Abstract
Geodiversity refers to the variety of geological and physical elements as well as to geomorphological processes of the earth surface. Heterogeneity of the physical environment has an impact on plant diversity. In recent years, the relations between geodiversity and biodiversity has gained attention in conservation biology, especially in the context of climate change. In this study, we assessed the spatial and temporal change in plant’s community structure in a semi-arid region, Sayeret Shaked Long Term Ecosystem Research (LTER) station, Israel. Vegetation surveys were conducted on different hillslopes, either with or without rock covers in order to study the spatial trends of hillslope geodiversity. The surveys were conducted for two consecutive years (2016 and 2017), of which the second year was drier and hotter and therefore permitted to investigate the temporal change of plant’s community structure. The results of the spatial trends show that (1) geodiversity increases vegetation biodiversity and promotes perennial plants and those of the temporal change show that (2) the positive effect of geodiversity on plants’ community structure and species richness is greater in the drier year than that in a wetter year. The main insight is that in these drylands, hillslopes with higher geodiversity appear to buffer the effect of drier years, and supported a more diverse plant community than lower geodiversity hillslopes.
Similar content being viewed by others
Introduction
Ecosystems are defined as complex communities of plant, animal and micro-organisms as well as the environments they are living and interacting with. The recognition and integration of the non-living features as part of the ecosystem is nowadays crucial1 even thus the abiotic world is often considered as function of the biotic one and not as its own2. An increase awareness on the relevance of the abiotic services led to the recognition in recent years of the interconnections between species, habitats and natural processes3,4. In fact, recently the term and concept of geodiversity was adopted in the scientific community to define the complex geomorphological features that contribute to ecosystem services1,2,4,5.
Geodiversity is defined as the natural assemblage of abiotic conditions within an ecosystem including its geological, geomorphological, and pedological features6. Geodiversity encompasses the substrates, landforms, and physical processes that govern habitat development and sustainability5. It can be addressed as variability between and among sites and it interested all Earth’s zones: litho-, hydro-, cryo- and atmo-sphere. The concept of geodiversity includes not only all its components but their relations and the link with the ecosystem they sustain7. In this framework, the vary geodiversity features play a crucial role in the conservation of habitats and landscape1,8,9,10.
An important component of geodiversity is the soil stoniness. The position of stones on the ground surface regulates hydrological process, as it affects infiltration and evaporation11,12,13. In fact, during rainfall, stones at the soil surface intercept raindrops11, reducing the splash formed by the impact force of raindrops on the soil14 with the consequences for processes of water overland flow and soil erosion15,16. On the other hand, stones increase water intake rates by preventing surface sealing and crusting17. Also, stones on the ground surface act as an isolation by reducing the soil temperature during the day and increasing the temperature during the night18. This temperature regulation resulted in effects on soil–water evaporation19. These processes are dependent on the stones’ size distribution and cover percentage20. Linked to the stoniness on the ground surface, the distribution of the stones throughout the soil profile is an important component of geodiversity21,22,23. The presence or absence of rock fragments affect the distribution of soil–water throughout the profile and the ramification of roots, and have an impact on soil temperature24,25,26. Characterization of soil profile is therefore crucial for understanding the complex impact of stoniness.
Heterogeneity of the physical environment, alongside with climatic variables, have a crucial effect on vegetation living conditions and biodiversity5,27. It was demonstrated that geodiversity affects the distribution of vegetation28,29, composition of soil microbes, and the resistance of plant to drought30,31,32. In drylands, understanding the relation between geodiversity-governed water distributions21 and plants viability is highly important. Recent studies from the semi-arid north-western Negev proposed that heterogeneous land units, dominated with partially-embedded stones in their ground surface, increase the spatial redistribution of water, with the resultant increase in water availability for shrubs32, improving their durability to prolong drought events21. Specifically, mass mortality of Noaea mucronata (Forssk.) shrubs was reported for hillslopes with low geodiversity level21,32. Recent studies proposed evidence on the buffering effect of geodiverse locations on vegetation diversity in a changing environment33,34 as geodiversity can improve adaptability of species and acts as microclimate refugia35,36. More empirical data from different climate zones are needed to support the geodiversity–biodiversity relations and to be implemented in conservation projects of natural ecosystem6,37,38.
The N. mucronata is a perennial shrub that dominates the north-western Negev (Fig. 2), which shows an adaptive response to dryland environments at numerous levels. The adaptation to water-limited environments shaped the plant’s metabolism and morphology. Aboveground adaptions comprise changes in leaf morphology, where the small narrow winter leaves that emerge after the rain (approximately in November) shed or turn into thorns during the dry season, reducing water loss through transpiration39. The seasonal difference in leaf morphology result in a total plant size variation from summer (smaller) to winter (larger). The green stems transform into grayish fissured bark, which enables the growth of young branches once the wet season starts39. The physiological traits of adaptions include the C4 carbon fixation pathway, which correlates with high efficiency in CO2 fixation and low transpiration loss under high temperature conditions40. Moreover, at the root level, N. mucronata adapt in association with ectomycorrhizal fungi, which improves the plants’ water and nutrient uptake41,42. The N. mucronata is considered as a landscape engineer43,44,45, which improves habitat conditions and facilitates herbaceous community in its surroundings by modifying microclimate (reduction of temperature stress due to the shading effect) and soil properties (increasing infiltration and reducing evapotranspiration)43,46,47,48. At the same time, it was reported that the N. mucronata may impose some negative effects, such as the suppression of annual plants49 through shading and competition50.
Our study investigates the spatial and temporal effect of geodiversity on plant community structure and on our model species N.mucronata. The importance of our results is its link with the climatically conditions and the novelty of the semi-arid region case. The study objectives were to assess during two compared year, a wetter and a drier one, the effect of geodiversity features such as stoniness and depth of the soil profile (1) on the physiological conditions of N. mucronata shrubs, and (2) on plant’s community structure and diversity. Our overall hypotheses are that an increase of geodiversity will (1) improve the physiological conditions of N. mucronata shrubs and (2) enhance plant diversity.
Materials and methods
Regional settings
The study was conducted between February 2016 and May 2017 in the fenced area (no access for livestocks since 1990s) of the Sayeret Shaked Park (~ 20 ha) (Fig. 1) with the permission of the Israel’s LTER Network. The park is located in the semi-arid north-western Negev of Israel (31° 17′ N, 34° 37′ E; 200 m.a.s.l.), where annual average rainfall is ~ 165 and standard deviation of 58 mm/year21. The site’s soil is aeolian loess, with sandy loamy to loamy sand texture51. The park is covered with dwarf shrubs (0.1–0.6 m tall) such as Atractylis serratuloides Cass., Thymelaea hirsuta (L.) Endl., and the dominant N. mucronata shrubs. The site has a large number of annuals, geophyte (e.g., Asphodelus ramosus L.) and hemicryptophyte species, as well as a rich community of biological crusts50,52,53,54,55. In a previous study, a geomorphological survey followed by hillslopes mapping took place in Sayeret Shaked starting in July 201521. The results showed the presence of either hillslopes without rock fragment cover and hillslopes with substantial (~ 30%) rock fragment cover (Fig. 2). Following the study, three low-geodiversity hillslopes, with a thick (> 1 m) and non-stony soil layer, without rock fragment cover or rock fragment content in the soil profile (homogeneous: HM; Fig. 2A), and three high-geodiversity hillslopes, with a thin (~ 0.1 m) stony soil layer, rock fragment cover (~ 30%) and rockfragment content in the soil profile (~ 35%) (heterogeneous: HT; Fig. 2B) were selected for the study. The mean (± SE) slope incline of all the hillslope is 5° (±)22. Distance between two adjacent hillslopes was at least 100 m. On each of these hillslopes, a 400 m2 (20 × 20 m) plot was established for data collection.
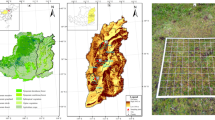
Maps of the study area. Sayeret Shaked LTER location in Israel (A), the location area (B) and the plots (C) where the red squares are the homogeneous plots and the blue squares the heteregenous ones (Google Earth). Maps data, Google 2021, CNES/Airbus, Maxar Tecnologliges, satellite image accesed through Google Earth.
A view of a homogeneous (A) and a heterogeneous (B) hillslopes. In the homogeneous hillslopes, the white spots are piles of snails in the vicinity of dead shrubs. The piles indicate the location of dead shrubs. At the same time, most of the shrubs in the heterogeneous hillslope are alive, and survived the prolonged drought of 2008–2009. N. mucronata during spring season (C). A detail of winter leaves (D). Photos courtesy of Prof. Hezi Yizhaq.
To study the effect of the geodiversity features on plant community, we assessed the plant diversity at a once-a-month frequency over the growing season (Feb–May/June) of two sequential years (2016–2017). In each of these years, the last cycle of data collection took place when the annual plants were dried out, and at the point where the N. mucronata ‘s ‘winter leaves’56 have disappeared. Data of precipitation, solar radiation, soil temperature and soil moisture at different depths (25 and 50 cm) were obtained from the meteorological station located in the LTER site.
Vegetation survey
The N. mucronata was the only perennial plant present in both HT and HM hillslope, making it the best-fit model species to assess the year-round differences in plant viability. In each plot (n = 6) we studied three individual shrubs, to a total of nine individuals per hillslopes type. In order to monitor the shrub’s morphological changes during the year, we measured the maximum length of green branches. Also, we measured the plant's size by measuring the maximum length from a green part to another on a north–south and east–west axes, as well as the shrub height. We multiplied the three axes to calculate the maximum plant size. In addition to these nine N. mucronata plants, we sampled leaves (winter leaves only) from other nine randomly selected N. mucronata plants to estimate their physiological condition, through measuring the relative water content (RWC), membrane stability (EC), carbon–nitrogen ratio (C:N), and chlorophyll content.
Biochemical analysis
Relative water content (RWC)
We added 3–5 g of N. mucronata young leaves of each individual to a 50 ml vial with a wet tissue to maintain humidity. The leaves were weighted for fresh weight using Sartorius AG Göttingen CP225D, Germany. The samples were submerged in de-ionized water for 24 h and then weighted for turgor weight. Additionally, the samples were dried at 65 °C for 24 h in the oven for dry weight.
C:N ratio
Few leaves from each shrub of N. mucronata were collected for total organic carbon (Corg) and total nitrogen content (Ntot) analysis, dried at 65 °C for 12 h and manually ground by mortar and pistil. Of these samples, 20 mg were put in a C–N analyzer (CHNS Elemental Analyzer, Thermo Scientific, USA).
Membrane stability index (MSI)
From each N. mucronata, 20–30 leaves were collected and placed in 50 ml vials filled with 20 ml of double distilled water (DDW). The electrolyte’s electric conductivity (EC) was measured with a probe (Eutech Instruments, CON 510, Singapore), as initial leakage (Ci). The samples were then placed on a laboratory shaker (TOS-4838PD, MRC Lab Instruments, Holon, Israel) for 12 h at 200 rpm and the EC was measured again as Cr. The samples were then autoclaved to blast cell membrane, and the maximum conductivity (Cm) was measured. Membrane stability index (MSI) was calculated according to Eq. (1):
Chlorophyll content
10 leaves of N. mucronata were collected and add into a 2 ml Eppendorf with 1 ml of Dimethyl sulfoxide (DMSO), covered with aluminum foils. In the laboratory, vials were kept in a 65 °C incubator for 72 h and then centrifuged for 14,000 rpm at 20 °C for 10 min. 200 μl of the supernatant was added to a 96 wells plate and the absorption was read with Epoch™ spectrophotometer (BioTek Instruments, Inc., Vermont, USA). Chlorophyll a (Chl a), contents was calculated using Eq. (2)57:
Vascular plant diversity
To measure plant diversity, we identified all the vascular plants to the species level along 3-m transects (one transect per plot), counted the number of individuals per species, and recorded the total cover per species. The monitoring of transects was conducted at a once-a-month frequency throughout the growing season (Feb–June 2016 and Feb–May 2017: a total of 11 sampling cycles). Due to overlapping plants, vegetation’s total cover could exceed 100%. We classified all plant species into three life forms: annual, perennial and herbaceous perennial. The use of any plants in this study was in accord with national guidelines. The formal identification of the plant material was performed by Prof. Rachmilevitch. We did not use voucher specimen.
In order to determine the plant diversity in the different hillslope, we calculate species richness (n) as the number of species present at the site, and species abundance as the total number of plants present at the site (N). In order to determine the diversity in the communities, we calculate the Shannon Diversity Index (Eq. 3) and Shannon Evenness Index (Eq. 4) and the Simpson Index (Eq. 5),58,59
where p is the proportion (n/N) of individuals of one particular species found (n) divided by the total number of individuals found (N), ln is the natural log, Σ is the sum of the calculations, and s is the number of species.
Data analysis
Nonparametric Mann–Whitney U test was performed to analyze the meteorological data (averaged solar radiation, total rain precipitation, averaged soil temperature and soil moisture at 25 and 50 cm depth) of the LTER station of year 2016 and 2017. Kruskal–Wallis one-way analysis of variance was used for averaged data of the different hillslopes (HM vs HT) of biochemical data (RWC, MSI and chlorophyll a content) as well as for estimated size of shrubs. Non-parametric Dunn's test for pairwise multiple comparisons was used to compared biochemical data of 2016 vs 2017 in the different hillslopes. Analysis of similarity (ANOSIM) was performed to explain plant diversity in the different hillslopes per hillslope and per year. Life form composition was analyzed with Pearson Chi-Square. All statistical were conducted using SPSS Statistics (SPSS, IBM Corp, Version 26.0. Armonk, NY, U.S.A.). Analysis of similarities was carried out in R60.
Results
The two years of sampling were characterized by two different rain regimes (Fig. 3B), where 2016 was substantially wetter compared to 2017 (223 and 96 mm, respectively: Fig. 3B, Mann–Whitney Ranks Sum Test p < 0.001). Data of total solar radiation (Fig. 3A) indicates too a significant difference between 2016 and 2017 (22.6 and 25.1 MJ/m2 year of Solar Radiation respectively, Mann–Whitney Ranks Sum Test p = 0.033). This resulted in increase of soil temperature in 2017 at both 25 and 50 cm soil depths (Fig. 3C), as well as the reduction of soil moisture at both depths (Fig. 3D).
Average solar radiation (± SE) (A), total rain (B), average soil temperature (± SE) (C), and average soil moisture (± SE) (D), for 2016 (n = 269) and 2017 (n = 337). For relative trend of meteorological parameters in the period 2000–2015 see SI 1.
We did not find significant differences in physiological measurements of N. mucronata plants between the two hillslope types throughout the two years of sampling (SI 2). For the phenological measurements, the estimated mean N. mucronata size was greater in HM hillslopes than that in HT hillslopes (Kruskal–Wallis one-way analysis of variance, p < 0.0001).
Nevertheless, once we plotted the data for different years (2016 vs 2017), we found a significant difference for most of the parameters between the two years, and especially regarding the C:N ratio and RWC. In 2016, N. mucronata had a higher C:N ratio (HT, Dunn’s Method: p < 0.05, HM Dunn’s Method: p < 0.05, Fig. 4A) and higher RWC (HT Holm–Sidak method: p < 0.001, HM Dunn’s Method: p < 0.05, Fig. 4B) than these in 2017. For the C:N ratio, this trend was similar for HT and HM hillslopes (a decrease of 26% and 28% from 2016 to 2017 in HT and HM, respectively). At the same time, the decrease in RWC was much smaller in HT hillslopes (5%) than that in HM hillslopes (11%).
Plant diversity
We found that plant diversity can be explained by hillslope type (Analysis of similarity: R = 0.36, p = 0.0001) (Fig. 5A,B). In addition, we measured the effect of sampling year (SI 6 A, B, C and D), which was also significant, though the value explained was lower than the hillslope type (R = 0.11, p = 0.002). Moreover, we found a significant difference in life form composition, considering all species found, between the hillslopes (Pearson chi-square: Chi Square: 56.7, df = 2, p < 0.0001). HT plots had a higher mean value of perennial (39% in HT compared to 3% in HM) as well as higher mean value of herbaceous perennial (13% in HT compared to 7.5% in HM) (Fig. 5A,B). Specifically, in HM we found two shrubby species (Pituranthos tortnousus and N. mucronata), and a total of 3 individuals, while in the HT we found six shrubby species (N. mucronata, A. articulata, Dianthus monadelphus subsp. judaicus, Salvia lanigera Poir., Helianthemum stipulatum (Forssk.) C. Chr. and Helianthemum lippii (L.) Dum. Courset) and a total of 47 individuals. At the same time, the mean value of annuals was significantly higher in HM (90% in HM compared to 48% in HT). The annual plant community in HM encompassed of 25 species and 110 individuals, while in the HT it encompassed 20 species and 58 individuals.
Life form composition in heterogeneous (A) and homogeneous (B) hillslopes. Differentiation among perennial (red), herbaceous perennial (blue), and annuals (green) is presented in the left circles, and species-level differentiation of annuals is presented in the right circles (only species that represent more than 2% were included in the figure). For the total list of the species SI 3–4.
The accumulated cover of four species contributed to 52.8% of the differences between the two hillslope types. These species included Stipa capensis Thunb. (19.6%), N. mucronata (18.8%), Anabasis articulata (Forssk.) Moq. (8.6%), and Onobrychista crista-gali (L.) Lam. (5.8%) (Similarity percentage analysis, SI 5). The main differences were caused by two perennial plants that were absent from the HM transects: N. mucronata and A. articulata. Additionally, once we plotted the annuals’ diversity data per year (SI 6 A–D), we found that the annual community structure in the HT hillslopes during the shift from 2016 to 2017 became more even, with a more uniform abundance of species (SI 6 A and B). An opposite trend was found for the HM hillslopes, where an increase in abundance of dominant species was observed (SI 6 C and D).
Species richness and abundance are reported in Table 1. Species richness was similar in HT and HM hillslopes (31 and 30 species, respectively). Once comparing 2016 to 2017 year—with equal species richness in both of the hillslope types but different structures and abundance in 2016—HT hillslopes faced an increase in 2017, while HM hillslopes faced a decrease.
To gain more insights about the community structure, we calculated the Shannon Diversity Index and Simpson Diversity Index (Table 1). Overall, HT hillslope had a higher diversity (Shannon Diversity Index in HT was 3.5% higher than that in HM). Further, once we calculated the yearly data, we found that in 2017 the drop of diversity in HM hillslopes was significant, whilst a significant increase was observed for HT hillslopes. Moreover, in 2017, the Shannon Evenness Index showed an increase in HT hillslopes and a decrease in HM hillslopes. This suggests that in HM hillslopes, the functional group encompass few dominant species with high abundance and few sparser species with low abundance. In HT hillslopes, the community structure was more even.
Discussion
The aims of our study were to assess the spatial effect of geodiversity, express as stoniness and depth of the soil profile in different hillslopes, on the physiological conditions of N. mucronata shrubs and plant’s community structure and diversity. We showed that the geodiversity features promote a more diverse plant community (Fig. 5A,B). During the two years monitoring (2016–2017) the second year happened to be significant drier with repercussion on soil temperature and moisture at different depth (Fig. 3). This fact allowed us to have insights about the temporal effect of geodiversity features to mitigate drier years. In fact, annual communities on hillslopes with lower geodiversity tend to suffer a decrease in diversity during the drier year (SI 6 and Table 1). Previous studies from the semi-arid north-western Negev suggested that hillslope-scale geodiversity improves the source–sink relations29 and positively affect soil quality and geo-ecosystem functions. Specifically, it was reported that the HT hillslopes had 22% greater mean hygroscopic moisture22 then that in HM hillslopes. This result can indirectly indicates a similar availability of soil water for plants. The comparatively favorable conditions in the HT hillslopes were proposed to be the key of survival of N. mucronata plants during the mass mortality that occurred during different drought events started in 199932.
Geodiversity components such as geomorphology, topography, geology, and hydrology are associated with energy and nutrients, which regulate biodiversity61,62. Nevertheless, only recently, the impact of geodiversity on biodiversity has gained attention63,64,65,66. Considering global climatic change, it was proposed that high-geodiversity land units are potentially more capable to support biodiversity because of their intrinsic resilience65,67,68,69. In our study, the favorable soil conditions in HT hillslopes were not straightforward translated into a better physiological state of plants (SI 2), as we expected in our first hypothesis. Our biochemical analysis did not show a prominent effect of geodiversity features over the physiological conditions of N.mucronata, when we compared the plots (HT vs HM) (SI 2). Despite that, during 2017, the shrubs in HM hillslopes faced stronger water stress compared to these in HT hillslopes (Fig. 4B). The positive effect of increased geodiversity on hygroscopic moisture22, and the overall higher soil–water content in HT hillslopes70, might explain the reduced water stress for shrubs in HT hillslopes32,71.
The shrub patch is the driver of the cyclic succession of plants community48,72,73,74. According to the biodiversity cyclic hypothesis48, once the patch is consolidated, the dissimilarity in community structure between the shrubby patches and interpatch spaces increases. Therefore, shrublands patchiness is critical for sustaining spatial heterogeneity75. In our study region, the HT hillslopes have higher patchiness, mainly due to higher geodiversity that supports shrub durability to droughts. Regarding our second objective on the effect of geodiversity on the plant community structure, we found that the perennials in HM were 2% of the community structure, whilst being 38% in HT (Fig. 5A,B). In HM, the annuals contributed 90% of the total community structure, while in HT they contributed 48% (Fig. 5A,B). The high cover of annuals in the HM hillslopes compared to the HT hillslope displays one possible response of the ecosystem to prolonged droughts that is a transition from shrubland to grassland. This transition did not occur in the HT hillslopes due to the effect of geodiversity that increase their resistance to drought. The ecosystem in the HM does not collapse to the bare soil as predicted by many mathematical models of vegetation patterns in drylands that include only wood vegetation but it is actually shifted to another stable state76,77.
The annual community structure faced a shift from the wetter 2016 to the dryer 2017 in both hillslopes (SI 6 A–D). In HT hillslopes, the annuals community become more even, having fewer dominant species with high abundance (SI 6 A and B). At the same time, in HM hillslopes, the annuals’ community structure became less even (SI 6 C and D). In our diversity analysis, we showed that in the drier year, plant diversity increased in HT hillslopes (Table 1) and decreased in HM hillslopes. Different simulation studies, where plant communities underwent abiotic stresses related to climate changes—such as higher temperature or lower precipitations—showed similar results78,79. It was shown that community response to changes is rapid (two seasons), and that stresses can change patterns of plant dominance and evenness78,79. Changes in dominance in community composition can have consequences for species coexistence and ecosystem functions80. Recent studies show that locations with high geodiversity have the potential role to act as hotspot for biodiversity and to maintain community’s structure due to their function to buffer climatic changes66,81. In our study, we show that the effect of geodiversity features on community structure and species richness is greater in the drier year than that in a wetter year. Our results suggested that hillslopes with higher geodiversity might be in situ refugia that facilitate the existing community and in shifting climate periods might serve as biodiversity spots82,83,84,85,86.
Our results aligned with the findings of recent studies that suggest that integrating geodiversity in conservation nature projects becomes a necessity to protect ecosystems and their services10,65,87 and offer the opportunity to broad the data availability. In fact the connection between biodiversity and geodiversity allow to use geodiversity measurements, more spatial consistent and therefore available at broader scale, and relating them to biodiversity64,88.
Conclusion
The motivation of this study was to understand how different geodiversity features, expressed by the degree of stoniness and soil thickness, affect the physiological state of N. mucronata and the plant life form variability. In conclusion, our data show that in a semi-arid regions, hillslopes with higher geodiversity better buffer the effect of drier years, and supported a more diverse plant community compared to lower geodiversity hillslopes. The potential role of sites with higher geodiversity to mitigate climate change effects in terms of persistence of biodiversity should be take into consideration when planning for conservation actions and ecosystem management84,89,90 In this context, additional studies should be conducted in other drylands of the world in order to verify the mechanisms through which geodiversity regulates the structure and composition of vegetation community.
References
Gray, M., Gordon, J. & Brown, E. Geodiversity and the ecosystem approach: The contribution of geoscience in delivering integrated environmental management. Proc. Geol. Assoc. 124, 659–673 (2013).
Gray, M. Valuing geodiversity in an ‘ecosystem services’ context. Scott. Geogr. J. 128, 177–194 (2012).
Warren, A. & French, J. R. Habitat Conservation: Managing the Physical Environment (Wiley, Hoboken, 2001).
Gordon, J. E., Barron, H. F., Hansom, J. D. & Thomas, M. F. Engaging with geodiversity—Why it matters. Proc. Geol. Assoc. 123, 1–6 (2012).
Hjort, J., Gordon, J. E., Gray, M. & Hunter, M. L. Why geodiversity matters in valuing nature’s stage. Conserv. Biol. 29, 630–639 (2015).
Gray, M. Geodiversity: Valuing and Conserving Abiotic Nature 448 (Wiley, 2004).
Serrano, E. & Ruiz-Flano, P. Geodiversity. A theoretical and applied concept. Geogr. Helv. Jg 62, 140–147 (2007).
Comer, P. J. et al. Incorporating geodiversity into conservation decisions. Conserv. Biol. 29, 692–701 (2015).
Pătru-Stupariu, I. et al. Integrating geo-biodiversity features in the analysis of landscape patterns. Ecol. Indic. 80, 363–375 (2017).
Chakraborty, A. & Gray, M. A call for mainstreaming geodiversity in nature conservation research and praxis. J. Nat. Conserv. 56, 125862 (2020).
Poesen, J., Torri, D. & Bunte, K. Effects of rock fragments on soil erosion by water at different spatial scales: A review. CATENA 23, 141–166 (1994).
Zhang, Y., Zhang, M., Niu, J., Li, H. & Xiao, R. Rock fragments and soil hydrological processes: Significance and progress. CATENA 147, 153–166 (2016).
Xia, L. et al. Effects of rock fragment cover on hydrological processes under rainfall simulation in a semi-arid region of China. Hydrol. Process. 32, 792–804 (2018).
Lavee, H. & Poesen, J. W. A. Overland flow generation and continuity on stone-covered soil surfaces. Hydrol. Process. 5, 345–360 (1991).
Agassi, M. & Levy, G. Stone cover and rain intensity—Effects on infiltration, erosion and water splash. Soil Res. 29, 565–575 (1991).
Mandal, U. K. et al. Soil infiltration, runoff and sediment yield from a shallow soil with varied stone cover and intensity of rain. Eur. J. Soil Sci. 56, 435–443 (2005).
Cerdà, A. Effects of rock fragment cover on soil infiltration, interrill runoff and erosion. Eur. J. Soil Sci. 52, 59–68 (2001).
Jury, W. A. & Bellantuoni, B. Heat and water movement under surface rocks in a field soil: I. Thermal effects. Soil Sci. Soc. Am. J. 40, 505–509 (1976).
Yuan, C., Lei, T., Mao, L., Liu, H. & Wu, Y. Catena soil surface evaporation processes under mulches of different sized gravel. CATENA 78, 117–121 (2009).
Poesen, J. & Lavee, H. Rock fragments in top soils: Significance and processes. CATENA 23, 1–28 (1994).
Yizhaq, H., Stavi, I., Shachak, M. & Bel, G. Geodiversity increases ecosystem durability to prolonged droughts. Ecol. Complex. 31, 96–103 (2017).
Stavi, I., Rachmilevitch, S. & Yizhaq, H. Geodiversity effects on soil quality and geo-ecosystem functioning in drylands. CATENA 176, 372–380 (2019).
Preisler, Y. et al. Mortality versus survival in drought-affected Aleppo pine forest depends on the extent of rock cover and soil stoniness. Funct. Ecol. 33, 901–912 (2019).
Sauer, T. J. & Logsdon, S. D. Hydraulic and Physical Properties of Stony Soils in a Small Watershed. Soil Sci. Soc. Am. J. 66, 1947–1956 (2002).
Arnau-Rosalén, E., Calvo-Cases, A., Boix-Fayos, C., Lavee, H. & Sarah, P. Analysis of soil surface component patterns affecting runoff generation. An example of methods applied to Mediterranean hillslopes in Alicante (Spain). Geomorphology 101, 595–606 (2008).
Ceacero, C. J., Díaz-Hernández, J. L., de Campo, A. D. & Navarro-Cerrillo, R. M. Soil rock fragment is stronger driver of spatio-temporal soil water dynamics and efficiency of water use than cultural management in holm oak plantations. Soil Tillage Res. 197, 104495 (2020).
Burnett, M. R., August, P. V., Brown, J. H. & Killingbeck, K. T. The influence of geomorphological heterogeneity on biodiversity I. A patch-scale perspective. Conserv. Biol. 12, 363–370 (2008).
Engelbrecht, B. et al. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82 (2007).
Stavi, I., Rachmilevitch, S. & Yizhaq, H. Small-scale geodiversity regulates functioning, connectivity, and productivity of shrubby, semi-arid rangelands. L. Degrad. Dev. 29, 205–209 (2018).
Dubinin, V., Stavi, I., Svoray, T., Dorman, M. & Yizhaq, H. Hillslope geodiversity improves the resistance of shrubs to prolonged droughts in semiarid ecosystems. J. Arid Environ. 188, 104462 (2021).
Ochoa-Hueso, R. et al. Soil fungal abundance and plant functional traits drive fertile island formation in global drylands. J. Ecol. 106, 242–253 (2018).
Stavi, I., Rachmilevitch, S., Hjazin, A. & Yizhaq, H. Geodiversity decreases shrub mortality and increases ecosystem tolerance to droughts and climate change. Earth Surf. Process. Landforms 43, 2808–2817 (2018).
Suggitt, A. J. et al. Extinction risk from climate change is reduced by microclimatic buffering. Nat. Clim. Change 8, 713–717 (2018).
Bailey, J. J., Boyd, D. S. & Field, R. Models of upland species’ distributions are improved by accounting for geodiversity. Landscape Ecol. https://doi.org/10.1007/s10980-018-0723-z (2018).
Lenoir, J. et al. Local temperatures inferred from plant communities suggest strong spatial buffering of climate warming across Northern Europe. Glob. Change Biol. 19, 1470–1481 (2013).
Lawler, J. J. et al. The theory behind, and the challenges of, conserving nature’s stage in a time of rapid change. Conserv. Biol. 29, 618–629 (2015).
Nichols, W. F., Killingbeck, K. T. & August, P. V. The influence biodiversity of geomorphological heterogeneity: II. A landscape perspective. Soc. Conserv. Biol. 12, 371–397 (1998).
Alahuhta, J., Toivanen, M. & Hjort, J. Geodiversity–biodiversity relationship needs more empirical evidence. Nat. Ecol. Evol. 4, 2–3 (2020).
Evenari, M., Shanan, L., Tadmor, N. & Shkolnik, A. The Negev: The Challenge of a Desert (Harvard University Press, 1982).
Akttani, H., Trimborn, P. & Ziegler, H. Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with their ecological, phytogeographical and taxonomical importance. Plant Syst. Evol. 206, 187–221 (1997).
Harley, J. The Biology of Mycorrhiza (Leonard Hill, 1969).
Mejsti, V. K. & Cudlin, P. Mycorrhiza in some plant desert species in Algeria. Plant Soil 71, 363–366 (1983).
Segoli, M., Ungar, E. D. & Shachak, M. Shrubs enhance resilience of a semi-arid ecosystem by engineering and regrowth. Ecohydrology 1, 330–339 (2008).
Gilad, E., Von Hardenberg, J., Provenzale, A., Shachak, M. & Meron, E. Ecosystem engineers: From pattern formation to habitat creation. Phys. Rev. Lett. 93, 098105 (2004).
Wright, J. P., Jones, C. G., Boeken, B. & Shachak, M. Predictability of ecosystem engineering effects on species richness across environmental variability and spatial scales. J. Ecol. 94, 815–824 (2006).
Katra, I., Blumberg, D. G., Lavee, H. & Sarah, P. Spatial distribution dynamics of topsoil moisture in shrub microenvironment after rain events in arid and semi-arid areas by means of high-resolution maps. Geomorphology 86, 455–464 (2007).
Hoffman, O., de Falco, N., Yizhaq, H. & Boeken, B. Annual plant diversity decreases across scales following widespread ecosystem engineer shrub mortality. J. Veg. Sci. 27, 578–586 (2016).
Shachak, M. et al. Woody species as landscape modulators and their effect on biodiversity patterns. Bioscience 58, 209–221 (2008).
Madrigal-González, J., García-Rodríguez, J. A. & Alarcos-Izquierdo, G. Testing general predictions of the stress gradient hypothesis under high inter- and intra-specific nurse shrub variability along a climatic gradient. J. Veg. Sci. 23, 52–61 (2012).
Boeken, B. & Shachak, M. The dynamics of abundance and incidence of annual plant species during colonization in a desert. Ecography (Cop.) 21, 63–73 (1998).
Golodets, C. & Boeken, B. Moderate sheep grazing in semiarid shrubland alters small-scale soil surface structure and patch properties. CATENA 65, 285–291 (2006).
Boeken, B. & Shachak, M. Desert plant communities in human-made patches-implications for management. Ecol. Appl. 4, 702–716 (1994).
Hoffman, O., Yizhaq, H. & Boeken, B. Small-scale effects of annual and woody vegetation on sediment displacement under field conditions. CATENA 109, 157–163 (2013).
Zaady, E., Arbel, S., Barkai, D. & Sarig, S. Long-term impact of agricultural practices on biological soil crusts and their hydrological processes in a semiarid landscape. J. Arid Environ. 90, 5–11 (2013).
Zaady, E., Stavi, I. & Yizhaq, H. Hillslope geodiversity effects on properties and composition of biological soil crusts in drylands. Eur. J. Soil Sci. https://doi.org/10.1111/ejss.13097 (2021).
Feinbrun-Dothan, N. & Danin, A. Analytical Flora of Eretz-Israel (Cana Publishing Ltd, 1991).
Solovchenko, A., Merzlyak, M. N., Khozin-Goldberg, I., Cohen, Z. & Boussiba, S. Coordinated carotenoid and lipid syntheses induced in parietochloris incisa (chlorophyta, trebouxiophyceae) mutant deficient in δ5 desaturase by nitrogen starvation and high light. J. Phycol. 46, 763–772 (2010).
Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Simpson, E. Measurement of diversity. Nature 163, 688 (1949).
R Core Team. R: A language and environment for statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2020).
Richerson, P. J. & Lum, K. Patterns of plant species diversity in California: Relation to weather and topography. Am. Nat. 116, 504–536 (1980).
Kerr, J. T. & Packer, L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 385, 252–254 (1997).
Alahuhta, J. et al. The role of geodiversity in providing ecosystem services at broad scales. Ecol. Indic. 91, 47–56 (2018).
Zarnetske, P. L. et al. Towards connecting biodiversity and geodiversity across scales with satellite remote sensing. Wiley Online Libr. 28, 548–556 (2019).
Schrodt, F. et al. To advance sustainable stewardship, we must document not only biodiversity but geodiversity. Proc. Natl. Acad. Sci. U. S. A. 116, 16155–161658 (2019).
Read, Q. D. et al. Beyond counts and averages: Relating geodiversity to dimensions of biodiversity. Wiley Online Libr. 29, 696–710 (2020).
Antonelli, A. et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 11, 718–725 (2018).
Knudson, C., Kay, K. & Fisher, S. Appraising geodiversity and cultural diversity approaches to building resilience through conservation. Nat. Clim. Change 8, 678–685 (2018).
Beier, P., Hunter, M. L. & Anderson, M. Special section: Conserving nature’s stage. Conserv. Biol. 29, 613–617 (2015).
Dubinin, V., Svoray, T., Stavi, I. & Yizhaq, H. Using LANDSAT 8 and VENµS data to study the effect of geodiversity on soil moisture dynamics in a semiarid shrubland. Remote Sens. 12, 3377 (2020).
Renne, R. R. et al. Soil and stand structure explain shrub mortality patterns following global change–type drought and extreme precipitation. Ecology 100, e02889 (2019).
Gutterman, Y., Golan, T. & Garsani, M. Porcupine diggings as a unique ecological system in a desert environment. Oecologia 85, 122–127 (1990).
Armas, C., Pugnaire, F. I. & Sala, O. E. Patch structure dynamics and mechanisms of cyclical succession in a Patagonian steppe (Argentina). J. Arid Environ. 72, 1552–1561 (2008).
Pickett, S. & White, P. The Ecology of Natural Disturbance and Patch Dynamics (Academic Press, 1985). https://doi.org/10.1016/C2009-0-02952-3.
Segoli, M., Ungar, E. D., Giladi, I., Arnon, A. & Shachak, M. Untangling the positive and negative effects of shrubs on herbaceous vegetation in drylands. Landsc. Ecol. 27, 899–910 (2012).
Rodríguez, F., Mayor, Á. G., Rietkerk, M. & Bautista, S. A null model for assessing the cover-independent role of bare soil connectivity as indicator of dryland functioning and dynamics. Ecol. Indic. 94, 512–519 (2018).
Zelnik, Y. R., Kinast, S., Yizhaq, H., Bel, G. & Meron, E. Regime shifts in models of dryland vegetation. Philos. Trans. R. Soc. A https://doi.org/10.1098/rsta.2012.0358 (2013).
Walker, M. D. et al. Plant community responses to experimental warming across the tundra biome. PNAS 103, 1342–1346 (2006).
Kardol, P. et al. Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Glob. Change Biol. 16, 2676–2687 (2010).
Hillebrand, H., Bennett, D. M. & Cadotte, M. W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 89, 1510–1520 (2008).
Stavi, I., Yizhaq, H., Szitenberg, A. & Zaady, E. Patch-scale to hillslope-scale geodiversity alleviates susceptibility of dryland ecosystems to climate change: Insights from the Israeli Negev. Curr. Opin. Environ. Sustain. 50, 129–137 (2021).
Loarie, S. R. et al. Climate change and the future of California’s endemic flora. PLoS ONE 3, 2502 (2008).
Loarie, S. R. et al. The velocity of climate change. Nature 462, 1052–1055 (2009).
Ashcroft, M. B., Chisholm, L. A. & French, K. O. Climate change at the landscape scale: Predicting fine-grained spatial heterogeneity in warming and potential refugia for vegetation. Glob. Change Biol. 15, 656–667 (2009).
Correa-Metrio, A., Meave, J. A., Lozano-García, S. & Bush, M. B. Environmental determinism and neutrality in vegetation at millennial time scales. J. Veg. Sci. 25, 627–635 (2014).
Baumgartner, J., Esperon-Rodriguez, M. & Beaumont, L. Identifying in situ climate refugia for plant species. Ecography (Cop.) 41, 1850–1863 (2018).
Alahuhta, J. et al. The Role of Geodiversity in Providing Ecosystem Services at Broad Scales (Elsevier, 2018).
Parks, K. E. & Mulligan, M. On the relationship between a resource based measure of geodiversity and broad scale biodiversity patterns. Biodivers. Conserv. 19, 2751–2766 (2010).
Keppel, G. et al. The capacity of refugia for conservation planning under climate change. Front. Ecol. Environ. 13, 106–112 (2015).
Mokany, K. et al. Past, present and future refugia for Tasmania’s palaeoendemic flora. J. Biogeogr. 44, 1537–1546 (2017).
Author information
Authors and Affiliations
Contributions
N.D.F.: conceptualization, data curation, validation; writing—original draft preparation and editing. R.T.-B.: methodology, design, acquisition data, conceptualization. A.H.: acquisition data. H.Y.: writing—reviewing and editing. I.S.: writing—reviewing and editing. S.R.: supervision, identification plant material, writing—reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Falco, N., Tal-Berger, R., Hjazin, A. et al. Geodiversity impacts plant community structure in a semi-arid region. Sci Rep 11, 15259 (2021). https://doi.org/10.1038/s41598-021-94698-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94698-0
This article is cited by
-
Assessing the relation between geodiversity and species richness in mountain heaths and tundra landscapes
Landscape Ecology (2023)
-
Climate and aridity measures relationships with spectral vegetation indices across desert fringe shrublands in the South-Eastern Mediterranean Basin
Environmental Monitoring and Assessment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.