Abstract
Used as traditional Chinese medicine, Astragalus membranaceus (Fisch.) Bge. (A. membranaceus) roots are also used as tonic food material in a wide range of applications, while the leaves are left in the field, unused. Therefore, comprehensively exploring and utilizing the leaves will inevitably reduce the associated resource waste and environment pollution. In this study, the plant leaves were processed into tea using green tea processing technology. Bioactive components, antioxidant and antibacterial activities of the Leaf Tea (LT) and Dry Leaves (DL) were studied, and compared to that of the Dry Roots (DR). The results showed that the polysaccharides content (POL) in the DR (20.44%) was twice as high as the DL (10.18%) and LT (8.68%). However, the DL contained 36.85% more water-soluble extracts (WSE), 35.09% more ethanol-soluble extracts (ESE), 409.63% more total flavonoid content (TFC), 221.01% more total phenolic content (TPC) and 94.34% more proteins, and the LT contained 26.21% more WSE, 40.64% more ESE, 326.93% more TFC, 191.90% more TPC and 37.71% more proteins. The total amino acid (AA) content in the DR was 8.89%, while in that of the DL and LT were 24.18% and 28.96% respectively, nearly 3-times higher than that of the DR. The antioxidant activity of DR was much lower than those of DL and LT, both of which had antioxidant activity closer to that of Vitamin C (VC) and the antioxidant activities were even stronger when the optimal concentration was reached. Except for Aspergillus niger and Staphylococcus aureus, the DL and DR exhibited inhibition activities to Salmonella, Bacillus subtilis, Escherichia coli and yeast, while the LT had antimicrobial activities against all the strains except for A. niger. In summary, compared with the most commonly used DR, the DL and LT from A. membranaceus contained higher bioactive components, and stronger antioxidant and antimicrobial activities. Producing leaf tea may be an appropriate way to economically and reasonably utilize the plant leaves which are by-products.
Similar content being viewed by others
Introduction
Astragali Radix (Huangqi) is the dry roots of the leguminous Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (A. membranaceus var. mongholicus) or Astragalus membranaceus (Fisch.) Bge. (A. membranaceus)1. It has been used as traditional medicine and tonic food material in China, Japan, Korea, and some Southeast Asia countries for more than 2000 years2,3, It can reinforce body immunity and has curative effect on (diuresis and edema, stagnation and obstruction as well as detoxification and purulence4,5). In addition, Astragali Radix has tonifying middle and Qi, and is used to treat deficiency, vitalise your energy and relief fatigue, eating less and loose stools1,6, which can improve anti-viral activity, protect liver and stomach7,8,9, inhibit cancer10, exert anti-aging and improve exercise performance5. Based on the above functions, Astragali Radix was recommended by the National Administration of Traditional Chinese Medicine11 as a Chinese traditional herbal medicine in the prevention of infection associated with the novel coronavirus COVID-19.
In recent years, there have been many reports on the chemical constituents and pharmacological active ingredients, including polysaccharides12, saponins13, amino acids, trace elements and flavonoids14 of the dry roots of A. membranaceus3. Similar to the roots, A. membranaceus leaves also possess medicinal or health promoting benefits. However, evidence of the bioactive components, antioxidant and antibacterial activities of the leaves of this medicinal plant is very limited. Additionally, little comparative studies exist between the leaves and the roots. Astragali Radix is an integral component in many pharmaceutical, beverage, and cosmetics industries2,15. During its cultivation, some the shoots and leaves are cut off to promote underground growth and bioactive compound biosynthesis. The removed leaves from A. membranaceus are underutilized in most producing areas16. Based on ancient record and modern science studies, A. membranaceus leaves can be used as a food source17,18,19. To promote A. membranaceus leaves as a nourishing healthy food resource, a comprehensive scientific study of the leaf processing procedures, the quantity and types of active ingredients they contain must be undertaken.
Therefore, the purpose of this study was to detect and comparatively analyze the bioactive constituents of A. membranaceus leaves, leaf tea and roots, and to ascertain their antioxidant and antibacterial activities in vitro.
Materials and methods
Plant materials and product processing
The A. membranaceus plants were cultivated by local farmers in Huining, Gansu province, P.R. China (35° 40′ N, 105° 21′ E), samples collection did not require permission. The fresh leaves of A. membranaceus were sampled in June and transported to laboratory for processing while the roots were harvested on Oct. 20, 2019. The plants were cultivated in summer 2017 and were harvested for the shoots in June 2019. The shoots were cut 20 cm above the ground level.
The leaf tea (LT) was processed according to the standard green tea processing procedure20. The carefully selected healthy-fresh leaves were placed on a cloth for 2 h to dissipate some moisture, blanched for 6 min at 200 °C, rolled by hand and oven dried to constant weight at 60 °C for 3 days to make the dry leaf product (DL). The healthy fresh roots were oven dried to constant weight at 60 °C to make the dry root product (DR).
All the above products, LT, DL and DR, Fig. 1, were crushed separately, sifted through 80 mm meshes, packaged in self-sealing bags, and stored at − 80 °C for subsequent analysis.
Chemicals and reagents
The chemicals H3PO4, K2S2O8, K4Fe (CN)6·3H2O, NaNO2, NaOH, Na2CO3, CuSO4, K2SO4, H3BO3, AlCl3, NaH2PO4, Na2HPO4, H2SO4, HCl, vitamin C (VC), methanol, ethanol and phenol were purchased from Shanghai Hushi Chemical Ltd (Shanghai, China). Beef peptone was purchased from AOBOX (Beijing, China). Gallic acid and rutin were purchased from Solarbio (Beijing, China). Folin & Ciocalteu's phenol reagent were purchased from Sigma (America). DPPH, agar powder and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan).
Bioactive composition determination
Contents of polysaccharides, protein, moisture and ash of the samples were determined according to the Association of Official Analytical Chemists (AOAC) methods20,21.
The total phenol content (TPC) was analyzed using the Folin-Ciocalteu (F–C) reagent method. A sample of the 0.10 g powder of each product received 3 mL of 70% methanol at 70 °C and extracted three times at 10 min interval using the sonic wave degradation method. The samples were centrifuged for 10 min at 3500 r/min, and the supernatant of three times centrifugation was transferred to a 10 mL volumetric bottle. The absorbance of the reaction mixture was measured at 760 nm using a spectrophotometer (Shimadzu UV-2450, Japan)20,22. Absorbance values were converted to contents using a standard curve expressed by gallic acid (y = 0.047 + 0.478x; r2 = 0.999).
Total flavonoid content (TFC) detection was based on the AlCl3 colorimetric method20,23. A 0.10 g sample was mixed with 3 mL 95% ethanol at 50 °C. The supernatant was extracted three times by ultrasound for 30 min each time, and centrifuged for 10 min at 4000 r/min. The supernatant was transferred to a 10 mL volumetric bottle. The absorbance of the sample was measured at 510 nm. The absorbance was converted to content using a standard curve expressed by rutin (y = 0.005 + 0.294x; r2 = 0.999). The ethanol- and water-soluble extracts were measured according to the Chinese Pharmacopoeia method1. A 2.0 g sample was put in a 100 mL Erlenmeyer flask containing 50 mL water (for the water-soluble extract) or 45% ethanol (for the ethanol-soluble extract). The plug was closed and connected to a reflux condenser, heated for 1 h to slightly boil and filtered. The residue of the 25 mL sample was oven dried to constant weight at 60 °C.
As referenced in the national standard GB 5009.124-201624, amino acid contents of the DL, LT and DR, (Fig. 1), from A. membranaceus were determined by automatic amino acid analyzer (Hitachi L-8900, Japan).
Antioxidant activity determination
For the antioxidant activity analysis, samples were prepared according to the following method: A 1.0 g sample and 50 mL pure water (water extract antioxidant activity analysis) or 70% ethanol (ethanol extract antioxidant activity analysis) were put into a 100 mL Erlenmeyer flask, the plug closed and heated to slight boiling for 1 h. The extract was transferred to a 50 mL volumetric bottle, using VC as the positive control.
2,2-Azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) radical scavenging activity
A 2,2-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical solutions were prepared as follows: 4.0 mL 7.0 mM ABTS and 2.0 mL 7.0 mM potassium per sulfate were mixed together. The mixture was kept at room temperature in dark for 12 h, while ABTS cation formed. The sample was then diluted before analysing with ethanol to give an absorbance of 0.700 ± 0.02 at 734 nm. The reaction mixture was developed by mixing a 10 μl product sample or control (70% methanol) with a 1.0 mL ABTS radical solution. Samples and blanks were placed in vortexes for 6 min, and measured at 734 nm. A 70% methanol was used as the blank20. The ABTS radical scavenging activity expressed as a percent was calculated as follows:
Inhibition of 2,2-diphenyl-1-picrylhy-drazyl (DPPH⋅) radicals
2,2-diphenyl-1-picrylhy-drazyl (DPPH⋅) was dissolved in methanol to 0.2 mM. 2 mL of the solution were mixed with 2 mL of the diluted sample at 25 °C for 30 min. At the end of the reaction, the absorbance was read at 510 nm20,25. Inhibiting percentages of the DPPH⋅ radical were calculated with the following formula.
where: Ao is absorbance of the mixed reaction solution with 2 mL DPPH⋅ and 2 mL water; Ax is absorbance of the final DPPH⋅ inhibition reaction solution with a 2 mL sample and 2 mL DPPH⋅; Axo is absorbance of the agent used in the test with no DPPH⋅ but a 2 mL sample and 2 mL pure water.
Ferric ion reducing antioxidant power (FRAP)
Reducing power of samples were measured using FRAP assay20,26. In a nutshell, a FRAP reagent was freshly prepared by mixing an acetate buffer (0.3 M, pH 3.6). 1.0 mL of FRAP reagent and 0.05 mL of properly diluted sample solution were added and mixed well. After reaction for 30 min at 37 °C, the absorbance was read at 593 nm. FRAP was expressed by VC (y = − 0.013 + 0.006x, r2 = 0.998).
Antibacterial activities
Microbial strains sources and the tested product sample preparation
Six microbial strains including Salmonella, Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), yeast, Aspergillus niger (A. niger) were obtained from the Northwest Institute of Ecology and Environmental Resources, Chinese Academy of Sciences.
Samples were prepared as follows: a 1.0 g sample and 50 mL distilled water were put into a 100 mL conical bottle. The bottle was sealed and heated to boil for 1 h and the extract was transferred to a 50 mL volumetric bottle.
Microbial activation and antibacterial activity test
Salmonella, B. subtilis, S. aureus, E. coli were cultured on beef-protein medium for 24 h at 37 °C. A. niger and yeast were cultured on Potato Dextrose Agar (PDA) for 48 h at 28 °C. The strain density was 1.6–2.5 × 109 cfu/mL. 100 μl strains were incubated in each plate.
Sterilized filter paper discs with 6 mm diameter were dipped in the sample extract and irradiated for 40 min under UV light. Then the soaked discs were placed in a microbial strain plate. The inhibition diameter was measured with a vernier caliper and the MIN and MAX effects were tested27.
Statistical analysis
Significant differences among the treatments were analyzed by Duncan multiple comparison based on one-way ANOVA. The Regression analysis with SPSS 20.0 software, indicated that the inhibition of water-soluble extracts (WSE) and ethanol-soluble extracts (ESE) to free radical ABTS and DPPH⋅ were performed according to method developed in previous research20.
Results and discussion
Bioactive composition analysis
The main bioactive components in the three products are listed in Table 1. The main chemical constitutes of DL and LT were quite similar; although significant differences were noted in indicators such as protein (DL > LT, difference = 8.22, P < 0.01), ESE (LT > DL, difference = 1.79, P < 0.01) and WSE (DL > LT, difference = 4.07, P < 0.05). Significant differences were not found in the TPC, TFC and POL contents (P > 0.05) of the samples. Among the bioactive constitutes, only POL contents in LT and DL were significantly lower (P < 0.01) while the other components in LT and DL were all significantly higher than those in DR (P < 0.01). Two major anti-inflammatory active fractions that may enhance wound healing were isolated from dry roots of A. membranaceus28. The extract from A. membranaceus var mongholicus roots has shown significant anti-viral and immune stimulate activities, from which a new saponin and iso-astragaloside (I) were separated3 Leaf products exhibited substantial total flavonoid with contents in the order DL (9.68%) > LT (8.11%) > DR (1.90%). Compared with DL and LT, the DR exhibited significantly higher POL, increased by 50.21% (P < 0.01) and 57.53% (P < 0.01), respectively. Compared with DR, DL and LT showed other higher nutrition components, leading to increased WSE of 14.10% and 10.03%, increased ESE of 11.27% and 13.05%, increased TFC of 7.78% and 6.21%, increased TPC of 3.99% and 3.46%, and increased Protein of 13.70% and 5.48%, respectively. The abundant TPC in LT and DL detected in this study were in accordance with the results of study examining the fleshy roots of Raphanus raphanistrum21. In addition to abundant TPC, increased TFC and protein in LT and DL was also detected in this study; these results were in accordance with studies of leaf tea and dry leaf from C. pilosula20. Differing from C. pilosula products, the contents of WSE and ESE were reversed in LT and DR from A. membranaceus, resulting in a maximum amount of WSE in DL and a maximum amount of ESE in LT20.
Amino acids are essential for human growth and development as well as reproduction and health29. Table 2 shows the amino acids (AA) in DL, LT and DR from A. membranaceus. The results showed that the 17 amino acid contents in DL and LT were all very similar leading to a non-significant difference in the total value (P > 0.01). Compared with DL (24.18%) and LT (28.96%), the total AA content in DR was significantly lower 8.89% (P < 0.01), which were nearly one third times lower than the formers. This indicates that all the three products are rich in amino acids (Table 2), because their total amino acid contents are far higher than those of famous Chinese teas including Maojian (slightly more than 0.02%), Biluochun (close to 0.03%), Longjing (slightly more than 0.03%)30. DR, DL and LT also presented more abundant amino acids than their respective products of dry root (5.36%), dry leaves (19.01%) and leaf tea (21.11%) from C. pilosula20.
The average data are presented in the table. The total described the sum of contents for all the amino acids in each product. DL, the abbreviations are the same as presented in Table 1.
Low ash content is desirable for Chinese Pharmacopoeia1. The crude ash content showed a minimum value in medicinal DR, but in the by-products of LT and DL the values increased by 3.98% and 3.38% respectively greater than that of DR. However, this does not affect the utilization value of leaf products, because these products are consumed by soaking rather than eating leaves as a whole, while ash is insoluble in water. The total ash content is mainly constituted by the mineral element, so the mineral element contents in leaves and leaf tea were higher than those in roots2. Similar results were demonstrated in the products of medicinal plant C. pilosula20.
The above results show that in addition to being rich in amino acids, dry leaf and leaf tea also have higher TFC, a natural antibiotic, providing an important basis for the development and utilization of LT products in the future.
Antioxidant activity analysis
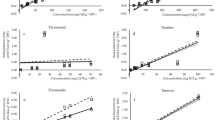
The antioxidant activities of the solutions extracted from the three products were measured using ABTS, DPPH⋅ and FRAP, using VC as a positive control. Figure 2 shows inhibitions of the samples to ABTS radicals. This could suggest that DR also presented lower scavenging activities than those in WSE and ESE from samples of DL and LT.
Inhibition of 2,2-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical for ethanol water-soluble extracts samples (A) and ethanol-soluble extracts samples (B) from DL (filled triangle), processed LT (open triangle) and medicinal DR (open circle) of A. membranaceus, compared with Vc (closed circle); DL, dry leaves; LT, leaf tea ; DR, dry roots; Vc, vitamin C.
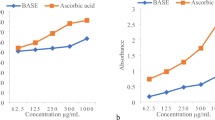
When 20,000 μg/mL was reached, ESE from the DR product showed a 51.89% inhibition rate to ABTS free radical, while the value of WSE was 56.52%. WSE and ESE of LT and DL had very similar scavenging ability to free radicals, and both have much stronger scavenging ability to free radicals than the respective root extracts. Once LT and DL concentration reached a certain level, the abilities noticeably reached that of VC. The highest inhibition rate of 99.94% in VC appeared at concentration of 300 μg/mL (Fig. 2). The highest value was 99.49% at concentration of 15,000 μg/mL in DL, and 99.10% at concentration of 20,000 μg/mL in LT for WSE samples (Fig. 2A).
However, for ESE samples, the highest inhibition rates of 89.62% and 94.28% in DL and LT respectively all appeared at a concentration of 20,000 μg/mL (Fig. 2B). Therefore, we concluded that LT and DL could inhibit ABTS radical efficiently at lower concentrations than DR. Furthermore, the inhibition effect of DL and LT were quite similar, and the highest inhibitions reached as high as VC at certain concentrations. The same inhibitions result also appeared in the corresponsive products of another medicinal and edible C. pilosula20.
The DPPH⋅ radical inhibition ability of various products is presented in Fig. 3. The highest inhibition rate of VC was 95.75% at 30 μg/mL. The highest DPPH⋅ radical inhibition rates of DL and LT were 90.78% and 91.47% for ESE samples as well as 93.19% and 80.25% for WSE samples at 1000 μg/mL. The maximum inhibition rates of DR for ESE and WSE samples were only 70.94% and 69.24% at 1000 μg/mL. Therefore, we can draw the conclusion that DR presented much lower scavenging vigor than DL and LT for both water-soluble and ethanol-soluble extracts. At a certain concentration, the highest inhibition of DL and LT to free radicals was approximately equal to the level of VC. These active functions occurred in the leaf products of medicinal plants A. membranaceus and C. pilosula20, both of which were added to the list of Chinese medicine and food materials of the same origin.
Inhibition of 2,2-diphenyl-1-picrylhy-drazyl (DPPH) radical for water-soluble extracts samples (A) and ethanol-soluble extracts samples (B) from DL (filled triangle), processed LT (open triangle) and medicinal DR (open circle) of A. membranaceus, compared with Vc (closed circle)); DL, dry leaves; LT, leaf tea ; DR, dry roots; Vc, vitamin C.
The IC50 of ABTS and DPPH⋅ inhibition is shown in Table 3. In terms of the IC50 values of ABTS, those in DL and LT were higher than that of DR (P < 0.01). The maximum IC50 of WSE appeared in the DL sample (1101.69 μg/mL), but the maximum IC50 of ESE was noted in LT product (2163.60 μg/mL). The WSE of DR exhibited the highest IC50 values of DPPH⋅ than other two leaf products (P < 0.01), reaching a value of 40.97 μg/mL. Compared with IC50 of DR to DPPH⋅ (32.20 μg/mL), the values in ESE exhibited 64.41 μg/mL from LT and 53.90 μg/mL from DL, resulting in no significant difference from each other (P < 0.01), but the LT is significantly higher (P < 0.01). However, the IC50 to DPPH⋅ in ESE from the roots of C. pilosula was up to 417.91 μg/mL, and the IC50 was not detected within the experiment concentration extent20. From the above it could be deduced that the leaf of A. membranaceus have much more potential utilization value in developing antioxidant bio-products than the DR.
FRAP test was to determine whether the samples have efficient reducing power, thus further revealing their authentic antioxidant ability. The reducing power was calculated according to the absorbance of reaction mixture at 593 nm by VC standard curve (y = − 0.013 + 0.006x, r2 = 0.998). The FRAP activity was expressed by x value calculated from above equation, finally shown in Table 4. When the concentration was at 20.0 mg/mL, we observed that the reducing powers of DL and LT to FRAP were very close both for WSE (546.66 μg/mL, 645.03 μg/mL) and for ESE (612.40 μg/mL, 645.33 μg/mL), and these trends also appeared the same to ABTS and DPPH⋅ inhibition. However, when the concentration of DR was at 20.0 mg/mL, the FRAP value for WSE and ESE were 51.33 μg/mL and 86.44 μg/mL, respectively. Obviously, DR showed much lower reducing power than DL and LT. The reducing powers of DL and LT were very similar, even approximated up to the level of VC when getting at a certain concentration. These same trends of reducing power to FRAP also existed in products of C. pilosula as observed in a recent study20.
Regarding the scavenging activities of ABTS, DPPH⋅ and FRAP, the results indicate that DL and LT have similarly very high antioxidant activities, being able to reach the level of VC at a certain concentration. DR antioxidant activities were significantly lower (P < 0.01)than the other two, and the inhibition capacities of ABTS, DPPH⋅ and FRAP were in accordance with the results of Coronopus didymus31. Therefore, DL and LT can be more widely and comprehensively developed and utilized in the future.
Antimicrobial activity analysis
The extracts were diluted to gradient concentrations at 0.02–20.00 mg/mL based on 20.00 mg/mL concentration prepared by LT, DL and DR20,27. The results showed that the different concentrations of extracts from three (3) products had different inhibitory effects on six (6) strains. Table 5 showed the diameters of antibacterial zones determined in vitro experiment. The extracts of some samples showed inhibition to some strains at high concentration, but did not at lower concentration (Table 5). However, the extracts of other samples showed inhibition to some strains at low concentration, but did not at high concentration. DL and DR showed no antimicrobial activity both to S. aureus and A. niger but for A. niger. LT could play antibacterial activity to other bacteria and yeast, showing the varied antibacterial activities with the concentrations (Tables 5, 6).
The MAX and MIN of tested concentration extents are listed in Table 6. The extracts of DL, LT and DR all had antibacterial activities against Salmonella at a concentration of 20.00 mg/mL, however, their MIN antibacterial concentrations appeared at 0.63 mg/mL, 2.50 mg/mL, and 2.50 mg/mL, respectively, indicating DL has stronger bacteriostatic effect. The sensitivities of the extracts from DL, LT and DR to B. subtilis were quite similar, having 0.63 mg/mL for the MIN and at least 20.00 mg/mL for the MAX. All the extracts of DL, LT and DR have antibacterial effects on E. coli at low concentrations of 0.02 mg/mL. Differing from the MIN, the MAX concentration varied with the products. It was 0.63 mg/mL for DL, 0.31 mg/mL both for LT and DR products. The extracts of DL, LT and DR had antimicrobial effect on yeast, but the effects varied with the concentrations, and the MIN and MAX concentrations appeared at 0.02 mg/mL and 0.63 mg/mL for DL, 0.04 mg/mL and 1.25 mg/mL for LT, and 0.08 mg/mL and 10.00 mg/mL for DR. Among the extracts, only the extracts of LT showed antibacterial activity on S. aureus, and the effective concentrations ranged 0.63 mg/mL to 10.00 mg/mL. Therefore, DL and LT were more sensitive to some bacteria and yeast; this proved that they have stronger and efficient antibacterial activity (Table 6). On the basis of exploiting bioactive potential of wild radish, Raphanus raphanistrum using hydroethanolic and decoction extracts, Iyda found both the samples proved to inhibit several Gram-positive and Gram-negative bacteria and revealed antioxidant activity21, while cytotoxicity against non-tumour cell was not observed. In our study, a systematic toxicological test was carried out on the three products from medicinal plant A. membranaceus in vivo and the results reveal no toxicity.
Conclusions
The usefulness of Astragali Radix root as valuable traditional Chinese medicine cannot not be underestimated. Its continuous root growth stimulation through the pruning of branches and leaves generates substantial amount of biomass. To promote the utilization of A. membranaceus leaves, the leaves were developed into a value added product—leaf tea. The evaluated chemical constituents, antioxidant capacities and antimicrobial activities of the leaves, leaf tea and roots were showed that the leaves of A. membranaceus had high nutritional value. Also a comparison of DL, LT and DR showed similarities in the chemical constituents of LT with DL. Compared with the DR, the nutrient contents in DL and LT exhibited higher bioactive chemicals (including polysaccharides, polyphenolic, flavonoids, protein and amino acids) and strong antioxidant effects. In this study, significantly higher protein values (DL: 28.21%, LT: 19.99%, DR: 14.52%), TPC (DL: 5.79%, LT: 5.26%, DR: 1.80%), TFC (DL: 9.68%, LT: 8.11%, DR: 1.90%) and total amino acids (DL: 24.18%, LT: 28.96%, DR: 8.89%) were detected in DL and LT. DL showed similar antioxidant and antibacterial activities as LT.
In addition TFC, POL, TPC, protein and amino acids, differing from the DL, LT exhibited stronger DPPH antioxidant activities and more broad inhibiting ability, an indication of its better health benefits.
Furthermore the two leaf products DL and LT are easy to obtain at certain concentration of antioxidant for best inhibition ability close to that of VC.
Compared with DL and DR, LT also has certain antimicrobial activities against bacteria and yeast.
It can be concluded that A. membranaceus leaves shows promising health benefits and can be effectively utilized as a medicinal plant as part of a varied healthy diet.
Change history
12 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-00118-8
References
C. P. Committee. 2015 Edition of Chinese Pharmacopoeia 302–303 (Chemical Industry Press, 2015).
Liu, B. Y., Zhou, H. J., Wang, P. S. & An, W. J. Ashes and water related to the quality of tea, Trop. Agric. Sci. Technol. 30, 22–26. https://doi.org/10.16005/j.cnki.tast.2007.03.015 (2017).
He, Z. Q. & Findlay, J. A. Constituents of Astragalus membranaceus. J. Nat. Prod. 54, 810–815. https://doi.org/10.1021/np50075a009 (1991).
Huang, H. P., Li, R. S. & Yang, D. C. The diuretic and hypotensive action of Astragalus membranaceus bge. Acta Pharm. Sinica. 12, 319–324 (1965).
Yeh, T. S. et al. Astragalus membranaceus improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules 19, 2793–2807. https://doi.org/10.3390/molecules19032793 (2014).
Shahzad, M., Shabbir, A., Wojcikowski, K., Wohlmuth, H. & Gobe, G. C. The antioxidant effects of Radix astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets. 17, 1331–1340. https://doi.org/10.2174/1389450116666150907104742 (2015).
Auyeung, K. K., Han, Q. B. & Ko, J. K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 44, 1–22. https://doi.org/10.1142/S0192415X16500014 (2016).
Lee, D. Y. et al. Anti-inflammatory cycloartane-type saponins of Astragalus membranaceus. Molecules 18(4), 3725–3732. https://doi.org/10.3390/molecules18043725 (2013).
Li, S. G. & Zhang, Y. Q. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym. 78, 343–348. https://doi.org/10.1016/j.carbpol.2009,04(0),pp.13 (2009).
Cui, R. T. et al. Suppressive effect of Astragalus membranaceus bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother. Pharmacol. 51, 75–80. https://doi.org/10.1007/s00280-002-0532-5 (2013).
National Administration of Traditional Chinese Medicine, Notice on Novel coronavirus pneumonia rehabilitation guidance for TCM rehabilitation (Trial Implementation) (2020), http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-23/13319.html.
Chen, R. Z. et al. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 77, 434–443. https://doi.org/10.1016/j.indcrop.2015.08.006 (2015).
Hirotani, M., Zhou, Y., Lui, H. & Furuya, T. Astragalosides from hairly root cultures of Astragalus membranaceus. Phytochemistry 36, 1407–1410. https://doi.org/10.1016/s0031-9422(00)89793-9 (1994).
Agyemang, K. J. et al. Recent advances in Astragalus membranaceus anti-diabetic research: Pharmacological effects of its phytochemical constituents. EC AM. 2013, 1–9. https://doi.org/10.1155/2013/654643 (2013).
Wu, H. H., Li, H. L., Hou, J. L. & Wang, W. Q. Research progress on germplasm resources of huangqi (Radix astragali). Guid. J. Trad. Chin. Med. Pharm. 24, 76–79 (2016).
Chen, H. H., Gong, S. X., Zhang, T. J., Xiao, T. T. & Wang, S. L. Chemical constituents and pharmacological function of stems and leaves of Radix astragali and their application. Drug Eva. Res. 34, 134–137 (2011).
Jiao, Y., Wen, J., Yu, X. H. & Zhang, D. S. Influence of flavonoid of Astragalus membranaceus’s stem and leaves on the function of cell mediated immunity in mice. J. Tradit. Chin. Med. 19, 356–358. https://doi.org/10.1007/BF02935185 (1999).
Liu, P., Zhao, H. & Luo, Y. Anti-aging implications of Astragalus membranaceus (huangqi): A well-known chinese tonic. Aging Dis. 8, 868–886. https://doi.org/10.14336/AD.2017.0816 (2017).
Wang, Q. H. et al. Three flavonoids from the leaves of Astragalus membranaceus and their antifungal activity. Mon. Chem. 146, 1771–1775. https://doi.org/10.1007/s00706-015-1473-0 (2015).
Yang, D. D. et al. Comparative analysis of chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crops Prod. 142, 111844. https://doi.org/10.1016/j.indcrop.2019.111844 (2019).
Iyda, J. H. et al. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 121, 714–722. https://doi.org/10.1016/j.foodres.2018.12.046 (2019).
Samsonowicz, M., Regulska, E., Karpowicz, D. & Leśniewska, B. Antioxidant properties of coffee substitutes rich in polyphenols and minerals. Food Chem. 278, 101–109. https://doi.org/10.1016/j.foodchem.2018.11.057 (2019).
Jiang, C., Zeng, Z., Huang, Y. & Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium Cristatum and their lipid-lowering activity. LWT. 98, 204–211. https://doi.org/10.1016/j.lwt.2018.08.007 (2018).
S. Fa. D. Administration, N. Ha. F. P. Commission, National food safety standards determination of amino acids in foods (2016). http://www.eshian.com/standards/37320.html.
Dong, X., Huang, Y., Wang, Y. & He, X. Anti-inflammatory and antioxidant jasmonates and flavonoids from lychee seeds. J. Funct. Foods. 54, 74–80. https://doi.org/10.1016/j.jff.2018.12.040 (2019).
Jin, L. et al. Antioxidant properties and color parameters of herbal teas in China. Ind. Crops Prod. 87, 198–209. https://doi.org/10.1016/j.indcrop.2016.04.044 (2016).
Babahmad, R. A. et al. Chemical composition of essential oil of Jatropha curcas L. leaves and its antioxidant and antimicrobial activities. Ind. Crops Prod. 121, 405–410. https://doi.org/10.1016/j.indcrop.2018.05.030 (2018).
Lai, P. K. K. et al. Isolation of anti-inflammatory fractions and compounds from the root of Astragalus membranaceus. Phytother. Res. 27, 581–587. https://doi.org/10.1002/ptr.4759 (2012).
Hou, Y. Q., He, W. L., Hu, S. D. & Wu, G. Y. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51, 1153–1165. https://doi.org/10.1007/s00726-019-02751-0 (2019).
Huang, Y., Wang, T. J., Fillet, M., Crommen, J. & Jiang, Z. J. Simultaneous determination of amino acids in different teas using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J. Pharm. Anal. 9, 254–258. https://doi.org/10.1016/j.jpha.2019.05.001 (2019).
Noreen, H., Semmar, N., Farman, M. & James, S. O. M. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 10, 792–801. https://doi.org/10.1016/j.apjtm.2017.07.024 (2017).
Acknowledgements
This work was supported by Gansu Provincial Department of Agriculture and Rural Areas Foundation to Prof. Yuan Chen for Evaluation of Gansu Provincial Special Advantage Agricultural Product- Longxi Huangqi (TYNPZ2020-18) and Foundation for a Chief Expert of Traditional Chinese Medicinal Industry in Modern Agricultural Industry System (GARS-ZYC-1); National Administration of Traditional Chinese Medicine (ZYBZH-Y-GS-11); Science and Technology Department of Gansu Province Support Project (1204FKCA123); Gansu Zhongtian Pharmaceutical Co., Ltd on the GAP Base Construction of Astragalus membranaceus (2017-2020) and SRTP Project of Gansu Agricultural University (202111018, 202101045).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. A.O.S. and B.H. prepared and wrote the manuscript, did all the lab work. J.J. and D.Y. prepared the experimental design and setup. F.-X.G. and Y.C. are supervising scientists.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Acknowledgments section. “This work was supported by National Administration of Traditional Chinese Medicine (ZYBZH-Y-GS-11); Science and Technology Department of Gansu Province Support Project (1204FKCA123); Gansu Provincial Department of Agriculture and Rural Areas Foundation to Prof. Yuan Chen for a Chief Expert of Traditional Chinese Medicinal Industry in Modern Agricultural Industry System (GARS-ZYC-1) and once again to Prof. Yuan Chen for Special Advantage Agricultural Products in Gansu Province -Evaluation of Longxi Huangqi (TYNPZ2020-18), Gansu Zhongtian Pharmaceutical Co., Ltd on the GAP Base Construction of Astragalus membranaceus (2017-2020) and SRTP Project of Gansu Agricultural University (202111018, 202101045).” now reads: “This work was supported by Gansu Provincial Department of Agriculture and Rural Areas Foundation to Prof. Yuan Chen for Evaluation of Gansu Provincial Special Advantage Agricultural Product- Longxi Huangqi (TYNPZ2020-18) and Foundation for a Chief Expert of Traditional Chinese Medicinal Industry in Modern Agricultural Industry System (GARS-ZYC-1); National Administration of Traditional Chinese Medicine (ZYBZH-Y-GS-11); Science and Technology Department of Gansu Province Support Project (1204FKCA123); Gansu Zhongtian Pharmaceutical Co., Ltd on the GAP Base Construction of Astragalus membranaceus (2017-2020) and SRTP Project of Gansu Agricultural University (202111018, 202101045).”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samuel, A.O., Huang, BT., Chen, Y. et al. Antioxidant and antibacterial insights into the leaves, leaf tea and medicinal roots from Astragalus membranaceus (Fisch.) Bge.. Sci Rep 11, 19625 (2021). https://doi.org/10.1038/s41598-021-97109-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97109-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.