Abstract
Cystic fibrosis (CF) is a multi-system disease that is characterized by lung disease due to recurrent airway infection and inflammation. Endocrine complications, such as CF bone disease (CFBD), are increasingly identified as patients are living longer. The cause of CFBD is multifactorial with chronic systemic inflammation theorized to be a contributing factor. Thus, we attempted to identify inflammatory biomarkers that are associated with CFBD. We conducted a retrospective observational study of 56 adult patients with CF with an average percentage predictive forced expiratory volume in one second (ppFEV1) of 73.7% (standard deviation: 30.0) who underwent baseline serum analysis for osteoprotegerin (OPG) and pro-inflammatory biomarkers (IL-1β, IL-6, IL-8 and TNF-α), and had repeated dual-energy x-ray absorptiometry (DXA) scans separated by at least 2 years to examine correlations between serum biomarkers and bone mineral density (BMD) measurements. Univariate linear regression model analysis demonstrated that serum IL-1β and IL-8, but not other pro-inflammatory markers, were negatively correlated with baseline BMD results. However, after accounting for confounding variables, only the relationship between IL-8 and left femoral neck BMD remained statistically significant. Additionally, IL-8 level was associated with BMD decline over time. These results suggest that IL-8 might play a unique role in the pathophysiology of CFBD relative to other pro-inflammatory cytokines but further study is warranted before firm conclusions can be made.
Similar content being viewed by others
Introduction
Cystic Fibrosis (CF) is a multisystem disorder that manifests predominantly in the lungs and pancreas, but also in other organs such as the intestines and liver. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) protein, a channel that is responsible for transcellular movement of chloride and bicarbonate, regulating epithelial surface fluid and mucous viscosity1.
As the life expectancy of CF patients increases due to advances in treatment and management of their condition, other complications, such as osteoporosis and osteopenia, collectively referred to as CF-related bone disease (CFBD), are becoming more prevalent. Based on retrospective analyses, 24% of CF adult patients have CFBD2,3, which further increases to 55–65% in CF patients > 45 years of age4,5. In addition to age, other risk factors have been associated with low bone mineral density (BMD) including male sex, low body mass index (BMI), low lung function6, and having dysglycemia as a comorbidity7. Consequently, the CF Foundation recommends routine screening with the dual-energy X-ray absorptiometry (DXA) scan to assess for bone mineral density (BMD) starting from the age of 98. Physiologically, bone density is a reflection of the mineral content in a given volume of bone, which is subject to a balance between osteoblasts mediating bone building activity and osteoclasts contributing to bone breakdown or resorption. There are likely multiple factors in CF that confers an imbalance between osteoblast and osteoclast activity that may result in CFBD, including CFTR dysfunction directly impairing new bone formation9 via reduced osteoblast activity10, as well as secondary causes, including malabsorption of calcium and vitamin D as a result of pancreatic insufficiency and recurrent pulmonary infections that lead to increased inflammatory bone resorption2,4,11,12.
The mechanism by which inflammation mediates bone resorption is complex and this relationship has been consistently documented in CF. At baseline, markers of bone mass turnover and formation, such as osteocalcin, are reduced in the CF population compared to the non-CF population13,14,15 while the receptor activator of nuclear factor kappa-Β ligand (RANK-L), a marker of bone resorption, is elevated in the CF population at baseline when compared to the non-CF population13,16,17. During periods of heightened systemic inflammation seen with pulmonary exacerbations, up-regulation of RANK-L and osteoclast activity have been demonstrated13,16,17. Circulating pro-inflammatory cytokines, such as interleukin (IL)-6, C-reactive protein (CRP), tumour necrosis factor (TNF)-α and IL-1β have been shown to be correlated with markers of bone resorption11,12 and inversely correlated to markers of bone formation18. Similarly, when human CF osteoblasts were provided with a pro-inflammatory stimulus, RANK-L production increased markedly while osteoprotegerin (OPG), a competitive inhibitor of RANK-L signalling, was found in relatively lower concentrations than RANK-L and as a result, osteoclastogenesis was favoured16. It remains unclear how these in vitro findings may impact clinical changes to BMD and CFBD development.
Here, we postulate that pro-inflammatory biomarkers, such as IL-1β, IL-6, IL-8, and TNF- α are inversely correlated to BMD measurements, the clinical manifestation of increased osteoclast activity. By longitudinally assessing a retrospective adult CF cohort with repeated DXA measurements over years, we further hypothesize that these pro-inflammatory markers may be associated with bone loss over time.
Results
Cohort selection
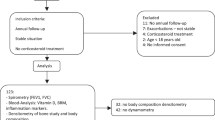
Based on the inclusion/exclusion criteria, 56 patients were included in this study. Of these, 5 subsequently went on to receive a lung transplant following baseline assessment. As BMD measurements after transplantation were excluded, 4 of these 5 individuals only contributed baseline BMD results to the analysis. Of the 52 patients who contributed repeated BMD measurements, the median number of BMD measurements per patient was 2 (minimum 2; maximum 5 measurements including baseline). The median time between first and last BMD measurements was 3.44 years (minimum 2 years and maximum 7.62 years).
Clinical and biochemical assessments
Baseline clinical and biomarker characteristics
For the 56 patients included, 37 (66%) subjects were male, the mean age was 35.6 years (standard deviation [SD] 12.2), and ppFEV1 was 73.7% (SD 30.0) (Table 1). Twenty-one (38%) subjects were homozygous for ΔF508 and 19 (34%) were heterozygous for ΔF508. Forty-two (75%) subjects had pancreatic insufficiency, 14 (26%) had CF-related diabetes (CFRD), and 35 (63%) were sputum positive for Pseudomonas aeruginosa (PsA). Six (11%) patients were on a bisphosphonate at baseline. 6 patients were started on CFTR modulator therapy during the period of study where only 1 patient was on treatment at the time of their baseline BMD assessment. Baseline BMD measurements as well as serum OPG and pro-inflammatory biomarker levels are reported in Table 1.
DXA measurements
At baseline, 12 (22%) subjects had BMD scores within the osteoporotic range while 19 (35%) were within the osteopenic range (Table 1). The majority of baseline z scores fell within normal limits (i.e. greater than − 1) (Supplemental Table S1). One subject had a history of previous fracture at the start of the study period.
Correlation between baseline clinical characteristics and biomarkers
Univariate linear regression analysis was performed to identify potential correlations between serum biomarkers and clinical characteristics, which may act as confounders in the relationship between serum biomarkers and BMD. ppFEV1 was inversely correlated with TNF-α, IL-6 and IL-1β while BMI was inversely correlated with OPG and IL-1β (Supplemental Table S2). CFRD status was positively associated with IL-8 such that those with CFRD had higher IL-8 levels. No other significant relationships between clinical characteristics and biomarker levels were found.
Correlation between baseline BMD measurements with baseline clinical characteristics and biomarkers
Univariate and multivariate linear regression modeling was next undertaken to determine which clinical characteristics or serum biomarkers were associated with baseline BMD results (Table 2).
In univariate analysis, BMI and ppFEV1 were positively associated with all baseline BMD measurements. Age was negatively associated with bilateral femoral neck BMD and male sex was negatively associated with L-spine BMD. Additionally, sputum positivity for Pseudomonas aeruginosa (PsA) was negatively associated with right femoral neck BMD. In multivariate analysis adjusted for other clinical covariates, age and male sex were negatively correlated with BMD and BMI remained positively correlated with BMD. Conversely, ppFEV1 no longer demonstrated a correlation with BMD following adjustment.
In univariate analysis, statistically significant negative correlations were observed between IL-8 with all BMD measurements and IL-1β with bilateral hip and femoral neck z scores (Table 2). In multivariate analysis adjusted for age, sex, ppFEV1, BMI and CFRD, both of these biomarkers demonstrated a weaker relationship with baseline BMD and only the negative correlation between IL-8 and left femoral neck z score remained statistically significant (Table 3).
Correlation between blood biomarkers and rate of BMD change over time
We next examined the relationship between clinical characteristics or baseline blood biomarkers with bone density change over time using a multivariate linear mixed effect models, adjusted for age, sex, ppFEV1, BMI and CFRD (Table 4). CFRD was positively associated with changes in L-spine and right hip BMD such that individuals with CFRD had an increase in BMD over time. As well, a positive correlation was found between IL-8 levels and changes in bilateral hip and femoral neck BMD. Categorical analysis of IL-8 levels divided into quartiles found that those with the highest IL-8 levels had the lowest BMD at baseline and the slowest rate of bone loss while those with the lowest IL-8 levels had the highest BMD at baseline and the fastest rate of bone loss over time (Fig. 1). Additionally, TNF-α was also positively correlated with changes in left femoral neck BMD over time (Table 4). No other relationships between clinical characteristics or baseline biomarkers and changes in BMD were demonstrated.
Predicted BMD measurements over time from linear mixed effect models using the first quartile, median, and the third quartile values of IL-8. (A) Right hip Z score; (B) left hip Z score; (C) right femoral neck Z score; (D) left femoral neck Z score. All other variables in the model were held constant at their means for continuous covariates, and categorical covariates at their proportions.
Six subjects were on bisphosphonates at baseline, which could have attenuated the rate of BMD change. Similarly, six individuals were initiated on CFTR modulators at various points during this study with one individual already on treatment at baseline, which may also have affected the rate of BMD change. Therefore, sensitivity analyses were performed to determine if individuals on bisphosphonates or CFTR modulators affected the multivariate analysis findings (Supplemental Table S3, S4). Exclusion of these subjects resulted in no significant change and the positive associations remained between IL-8 and bilateral hip and femoral neck BMD changes.
Discussion
CFBD is an increasingly common complication seen in CF patients caused in part by increased levels of systemic inflammation stemming from recurrent pulmonary exacerbations. While prior studies have demonstrated a relationship between systemic inflammation and markers of bone resorption during heightened states of inflammation (i.e. pulmonary exacerbations)12, in this study, we examined the association between systemic inflammation during clinical stability and BMD. Overall, our findings suggest that inflammation is associated with a reduced BMD at baseline but is not likely to be predictive of bone loss over time.
The pro-inflammatory cytokines examined in this study were selected due to their putative roles in the pathogenesis of inflammatory bone disease and osteoporosis by synergistically stimulating osteoclastogenesis and/or inhibiting osteoblasts19. Both serum IL-8 and IL-1β were found to be inversely correlated with baseline BMD in the crude analysis but only the relationship between IL-8 and left femoral neck BMD remained statistically significant following adjustment for clinical covariates. While it is possible that a larger sample size might have identified additional statistically significant relationships between inflammatory markers and baseline BMD, the relationship observed between serum IL-8 and left femoral neck BMD suggests that it may possibly play a unique role in the pathogenesis of CFBD. Based on the results of an in vitro study by Le Heron et al., inhibiting CFTR in osteoblasts results in the enhanced production of IL-8 and a reduction in OPG and thus may contribute to inflammation-driven bone loss9. This important finding suggests that CFTR dysfunction can directly impact on bone health, independent of inflammation stemming from pulmonary exacerbations, and that IL-8 could be one of the key mediators of this effect. Thus, the hypothesized role of IL-8 in the pathogenesis of CFBD deserves further study and validation.
Contrary to expectations based on our cross-sectional findings, the group of individuals with the highest IL-8 levels experienced a slower rate of bone loss over time relative to the group with the lowest IL-8 levels (Fig. 1). However, the group with the highest IL-8 levels started with a lower BMD to begin with and therefore the lower rate of change in BMD may have been a result of the “floor effect” as those individuals with the lowest BMD had the least to lose, whereas those individuals with the highest BMD had the most to lose. Furthermore, those with lowest baseline BMD may have received more aggressive interventions, such as use of bisphosphonates, over the course of the observational study compared to those with normal baseline BMD which could have attenuated the rate of bone loss in the group with the highest IL-8 level.
There are a number of limitations to our study that should be considered. As mentioned, this is a single center observational study focused on adults with a relatively small sample size and therefore it might be limited in terms of generalizability. In addition, BMD measurements were performed at different sites on different DEXA scanners throughout the study and therefore there may have been analytical differences in the reported BMD measurements. However, since the clinical variables that we identified to be correlated with BMD is consistent with what has been reported in the literature, the use of different DEXA scanners likely did not introduce significant variability to the data. Finally, our study focused on baseline biomarker measurements and thus it is not clear how ongoing fluctuations in these pro-inflammatory cytokines, such as during pulmonary exacerbations, might alter BMD over time and therefore a longitudinal study is required to examine this further.
In conclusion, serum IL-8 correlates with femoral bone BMD at baseline and may play an important role in inflammation-related bone disease in CF but further study is required before firm conclusions can be made. Given that both CFTR dysfunction and pulmonary exacerbations can result in an elevation of IL-89, CFTR modulators may have a beneficial effect on bone health by virtue of improving CFTR function and reducing pulmonary exacerbations. Future studies are required to fully understand the impact of CFTR modulators on CFBD.
Methods
Patient inclusion and exclusion criteria
Patients from the St. Paul’s Hospital Adult CF Clinic (Vancouver, Canada) enrolled in a single-centre CF Biomarker study between 2012 and 2018 had serum samples collected during non-consecutive stable clinic visits. The inclusion criteria for this sub-study focused on bone health were: patients aged 19 years or older with a baseline DXA scan performed within one year of a stable visit blood sample and at least one follow-up DXA scan at least 2 years later. Patients on immunosuppressants or with a history of lung transplantation at baseline were excluded. If a patient subsequently underwent lung transplantation after baseline, BMD measurements collected after transplantation were excluded. The research protocol was approved by the University of British Columbia Providence Health Care Research Institute Research Ethics Board (REB#H16-01063). Informed patient consent was obtained for the use of blood samples from the CF Biomarker study (REB#H12-00835). This project was conducted in full accordance with the above research ethics board guidelines and regulations and in accordance with the Declaration of Helsinki.
Clinical data collection
Clinical characteristics corresponding to baseline blood sample collection were collected through chart review and included: patient age, genotype, pancreatic status, weight, height, disease co-morbidities, lung transplant status, medications, percentage predicted forced expiratory volume in one second (ppFEV1) and forced vital capacity in liters (FVC). DXA scan results measured as part of clinical care were collected from electronic medical records. Lumbar spine, bilateral hip, and bilateral femoral neck BMD were reported in g/cm2 and as z scores.
Serum analysis
Blood samples stored at − 80 °C were thawed and underwent batched measurement. OPG, a marker of bone formation, and pro-inflammatory markers (IL-1β, IL-6, IL-8 and TNF-α) were analyzed. Serum OPG levels were measured using Human TNFRSF11B (OPG) ELISA Kits (Thermo Scientific, Fredrick, MD, USA) with an assay sensitivity of 1 pg/mL. Serum pro-inflammatory cytokine levels (IL-1β, IL-6, IL-8, and TNF-α) were measured using the V-Plex Human Proinflammatory Panel II (4-Plex) kits (Meso Scale Diagnostics, Rockville, ML, USA) with an assay sensitivity of 0.04–0.07 pg/mL. Assay mean coefficient of variability (CV) for OPG, TNF-α, IL-6, IL-8 and IL-1β were 6.15%, 8.31%, 11.00%, 12.60% and 5.45%, respectively.
Statistical analysis
Clinical characteristics were described using simple descriptive statistics (e.g. means and percentages). A univariate linear regression model was used to estimate crude relationships between baseline blood biomarker levels or clinical characteristics and baseline BMD measurements. A multivariable linear regression model was used to determine relationships between baseline blood biomarkers levels or clinical characteristics and baseline BMD measurements after adjustment for covariates. Multivariate linear mixed effect regression models, which accounts for correlated BMD from each patient, was then used to estimate the correlation between each of the baseline biomarkers or clinical characteristics and rate of BMD change from baseline to the last BMD measured by introducing an interaction term (i.e., slope between repeated BMD measurements over time). Random intercepts for within-patient variation, comprised of an unstructured covariance pattern, was treated as a random effect in the mixed effects model and known or suspected predictors of bone loss (age, sex, BMI, ppFEV1, CFRD status) were also included20,21.
Serum biomarker levels were log10 transformed prior to analysis. The level of significance was set at P < 0.05 for all statistical analyses and all reported P values reflect two-tailed tests. All analyses were conducted using R version 3.5.2 statistical programming22 and “lme4” package23.
References
Rosenstein, B. J. & Cutting, G. R. The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel. J. Pediatr. 132, 589–595 (1998).
Paccou, J., Zeboulon, N., Combescure, C., Gossec, L. & Cortet, B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: A systematic literature review with meta-analysis. Calcif. Tissue Int. 86, 1–7. https://doi.org/10.1007/s00223-009-9316-9 (2010).
Braun, C., Bacchetta, J. & Reix, P. Insights into cystic fibrosis-related bone disease. Arch. Pediatr. 23, 857–866. https://doi.org/10.1016/j.arcped.2016.05.001 (2016).
Jacquot, J., Delion, M., Gangloff, S., Braux, J. & Velard, F. Bone disease in cystic fibrosis: New pathogenic insights opening novel therapies. Osteoporos Int. 27, 1401–1412. https://doi.org/10.1007/s00198-015-3343-3 (2016).
Plant, B. J., Goss, C. H., Plant, W. D. & Bell, S. C. Management of comorbidities in older patients with cystic fibrosis. Lancet Respir. Med. 1, 164–174. https://doi.org/10.1016/S2213-2600(13)70025-0 (2013).
Sheikh, S., Gemma, S. & Patel, A. Factors associated with low bone mineral density in patients with cystic fibrosis. J. Bone Miner. Metab. 33, 180–185. https://doi.org/10.1007/s00774-014-0572-z (2015).
Rana, M., Munns, C. F., Selvadurai, H., Briody, J. & Craig, M. E. The impact of dysglycaemia on bone mineral accrual in young people with cystic fibrosis. Clin. Endocrinol. (Oxf.) 78, 36–42. https://doi.org/10.1111/j.1365-2265.2012.04484.x (2013).
Aris, R. M. et al. Guide to bone health and disease in cystic fibrosis. J. Clin. Endocrinol. Metab. 90, 1888–1896 (2005).
Le Heron, L. et al. Cystic fibrosis transmembrane conductance regulator (CFTR) regulates the production of osteoprotegerin (OPG) and prostaglandin (PG) E2 in human bone. J. Cyst. Fibros 9, 69–72. https://doi.org/10.1016/j.jcf.2009.11.005 (2010).
Velard, F. et al. Cystic fibrosis and bone disease: Defective osteoblast maturation with the F508del mutation in cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Crit. Care Med. 189, 746–748. https://doi.org/10.1164/rccm.201312-2144LE (2014).
Shead, E. F. et al. Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 174, 306–311 (2006).
Aris, R. M. et al. Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 162, 1674–1678. https://doi.org/10.1164/ajrccm.162.5.2002100 (2000).
Baroncelli, G. I. et al. Bone demineralization in cystic fibrosis: Evidence of imbalance between bone formation and degradation. Pediatr. Res. 41, 397–403. https://doi.org/10.1203/00006450-199703000-00016 (1997).
Jakovska, T., Mecevska-Jovcevska, J., Petlickovski, A., Fustik, S. & Zorcec, T. Prevalence of low bone mass and vitamin D deficiency in pediatric and adult patients with cystic fibrosis in Republic of Macedonia. Pril (Makedon Akad. Nauk. Umet Odd Med Nauki) 35, 151–158 (2014).
Greer, R. M. et al. Abnormalities of the PTH-vitamin D axis and bone turnover markers in children, adolescents and adults with cystic fibrosis: Comparison with healthy controls. Osteoporos Int. 14, 404–411. https://doi.org/10.1007/s00198-003-1388-1 (2003).
Delion, M. et al. Overexpression of RANKL in osteoblasts: A possible mechanism of susceptibility to bone disease in cystic fibrosis. J. Pathol. 240, 50–60. https://doi.org/10.1002/path.4753 (2016).
Gensburger, D. et al. Reduced bone volumetric density and weak correlation between infection and bone markers in cystic fibrosis adult patients. Osteoporos Int. 27, 2803–2813. https://doi.org/10.1007/s00198-016-3612-9 (2016).
Street, M. E. et al. Analysis of bone mineral density and turnover in patients with cystic fibrosis: Associations between the IGF system and inflammatory cytokines. Horm. Res. 66, 162–168 (2006).
Zupan, J., Jeras, M. & Marc, J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem. Med. (Zagreb) 23, 43–63. https://doi.org/10.11613/bm.2013.007 (2013).
Conroy, S. & Murray, E. Let the question determine the methods: Descriptive epidemiology done right. Brit. J. Cancer 20, 20 (2020).
Hernan, M. A. & Robins, J. M. Causal Inferences: What If (Chapman & Hall, 2020).
R Core Team, R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing [Internet]. 2011.
Bates, D., Machler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Acknowledgements
We would like to acknowledge and thank patients from the St. Paul’s Hospital Adult CF clinic for their study participation.
Funding
CF Canada Gilead Sciences Fellowship Award (G. Y. L.), and Michael Smith Foundation for Health Research Scholar award (B. S. Q.) provided funding for clinician research time to prepare this manuscript. Additionally, the Rare Disease Foundation provided Grant support for this study.
Author information
Authors and Affiliations
Contributions
G.Y.L. is responsible for writing the main manuscript text. S.D. is responsible for the formal analysis and preparation of Fig. 1 and all tables. J.F. and X.Y.H. are responsible for the data curation and investigation. J.J. is responsible for the investigation. A.G. and S.K. are responsible for editing the manuscript. B.S.Q. is responsible for the conceptualization, methodology, funding acquisition and supervision of this project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
SK is the Vice President of Scientific Innovation at Qu Biologics, a Canadian clinical-stage biotechnology company. All other authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lam, G.Y., Desai, S., Fu, J. et al. IL-8 correlates with reduced baseline femoral neck bone mineral density in adults with cystic fibrosis: a single center retrospective study. Sci Rep 11, 15405 (2021). https://doi.org/10.1038/s41598-021-94883-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94883-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.