Abstract
Our recent study on full-term toddlers demonstrated that daytime nap properties affect the distribution ratio between nap and nighttime sleep duration in total sleep time but does not affect the overall total amount of daily sleep time. However, there is still no clear scientific consensus as to whether the ratio between naps and nighttime sleep or just daily total sleep duration itself is more important for healthy child development. In the current study, to gain an answer to this question, we examined the relationship between the sleep properties and the cognitive development of toddlers born prematurely using actigraphy and the Kyoto scale of psychological development (KSPD) test. 101 premature toddlers of approximately 1.5 years of age were recruited for the study. Actigraphy units were attached to their waist with an adjustable elastic belt for 7 consecutive days and a child sleep diary was completed by their parents. In the study, we found no significant correlation between either nap or nighttime sleep duration and cognitive development of the preterm toddlers. In contrast, we found that stable daily wake time was significantly associated with better cognitive development, suggesting that sleep regulation may contribute to the brain maturation of preterm toddlers.
Similar content being viewed by others
Introduction
Children’s sleep architecture develops rapidly during the first 5 years of life bringing about dramatic changes in their sleep patterns. During this period, the duration and frequency of daytime naps diminishes and they begin to adopt a more consolidated nighttime sleep, like that in adults. In a previous study, we examined the sleep properties of full-term toddlers approximately 1.5 years of age and demonstrated that nap duration directly influences the distribution ratio between nap and nighttime sleep but does not affect overall total daily sleep duration1. There is, however, still an ongoing debate surrounding the two hypotheses on whether either nap or nighttime sleep contributes more to the proper cognitive development of children or whether appropriate intellectual development depends merely on daily total sleep duration2,3.
In the current study, to gain an answer to this question, we examined the relationship between the sleep properties and cognitive development of 101 toddlers who had been born prematurely (preterm toddlers) and whose physiological and psychological data had been systematically collected from birth. Focusing on the early developmental stage of approximately 1.5 years of age, when the basic sleep structure of young children has been reported to be established1,4,5,6,7,8,9,10,11,12,13,14, we examined the effects of sleep maturation on the cognitive development of the preterm toddlers in order to find which sleep variables, such as nap, nighttime sleep, total sleep duration, or other sleep variables, contribute to their cognitive development.
Results
Sleep properties of the preterm toddlers
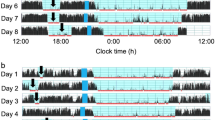
The characteristics of the 101 toddlers are shown in Table 1. No significant difference in characteristics by gender was detected (p < 0.05). The toddlers’ sleep arrangements are shown in Table 2. No significant difference in sleep arrangements by gender was detected except for “Putting children to sleep with formula” (p = 0.024), suggesting that more male toddlers were fed with formula at onset of nighttime sleep. The toddlers’ sleep variables such as bedtime, wake time, nighttime sleep duration, and nap duration are shown in Table 3 (Supplementary Data 1 and 2). No differences were found between boys and girls among the 15 different sleep variables (t-test, p > 0.05) except for daily variation in wake time, nap onset time and sleep efficiency. Boys were found to have more daily variation in wake time (p = 0.048), lower sleep efficiency (p = 0.035), and an earlier nap onset time (p = 0.017) compared to girls. Figure 1 demonstrates the representative daily activity-rest patterns of the approximately 1.5-year-old toddlers, indicating the existence of various nap patterns among the toddlers. There was a significant negative correlation between nap duration and nighttime sleep duration (r = − 0.517, p = 0.000), suggesting that longer nap duration induces shorter nighttime sleep duration (Fig. 2), as we previously reported1.
The actograms show representative daily activity-rest patterns of preterm toddlers with stable daily wake times (a) (an infant with DQ96) and unstable daily wake times (b) (an infant with DQ73). The vertical axis shows the 7 consecutive observation days and the horizontal axis shows the course of each 24 h day from 12:00 h (00:00 pm). Activity counts per minute are represented by the height of the vertical black bars on each actogram. The arrows and the blue rectangles indicate naps and bathing periods, respectively. The red underlines are the periods that were automatically judged as sleep periods by the actigraph software. Note that the wake times are recognized as relatively regular starts of the vertical black bars at around 6:00 h (06:00 am) in (a) but as irregular starts of the vertical black bars between 6:00 h (06:00 am) and 9:00 h (09:00 am) in (b).
Effects of sleep on cognitive development of the preterm toddlers
Before logistic regression analysis, univariate regression analysis was performed in order to select variables (Table 4). Next, to evaluate possible factors contributing to the toddlers’ cognitive development, we performed a logistic regression analysis for the effects of birth profiles, respiratory complications, sleep variables and sleep arrangements on the cognitive development (DQ (Developmental Quotient) scores of the KSPD test) of the preterm toddlers (Table 5). According to analysis of DQ scores in model 1, which was adjusted for birth profile characteristics such as gender and birth weight, no significant odds ratios (ORs) for toddlers with a DQ score of ≥ 93.4 (mean) were found. In model 2, which adds the respiratory complications of prolonged ventilation and non-significant chronic lung disease (CLD) to model 1, no significant ORs for toddlers with a DQ score of ≥ 93.4 (mean) were found. In model 3, which adds the sleep variables of daily variation (standard deviation) of wake time, daily variation (standard deviation) of sleep onset time, sleep onset time, and total sleep duration to model 2, the ORs for toddlers with a DQ score of ≥ 93.4 (mean) were 0.964 (p = 0.014) for daily variation of wake time, indicating that greater daily variation of wake time is a significant predictor of lower DQ in toddlers, but failed to find any significant correlations with other sleep variables. In model 4, which adds the sleep arrangement factors of co-sleeping with parents, child attending kindergarten, and nighttime formula feeding to model 3, the ORs for toddlers with a DQ score of ≥ 93.4 (mean) were also 0.964 (p = 0.014) for daily variation of wake time, again indicating that greater daily variation of wake time is a significant predictor of lower DQ in toddlers, but failed to find any significant correlations with other variables.
Discussion
The present study indicates three significant findings concerning the sleep properties of preterm toddlers at approximately 1.5 years of age. First, our study describes a new finding that only daily variation of wake time, a sleep regulatory variable, is significantly associated with the cognitive development (the DQ scores of the KSPD test) of preterm toddlers in the logistic regression analysis (Table 5). This is inconsistent with a current working hypothesis that the DQ scores of preterm toddlers are significantly influenced by nap and/or nighttime sleep duration2,3. Rather, the maturation of the sleep regulatory mechanism, which controls wake time, contributes to or reflects the levels of preterm toddlers’ cognitive development. This is partly supported by the findings of previous studies in which the daily variation of wake time of full-term infants has been reported to decrease as infants mature15. Present data also suggests that, among toddlers, the cortex, which is responsible for cognitive functions, may also play an important role in sleep/wake transition as the final destination of the output from the GABAergic and/or the orexinergic pathway. It has been known that the sleep/wake transition of animals is modulated by their cognitive status, which is affected by environmental conditions such as feeding, mating, and predation. In particular, the orexinergic neurons of the lateral hypothalamus (LH) have been reported to increase wake in response to stress such as from reduced food availability16. So far, however, rather than the cortex, the GABAergic neurons of the ventrolateral preoptic area (VLPO) and brainstem and/or the LH orexinergic neurons have been hypothesized to mainly control the transition between wake and sleep status in mammals16.
The second significant finding is that the current study with preterm toddlers also agrees with a finding from our previous study with full-term toddlers, namely that there is a significant negative correlation between nap duration and nighttime sleep duration1, suggesting that longer nap durations may also lead to shorter nighttime sleep durations in preterm toddlers (Fig. 2). This indicates that the balance between nap and nighttime sleep duration is a strong sleep regulatory mechanism and also that we may be able to control the nighttime sleep duration of preterm and term toddlers effectively by controlling their nap durations. This is quite different from adults’ sleep regulatory system in which the circadian sleep mechanism plays a more powerful role, resulting in that adults do not have naps but only nighttime sleep12.
The third significant finding is that sex-based differences existed among toddlers in their cognitive development and sleep variables (Table 1 & 3). In cognitive development, the female toddlers had higher DQ scores than the male toddlers. This is consistent with the results of previous studies, in which increased intraventricular hemorrhage (IVH) and prolonged ventilatory support from pulmonary diseases among male preterm infants was reported to have contributed to their reduced cognitive development17,18. A group from Karolinska University Hospital speculates that IVH and prolonged ventilatory support may enlarge the sexual brain dimorphism already existing at the early developmental stage, leading to delayed myelination and lower white matter volumes in male brains, which may result in lower cognitive functions in preterm male toddlers17. In sleep variables, female toddlers had significantly less daily variation in wake time, higher sleep efficiency and later nap onset time, which may reflect more mature sleep regulatory mechanisms being associated with toddlers’ cortical function as we previously discussed.
Several concerns warrant consideration in the present study. First, this study did not examine whether the cortical maturation of toddlers’ brains may affect either their sleep regulatory mechanism and/or cognitive functions. That is, there is a possibility that unstable wake time may simply reflect toddlers’ brain immaturity. To investigate this possibility, we would have to artificially improve or hamper toddlers’ intellectual development and evaluate its effects on sleep regulation. However, such an experimental design has not been scientifically established nor, even if it were, could be ethically approved for use in human studies. Second, although the sleep habits of toddlers are affected by those of their parents, especially their mothers19, the present study did not investigate the sleep habits of the parents themselves. Third, several sleep variables related to birth profiles, such as gestational age at birth, were not added as a dependent variable to the logistic regression analysis of the DQ scores of the toddlers to avoid multicollinearity between birth weight and gestational age at birth, although previous studies indicated significant association between gestational age at birth and brain development using psychological assessments and physiological measurements such as those made by EEG20,21,22,23 (Table 5 and Supplementary Data 3–5). Fourth, although the sleep habits of toddlers would also be affected by their temperament such as mood, adaptability to a new situation, attention span or sensory threshold to stimuli or pain, the present study did not investigate the effect of toddlers’ temperament on sleep variables21. Fifth, although co-sleeping with parents would have similar positive effects to those of kangaroo care on the cognitive development of preterm toddlers24, the present study was not able to investigate possible significant effect of co-sleeping on toddlers’ DQs as we could not be sure if the preterm toddlers had co-slept with their parents continuously since their discharge from NICUs, or whether they had begun to sleep separately before reaching one year of age in compliance with SIDS prevention recommendations25.
Methods
Participants
Preterm toddlers of approximately 1.5 years of age were recruited from Hokkaido University Hospital (Sapporo, Japan), Sapporo City Hospital (Sapporo, Japan), St. Luke’s International Hospital (Tokyo, Japan), Toho University Hospital (Tokyo, Japan), Japanese Red Cross Medical Center (Tokyo, Japan) and Kanazawa University Hospital (Kanazawa, Japan). Inclusion criteria were as follows: (1) preterm birth [defined as being born at less than 36 weeks’ gestational age and having a birth weight of less than 1500 g (very low birth weight)] and (2) the absence of chromosomal or other major genetic abnormalities, suspected neuromuscular disorders, intraventricular hemorrhage or significant chronic lung disease (CLD). Non-significant CLD was not considered a factor for exclusion. We defined non-significant CLD as requiring ventilation or/and oxygen at 36 weeks corrected gestational age but not at discharge. Exclusion criteria was parental language difficulties. Age correction was performed as follows: the duration between expected birth date and actual birth date was subtracted from the actual age to calculate the chronological developmental stage. Of 105 eligible toddlers, 4 were excluded because sleep data were invalid due to technical problems with the activity recording devices or incomplete descriptions in sleep diary. The final sample thus consisted of 101 preterm toddlers (44 boys, 57 girls). The ethics committees of Hokkaido University Hospital, Sapporo City Hospital, St. Luke’s International Hospital, Toho University Hospital, Japanese Red Cross Medical Center, Kanazawa University Hospital and Akita University Hospital approved the study protocol (UMIN000021153) and all procedures were carried out in accordance with the approved guidelines. Written informed consent was obtained from the parents.
Activity and sleep assessment
For activity and sleep assessment we used actigraphy and sleep diaries, as previously described1. Briefly, the parents were instructed to attach Actigraphs (Micro-mini RC, Ambulatory Monitoring Inc., NY, USA) to their child’s waist with an adjustable elastic belt for 7 consecutive days1. The activity data recorded by the Actigraph were later downloaded using ActMe software (ver. 3.10.0.3, Ambulatory Monitoring Inc., NY, USA), and then sleep measurements were analyzed using Action-W software (ver. 2.4.20, Ambulatory Monitoring Inc., NY, USA). Time intervals during the study when the Actigraph was removed, for example, during bathing, were recorded by parents in a sleep diary1. The sleep diary was composed of seven 24-h single-sheet schedules, on which parents were asked to record details such as time of nap, going in/out of bed, bathing and night wakings of which they were aware. Sleep diary data were used to define the scoring interval for actigraphic sleep measurement, according to the procedure outlined by Acebo and colleagues4.
Neurodevelopmental assessment
The assessment of the cognitive function of the preterm infants was performed at approximately 1.5 years of age using the Kyoto Scale of Psychological Development (KSPD) test, as previously described26. Briefly, experienced testers who were certified psychologists administered the KSPD test, blinded to the perinatal details of the toddlers. It usually takes approximately 20–40 min to administer. The KSPD is standardized for all subjects ranging from neonates to adults of 29 years of age. This scale consists of 328 items covering the Cognitive-Adaptive area (C-A), Language-Social area (L-S), and Postural-Motor area (P-M). The C-A section assesses non-verbal reasoning and visuospatial perception. The L-S section assesses interpersonal relationships, socialization and verbal abilities. The P-M section assesses fine motor functions. The developmental age is estimated according to the sum score of the three sections. The DQ is then calculated by dividing the developmental age by the chronological age and then multiplying it by 100. A DQ score of 100.6 ± 13.4 represents the mean ± 1 s.d. at the time of standardization26.
Statistical analysis
A Student’s t-test for continuous data or a χ2 test for categorical data was performed to compare the characteristics of participants by gender and a χ2 test was used to compare the sleep arrangements and sleep variables by gender (Table1, 2, and 3) after confirming that all data fulfilled the requirements for normality and equal variances. Univariate regression analysis was performed before logistic regression analysis (Table 4). The degrees of correlation between the cognitive development parameter (DQ scores of the KSPD test) and birth profiles, respiratory complications, sleep variables, and sleep arrangement factors were assessed using the Spearman correlation test. Only variables with relatively significant values (p < 0.2) in the Spearman correlation tests were included in logistic regression analysis. Logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals as estimates of effects, with the DQ scores of the toddlers as the outcome variable (Table 5). Statistical analyses were performed with SPSS Statistics 25.0 (IBM Corp. Armonk, NY, USA).
References
Nakagawa, M. et al. Daytime nap controls toddlers’ nighttime sleep. Sci. Rep. 6, 27246. https://doi.org/10.1038/srep27246 (2016).
Horváth, K., Myers, K., Foster, R. & Plunkett, K. Napping facilitates word learning in early lexical development. J. Sleep Res. 5, 503–509. https://doi.org/10.1111/jsr.12306 (2015).
Horváth, K., Liu, S. & Plunkett, K. A daytime nap facilitates generalization of word meanings in young toddlers. Sleep 39, 203–207. https://doi.org/10.5665/sleep.5348 (2016).
Acebo, C. et al. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep 28, 1568–1577. https://doi.org/10.1093/sleep/28.12.1568 (2005).
Harada, T., Hirotani, M., Maeda, M., Nomura, H. & Takeuchi, H. Correlation between breakfast tryptophan content and morning-evening in Japanese infants and students aged 0–15 yrs. J. Physiol. Anthropol. 26, 201–207. https://doi.org/10.1093/sleep/28.12.1568 (2007).
Asaka, Y. & Takada, S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 32, 150–155. https://doi.org/10.1016/j.braindev.2008.12.006 (2010).
Nakade, M., Takeuchi, H., Taniwaki, N., Noji, T. & Harada, T. An integrated effect of protein intake at breakfast and morning exposure to sunlight on the circadian typology in Japanese infants aged 2–6 years. J. Physiol. Anthropol. 28, 239–245. https://doi.org/10.1093/sleep/28.12.1568 (2009).
Akacem, L. D. et al. The timing of the circadian clock and sleep differ between napping and non-napping toddlers. PLoS ONE 10, e0125181. https://doi.org/10.1371/journal.pone.0125181 (2015).
Thorpe, K. et al. Napping, development and health from 0 to 5 years: A systematic review. Arch. Dis. Child. 100, 615–622. https://doi.org/10.1136/archdischild-2014-307241 (2015).
Fukuda, K. & Sakashita, Y. Sleeping pattern of kindergartners and nursery school children: Function of daytime nap. Percept. Mot. Skills 94, 219–228. https://doi.org/10.2466/pms.2002.94.1.219 (2002).
Yokomaku, A. et al. A study of the association between sleep habits and problematic behaviors in preschool children. Chronobiol. Int. 25, 549–564. https://doi.org/10.1080/07420520802261705 (2008).
Komada, Y. et al. Relationship between napping pattern and nocturnal sleep among Japanese nursery school children. Sleep Med. 13, 107–110. https://doi.org/10.1016/j.sleep.2011.10.017 (2012).
Staton, S. L., Smith, S. S., Pattinson, C. L. & Thorpe, K. J. Mandatory naptimes in child care and children’s nighttime sleep. J. Dev. Behav. Pediatr. 36, 235–242. https://doi.org/10.1097/DBP.0000000000000157 (2015).
Staton, S. L., Smith, S. S., Hurst, C., Pattinson, C. L. & Thorpe, K. J. Mandatory nap times and group napping patterns in child care: an observational study. Behav. Sleep Med. 11, 1–15. https://doi.org/10.1080/15402002.2015.1120199 (2016).
Miike, T. How to analyze and evaluate data from a self-reported sleep diary. In Practical and Clinical Pediatric Sleep Medicine Required for Pediatricians at Present (eds Miike, T. et al.) 120–129 (Shindan to Chiryo Sha Inc., 2015).
Eban-Rothschild, A., Giardino, W. J. & de Lecea, L. To sleep or not to sleep: Neuronal and ecological insights. Curr. Opin. Neurobiol. 44, 132–138. https://doi.org/10.1016/j.conb.2017.04.010 (2017).
Stålnacke, S. R., Tessma, M., Böhm, B. & Herlenius, E. Cognitive development trajectories in preterm children with very low birth weight longitudinally followed until 11 years of age. Front. Physiol. 10, 307. https://doi.org/10.3389/fphys.2019.00307 (2019).
Townsel, C. D., Emmer, S. F., Campbell, W. A. & Hussain, N. Gender differences in respiratory morbidity and mortality of preterm neonates. Front. Pediatr. 5, 6. https://doi.org/10.3389/fped.2017.00006 (2017).
Komada, Y. et al. Short sleep duration and irregular bedtime are associated with increased behavioral problems among Japanese preschool-age children. Tohoku J. Exp. Med. 224, 127–136. https://doi.org/10.1620/tjem.224.127 (2011).
Hediger, M. L., Overpeck, M. D., Ruan, W. J. & Troendle, J. F. Birthweight and gestational age effects on motor and social development. Paediatr. Perinat. Epidemiol. 16, 33–46. https://doi.org/10.1046/j.1365-3016.2002.00393.x (2002).
Lavanga, M. et al. The effect of early procedural pain in preterm infants on the maturation of electroencephalogram and heart rate variability. Pain 162, 1556–1566. https://doi.org/10.1097/j.pain.0000000000002125 (2021).
De Ridder, J. et al. Prediction of neurodevelopment in infants with tuberous sclerosis complex using early EEG characteristics. Front. Neurol. 11, 582891. https://doi.org/10.3389/fneur.2020.582891 (2020).
Pavlidis, E., Lloyd, R. O., Mathieson, S. & Boylan, G. B. A review of important electroencephalogram features for the assessment of brain maturation in premature infants. Acta Paediatr. 106, 1394–1408. https://doi.org/10.1111/apa.13956 (2017).
Messmer, P. R. et al. Effect of kangaroo care on sleep time for neonates. Pediatr. Nurs. 23, 408–414 (1997).
Moon, R. Y. & Task Force On Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: Updated 2016 recommendations for a safe infant sleeping environment. Pediatrics 138, 2016. https://doi.org/10.1542/peds.2016-2938 (2016).
Ishii, N. et al. Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics 132, 62–71. https://doi.org/10.1542/peds.2012-2857 (2013).
Acknowledgements
We thank all the participants and their parents for their generous contributions. This work was supported by Grants-in-Aid for Scientific Research (to H.O. # 26650176 and K.C. # H30W05) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants (to M.N.) from St. Luke’s Life Science Institute and the Public Health Research Foundation, and grants (to H.O., Y.Y., Y.K., and K.C.) from the JST Center of Innovation (COI).
Author information
Authors and Affiliations
Contributions
H.O., Y.Y., Y.A., I.K., H.Y., M.K. and K.C. conceived of the study and designed the experiments. A.A., H.O., Y.Y., M.N., Y.A., T.N., Y.M., Y.O., M.M., H.A., Y.K., K.M., R.S., M.H., T.I., R.F., K.K., M.O., M.T., K.M., I.K., H.Y., M.K., and K.C. performed and analyzed the experiments. A.A., H.O., Y.Y., M. N., Y.A., Y.M., A.M., T.T., K.M., I.K., H.Y., M.K. and K.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ando, A., Ohta, H., Yoshimura, Y. et al. Sleep maturation influences cognitive development of preterm toddlers. Sci Rep 11, 15921 (2021). https://doi.org/10.1038/s41598-021-95495-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95495-5
This article is cited by
-
Preterm toddlers have low nighttime sleep quality and high daytime activity
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.