East Asian patients with BCR-ABL1 negative myeloproliferative neoplasms. Sci Rep 11, 17819 (2021). https://doi.org/10.1038/s41598-021-97464-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97464-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.