Abstract
Preinjury anticoagulation therapy (AT) is associated with a higher risk for major bleeding. We aimed to evaluated the influence of preinjury anticoagulant medication on the clinical course after moderate and severe trauma. Patients in the TraumaRegister DGU ≥ 55 years who received AT were matched with patients not receiving AT. Pairs were grouped according to the drug used: Antiplatelet drugs (APD), vitamin K antagonists (VKA) and direct oral anticoagulants (DOAC). The primary end points were early (< 24 h) and total in-hospital mortality. Secondary endpoints included emergency surgical procedure rates and surgery rates. The APD group matched 1759 pairs, the VKA group 677 pairs, and the DOAC group 437 pairs. Surgery rates were statistically significant higher in the AT groups compared to controls (APD group: 51.8% vs. 47.8%, p = 0.015; VKA group: 52.4% vs. 44.8%, p = 0.005; DOAC group: 52.6% vs. 41.0%, p = 0.001). Patients on VKA had higher total in-hospital mortality (23.9% vs. 19.5%, p = 0.026), whereas APD patients showed a significantly higher early mortality compared to controls (5.3% vs. 3.5%, p = 0.011). Standard operating procedures should be developed to avoid lethal under-triage. Further studies should focus on detailed information about complications, secondary surgical procedures and preventable risk factors in relation to mortality.
Similar content being viewed by others
Introduction
It is well known that older trauma patients have poorer outcomes than younger ones1. Physiological changes, comorbidities, nutritional deficits and pre-medication are discussed as reasons for this increased mortality2,3,4. About 4% of trauma patients are on anticoagulation therapy (AT)5,6, mainly due to atrial fibrillation and other cardiovascular diseases (e.g. thromboembolic events, prosthetic heart valves)7,8,9. Demographic changes will lead to a further increase in patients on AT10,11.
As AT has the potential to increase the risk of major bleeding, it represents a major concern for the post-traumatic course, especially in patients with severe haemorrhage. Several studies have reported preinjury medication with warfarin as an independent predictor of mortality2,12. Moreover, impaired physiological reserve due to reduced cardiac function, loss of vessel elasticity and vasoconstriction3,13 might mask accelerated blood loss. In up to 20% of geriatric trauma patients, occult hypoperfusion has been described14. These risk factors may lead to under-triage, which is associated with a four-fold higher mortality in the geriatric trauma population compared to accurately triaged trauma patients15.
Despite the high relevance of AT treatment for the post-traumatic course, the specific impact of different drug groups remains largely unknown. We evaluated the impact of preinjury AT treatment on surgery, thromboembolic events and mortality.
Materials and methods
TraumaRegister DGU
The TraumaRegister DGU of the German Trauma Society (Deutsche Gesellschaft für Unfallchirurgie, DGU) was founded in 1993 with the aim of creating a multi-centre database for pseudonymized and standardized documentation of severely injured patients for the purposes of quality assurance and research16. Participating hospitals are primarily located in Germany (90%), but a rising number of hospitals from other countries have begun to contribute data as well, to include Austria, Belgium, China, Finland, Luxembourg, Slovenia, Switzerland, The Netherlands, and the United Arab Emirates. Currently, approximately 33,000 cases from more than 650 hospitals are entered into the database per year. Participation in the TR-DGU is voluntary; however, hospitals associated with the TraumaNetzwerk DGU are obligated to enter at least one basic dataset for quality assurance purposes.
Data were collected prospectively over four consecutive time phases from site of injury until discharge from hospital, as follows: (A) prehospital phase, (B) emergency room and initial surgery, (C) intensive care unit (ICU), and (D) discharge. Documentation included detailed information on demographics, injury pattern, comorbidities, pre- and in-hospital management, course of care while in the ICU, relevant laboratory findings (including data on transfusion), and clinical outcome for each individual. Inclusion criteria were: admission to hospital via emergency room with subsequent ICU/intermediate unit care, or entrance to the hospital with vital signs and death prior to admission to the ICU.
Infrastructure for documentation and data management was provided by the Academy for Trauma Surgery (AUC—Akademie der Unfallchirurgie GmbH), a company affiliated with the German Trauma Society. Scientific leadership draws from the Committee on Emergency Medicine, Intensive Care and Trauma Management (Sektion NIS) of the German Trauma Society. Participating hospitals submitted pseudonymized data into a central database via web-based application. Scientific data analysis was approved according to a peer review procedure established by the NIS Committee .
The present study is in line with the publication guidelines of the TR-DGU and registered as TR-DGU project ID 2018-006.
Definitions
The patients were treated according to the German Level 3 guideline on the treatment of patients with severe/multiple injuries (Version 1 from 2011 and 2016 Update)17. Injuries were coded according to the Abbreviated Injury Scale (AIS, Version 2005/2008, Association for the Advancement of Automotive Medicine, Barrington, IL). The severity of injuries was recorded according to the AIS as 1 (minor), 2 (moderate), 3 (severe, not life threatening), 4 (serious, life-threatening), 5 (critical, survival uncertain) and 6 (maximum, currently untreatable). Overall injury severity was calculated by the Injury Severity Score (ISS) as described by Baker et al.18. The outcome after severe brain damage has been assessed by using the Glasgow Outcome Scale (GOS)19.
Hypotension was defined as systolic blood pressure < 90 mmHg at hospital admission. Thrombembolic events included deep vein thrombosis, pulmonary embolism and ischemic stroke during hospital stay. The definition of coagulopathy included at least one of the following criteria: (A) International normalized ratio (INR) > 1.4, (B) Activated partial thromboplastin time (aPTT) ≥ 40 s, and (C) Quick ≤ 60%20, at hospital admission. Acidosis was defined as a pH value < 7.35 in the first blood gas analysis within the resuscitation room. A mass transfusion was coded, if ≥ 10 units of packed red blood cells (PRBC) were transfused within 24 h.
Mortality was reported as early (< 24 h) and total in-hospital mortality. Furthermore, we used the Revised Injury Severity Classification score, version II (RISC II) to predict the risk of death in severely injured patients that were primarily admitted to one of the reporting trauma centres21.
The variable “surgery” included all surgical procedures performed within the hospital stay. Emergency Surgery procedures (ESP) included:
-
Decompressive craniectomy
-
Laminectomy
-
Emergency thoracotomy
-
Laparotomy
-
Revascularisation
-
Embolisation
-
Stabilisation of pelvic fractures
-
Stabilisation of extremity fractures
Anticoagulants and antiplatelet drugs investigated in this study are presented in Table 1.
Inclusion criteria
The following inclusion criteria were applied:
-
AIS ≥ 3 in at least one body region
-
Age ≥ 55 years
-
Treated in a German hospital
-
Standard data entry form (Version 2015, admissions from 2015 to 2018)
-
Data availability regarding AT
-
Early (< 48 h) transfer out excluded
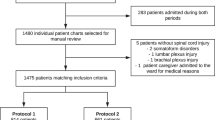
Patients were then grouped according to their AT-medication (APD, VKA or DOAC). Patients who took a combination of two drugs were excluded. Each group of patients was matched to control patients who did not receive an anticoagulation drug (Fig. 1).
Matched-pair analysis
Patients with and without AT were matched according to the following nine criteria (exact match):
-
Age groups (years): 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, ≥ 90
-
Sex (male vs. female)
-
AISHead (0–1/2/3/4/5–6)
-
AISThorax (0–1/2/3/4/5–6)
-
AISAbdomen (0–1/2/3/4/5–6)
-
AISExtremities (0–1/2/3/4/5–6)
-
Pre-trauma ASA classification (1–2 vs. 3–4)
-
Type of admission (primary vs. secondary)
-
Systolic blood pressure on hospital admission (divided into four categories (mmHg): < 90, 90–110, 111–179, ≥ 180)
Statistics
The subgroups resulting from the matching protocol according to the AT taken were compared and analyzed individually to controls. No statistical intergroup comparisons were performed. Categorical variables are presented as percentages if the underlying total is obvious. Metric data are presented as mean and median with standard deviation. Differences in categorical and metric variables were evaluated with the McNemar test and Wilcoxon test for paired data, respectively. A two-sided p-value < 0.05 was considered significant. The interpretation of results should generally focus on the clinical relevance of an observed difference rather than p-values alone. All statistical analyses were performed using SPSS statistical software (SPSS 25; IBM Inc., Armonk, NY, USA).
Consent for publication
The TR-DGU gave permission for publication. All authors read and approved the final manuscript and gave permission for publication.
Results
A total of 18,125 patients met the inclusion criteria. The matched-pair analyses were performed with 5,746 patients (2,873 pairs), resulting in 1,759, 677 and 437 pairs with APD, VKA and DOAC pre-medication, respectively. By definition, the pairs were identical in terms of the nine matching factors. Table 2 summarises each matched-pair group’s baseline characteristics and injury mechanisms.
Length of stay (LOS), ICU length of stay and intubation rates showed no differences within the three matched-pair analyses (Table 2). Patients on APD treatment had a significantly higher early in-hospital mortality rate (< 24 h) compared to the controls (5.3% vs. 3.5%, p = 0.011). However, total in-hospital mortality did not differ (14.0% vs. 13.6%, p = 0.73) (Table 2). The APD group had higher surgery rates compared to the controls (51.8% vs. 47.8%, p = 0.015), whereas ESP rates and time until ESP showed no differences. Prehospital hypotension incidence, coagulopathy, the number of received PRBC and prothrombin complex concentrate (PCC) transfusions were comparable between groups (Table 3).
Total in-hospital mortality (23.9% vs. 19.5%, p = 0.026), RISC II prognosis (25.6% vs. 19.4%, p < 0.001) (Table 2) and INR values (2.36 ± 1.07 vs. 1.08 ± 0.2, p < 0.001) were higher in the VKA group. Furthermore, this group presented with coagulopathy (84.7% vs. 6.1%, p < 0.001) and received PCC more frequently (26.3% vs. 1.9%, p < 0.001), and surgical treatment was more frequent compared to the controls (52.4% vs. 44.8%, p = 0.005). However, no differences in ESP rates were found. Patients on VKA less often presented with prehospital hypotension (2.9% vs. 5.5%, p = 0.038) (Table 4).
DOAC therapy was associated with a significant increase in the coagulopathy rate (37.5% vs. 5.2%, p < 0.001), number of received PCC transfusions (10.2% vs. 0.5%, p < 0.001) and incidence of surgical procedures (52.6% vs. 41.0%, p = 0.001). INR values were significantly higher in the DOAC group compared to the controls (1.41 ± 0.63 vs. 1.09 ± 0.39, p < 0.001). However, no differences in ESP rate, incidence of prehospital hypotension or thromboembolic events and PRBC transfusion rates were observed (Table 5). Furthermore, no differences could be seen in total in-hospital mortality, early mortality (< 24 h) and RISC II prognosis (Table 2).
Discussion
Demographic changes and comorbidities will result in an increased incidence of geriatric trauma patients with AT. The recent approval of novel anticoagulants has further increased the diversity of AT medication. Trauma care of these patients is still debated, with inconsistent data regarding the clinical course after moderate or severe trauma. To elucidate to what extent different anticoagulants (APD, DOAC or VKA) affect the clinical course after severe trauma, we performed a retrospective study based on trauma registry data.
The main findings of our matched-pair analyses can be summarised as follows:
-
1.
AT did not influence the incidence of ESP but resulted in higher surgery rates over the further clinical course in all medication groups.
-
2.
In contrast to APD and VKA, DOAC affected neither early nor total in-hospital mortality.
-
3.
Coagulopathy rates and PCC transfusion rates were significantly higher in patients with both DOAC and VKA treatment.
Patients with VKA and DOAC treatment had significantly higher coagulopathy rates. As increased INR values represent a VKA treatment goal and have also been associated with DOAC treatment22, this supported our expectations. Surprisingly, about 15% of VKA patients had an INR < 1.5 and were thus in the non-therapeutic range. This observation could be explained by two main reasons. First, a non-adherence to VKA therapy is described in up to 20% of the respective patients23. On the other hand, monitoring in the patient group we investigated (patients > 75 years of age) is mostly performed by the primary care physician. Monitoring takes place once a month. Between these monitoring appointments, non-therapeutic and overshooting INR values may occur. Moreover, only a few patients of this age perform close-meshed self-monitoring (e.g., CoaguChek or INRatio2 point-of-care-systems at least once a week)24.
As coagulopathy is part of the “lethal triad” and typically associated with severe bleeding, we assessed preclinical and in-hospital hypotension rates. Interestingly, the controls showed a significantly higher prehospital hypotension rate compared to patients with VKA treatment. This might be explained by the higher risk of vascular calcification in patients with low vitamin K levels25. Vitamin K is needed to synthesise matrix glad protein (MGP), a natural calcification inhibitor. In mice, targeted deletion of the MGP gene resulted in rapid and complete arterial calcification, resulting in death by 6 weeks26. Vitamin K antagonism with warfarin, which antagonises the vitamin K-dependent MGP carboxylation, leads to rapid arterial calcification in rats27. Furthermore, diets high in vitamin K have been shown to reverse aortic calcification and improve arterial elasticity in warfarin-treated rats, suggesting that the calcification in response to warfarin treatment is due to the inhibition of the vitamin K-dependent MGP γ-carboxylation28. Due to vessel calcification, elasticity decreases and hypertension might develop. The higher hypertension risk in patients with low vitamin K and D levels has been described earlier29. Thus, the observed difference might be biased by the patients’ comorbidities. Pathological hypertension may affect patients on VKA treatment. In cases of blood loss, these patients might present with physiological blood pressure rather than hypotension. Although vitamin K’s role in vascular calcification has been demonstrated in animal and in vitro studies, evidence from human studies needs to be further elucidated. Our data may present useful references for the trauma population.
As haemostasis control is the goal in anticoagulated patients during resuscitation, we also investigated PCC, FFP and PRBC transfusion rates. PCC transfusion rates were significantly increased in patients with VKA and also with DOAC treatment. This was expected as guidelines recommend PCC application for reversal of VKA- and DOAC-associated coagulopathy in trauma patients17. As ESP rates and time to ESP did not differ between patients on AT and controls, the decision to perform ESP likely was not influenced by AT and the transfusion therapy was sufficient to achieve haemostasis in most patients with VKA and DOAC treatment. This supports treatment algorithms, which recommend antagonisation of known pharmacological coagulopathies before surgery30.
We found a significantly higher incidence of overall surgical procedures in patients with AT in all groups. As the AIS was comparable between AT and control patients, different injury severities as a reason can be excluded. We assume that AT-related development of clinically relevant haematomas, ongoing bleeding or compartment syndromes were indications for the higher number of operations. This has been reported to cause surgical interventions and is likely to contribute to higher surgical rates in AT patients31,32. Furthermore, several studies have associated relevant anticoagulation with prolonged wound drainage, which is conducive for infection development, due to hematoma formation, which predisposes towards infection33,34. More numerous persistent drainage or infections, although mostly superficial, may have contributed to the observed higher surgical intervention rates in all three treatment groups compared to controls34. However, due to the TraumaRegister DGU design, we were unable to verify an increase in operations due to one of the above-mentioned reasons.
Although surgical procedures are indications for AT reversal35, PCC application may cause severe side effects. PCC application and a delayed AT restart after surgery were thought responsible for higher incidences of thromboembolic events in the 1990s and early 2000s36. Our results did not show higher thromboembolic event rates in trauma patients on AT, confirming Majeed et al.’s findings on thromboembolic events after PCC application37. A possible explanation might be that modern PCCs have a higher degree of security, which has been attributed to the inclusion of coagulation inhibitors such as protein Z and heparin. As a result, a balance of pro- and anti-coagulatory effects seems to have been achieved38.
Despite the different overall surgical rates, LOS and ICU treatment duration did not differ between the AT patients and the controls. Protocolised daily examinations during the ICU stay might have led to early identification of patients at risk for further interventions and surgery39,40. Improved general status might have resulted in transfer to the normal ward, so additional surgical interventions did not prolong the ICU stay. Moreover, the severity of these surgical interventions might not have been as invasive as those needed to stabilise the patient, so renewed ICU treatment was unnecessary. As the patients on VKA and APD showed a higher total in-hospital mortality rate, it might be assumed that the increased number of deceased patients resulted in a LOS bias in these patients. Nevertheless, due to the low mortality rates, especially in APD patients, this potential bias seems negligible.
For mortality analysis, we distinguished between early and total in-hospital mortality as previously recommended41. Early in-hospital mortality was significantly higher in patients on APD compared to the controls (5.3% vs. 3.5%, p = 0.011). This difference diminished during the hospital stay and culminated in nonsignificant differences in the total in-hospital mortality rate, possibly due to the beneficial effects of APD on the immune system during the further clinical course of critically ill patients. A lower rate of post-traumatic acute respiratory distress syndrome, sepsis and multiple organ failure has been published42,43. Thus, some protective characteristics of ADP might have contributed to lower overall mortality during the later clinical course (> 24 h). The diagnosis of haemorrhagic shock, characterised by hypotension, does not seem to be causal for early in-hospital mortality in APD patients. Head trauma is common in multiply injured patients and was a leading injury (more than 50%) in each medication group. A meta-analysis by Batchelor et al. reported a trend towards increased mortality rates in head trauma patients on APD medication, due to intracranial haematoma enlargement44,45. In contrast, DOAC-associated intracranial haemorrhage resulted in smaller baseline haematoma volumes and a lower likelihood of haematoma expansion compared to VKA-associated haemorrhage46. Hence, the in-hospital mortality rate was lower in DOAC-associated intracranial haemorrhage (adjusted risk difference in favour of DOACs, 5.7%). This difference was accentuated among patients pre-treated with antiplatelet therapy in addition to VKA treatment (adjusted risk difference in favour of DOACs, 15%)47. Beneficial effects of DOAC have also been described by Mullins et al., who found no difference in 30-day mortality in a matched-pair analysis of 124 patients with proximal femoral fractures48. Although no statistical comparison of mortality rates between analyses was permitted because of the isolated matched-pair analyses, cautious comparisons can be made because the matched-pairs had markedly comparable baseline data. Our findings support recent safety studies reporting bleeding complications and thromboembolic events in patients on DOACs compared to VKA treatment49. A potential explanation might be the different effects on the coagulation system50. Whereas exposed tissue factor induces thrombin generation that can overcome DOACs’ inhibitory effects, the markedly reduced levels of FVIIa by VKA impair this response and increase the likelihood of severe bleeding even in modest trauma. Althoughs statistical comparison of matched-pair analyses was not feasible within this study, the observed findings might support previous findings: PCC rates—as a potential reversal therapy—were markedly increased in VKA compared to controls (Table 4) and were also 2.5-fold higher compared to differences between DOACs and their controls (Table 5). Yet, furher studies need to investigate this findings in more detail. In line with previous studies, patients with VKA treatment showed a significantly higher total mortality compared to controls (23.9% vs. 19.5%, p = 0.026)51. Other reasons include higher thromboembolic event rates due to VKA reversal and higher surgery rates in patients with VKA treatment52. Although the present study did not reveal higher thrombembolic event rates in patients on AT, higher surgery rates in patients with VKA treatment were observed. It is likely that bleeding complications during the further clinical course potentially influence the total in-hospital mortality in this subgroup, and careful monitoring of patients with VKA treatment within the Tertiary Trauma Survey remains crucial for early identification of patients at risk of ongoing bleeding53. Further studies exploring traumatic bleeding in patients with non-vitamin K oral anticoagulants are warranted54.
The large number of included patients, the strict inclusion criteria and the rigorous matching protocol were sufficient to provide clinically relevant data for geriatric trauma patients on AT. There were some limitations, however. Other combinations of matching criteria and extended matching criteria would have been possible, but additional criteria would have resulted in a loss of statistical power. The criteria were therefore carefully selected and, in our opinion, represent a good balance between goal-oriented matching results and statistical power. Although geriatric trauma is usually defined as patients older than 65 years55, vascular diseases leading to AT by DOAC predominantly occur by the age of 55 years56. Thus, this lower age threshold was included. Additionally, patients who died before hospital admission are not included in the TR-DGU. Therefore, preclinical causes of death cannot be investigated. This risks overlooking AT-related prehospital deaths of trauma patients. Another limitation results from the reduced data set (standard data entry form) of the TR-DGU that we used. Detailed information on the diseases for which the medications were prescribed or specific laboratory parameters (e.g. kidney function) were not recorded in this data set. No detailed information about comorbidities leading to AT and their potential influence on the clinical course and mortality was available. Moreover, registry studies are restricted to documented data quality. The TR-DGU is not designed to monitor anticoagulant use, and time since the last medication ingestion was not recorded. Additionally, subgroup analyses for different DOAC (Factor IIa and Xa inhibitors), PCC (three-factor PCC; four-factor PCC and activated PCC), and APD (single vs. dual APD treatment) could not be performed due to missing data. Nevertheless, the TR-DGU data quality is very high.
Conclusion
Although not influencing ESP or the time span until ESP, AT significantly affects the entire clinical course with higher overall surgical rates compared to controls. Further research should focus on improvement of diagnostic procedures and treatment algorithms for the early phase of trauma care. Prospective studies are needed to investigate possible risk factors and complications in trauma patients with AT to ultimately reduce the higher overall surgical and mortality rates.
Data availability
Data are provided by the TraumaRegister DGU. Data are available from the TraumaRegister DGU for researchers who meet the criteria for access to confidential data.
Abbreviations
- AT:
-
Anticoagulant therapy
- AIS:
-
Abbreviated injury scale
- APD:
-
Antiplatelet drugs
- ASA:
-
American Society of Anesthesiologists
- AUC:
-
AUC—Academy for Trauma Surgery
- DGU:
-
German Trauma Society
- DOAC:
-
Direct oral anticoagulant
- ESP:
-
Emergency surgical procedures
- ICU:
-
Intensive Care Unit
- ISS:
-
Injury Severity Score
- LOS:
-
Length of stay
- PCC:
-
Prothrombin complex concentrates
- PRBC:
-
Packed red blood cells
- RISC II:
-
Revised Injury Severity Classification Score II
- SD:
-
Standard deviation
- TR-DGU:
-
TraumaRegister DGU
- TTS:
-
Tertiary Trauma Survery
- VKA:
-
Vitamin K antagonist
References
Hashmi, A. et al. Predictors of mortality in geriatric trauma patients: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 76, 894–901. https://doi.org/10.1097/TA.0b013e3182ab0763 (2014).
Bonville, D. J. et al. Impact of preinjury warfarin and antiplatelet agents on outcomes of trauma patients. Surgery 150, 861–868. https://doi.org/10.1016/j.surg.2011.07.070 (2011).
Hildebrand, F. et al. Impact of age on the clinical outcomes of major trauma. Eur. J. Trauma Emerg. Surg. 42, 317–332. https://doi.org/10.1007/s00068-015-0557-1 (2016).
Joseph, B. et al. Redefining the association between old age and poor outcomes after trauma: The impact of frailty syndrome. J. Trauma Acute Care Surg. 82, 575–581. https://doi.org/10.1097/ta.0000000000001329 (2017).
Dossett, L. A., Riesel, J. N., Griffin, M. R. & Cotton, B. A. Prevalence and implications of preinjury warfarin use: An analysis of the National Trauma Databank. Arch Surg. 146, 565–570. https://doi.org/10.1001/archsurg.2010.313 (2011).
Wood, B. et al. The Anticoagulated trauma patient in the age of the direct oral anticoagulants: A Canadian perspective. Scand. J. Trauma Resuscit. Emerg. Med. 25, 76. https://doi.org/10.1186/s13049-017-0420-y (2017).
Eckman, M. H. et al. Atrial fibrillation decision support tool: Population perspective. Am. Heart J. 194, 49–60. https://doi.org/10.1016/j.ahj.2017.08.016 (2017).
Lippi, G., Mattiuzzi, C., Cervellin, G. & Favaloro, E. J. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann. Transl. Med. 5, 322. https://doi.org/10.21037/atm.2017.06.65 (2017).
Fan, P. et al. Recent progress and market analysis of anticoagulant drugs. J. Thorac. Dis. 10, 2011–2025. https://doi.org/10.21037/jtd.2018.03.95 (2018).
Bonne, S. & Schuerer, D. J. Trauma in the older adult: Epidemiology and evolving geriatric trauma principles. Clin. Geriatr. Med. 29, 137–150. https://doi.org/10.1016/j.cger.2012.10.008 (2013).
Adams, S. D. & Holcomb, J. B. Geriatric trauma. Curr. Opin. Crit. Care 21, 520–526. https://doi.org/10.1097/mcc.0000000000000246 (2015).
Lecky, F. E. et al. The effect of preinjury warfarin use on mortality rates in trauma patients: A European multicentre study. Emerg. Med. J. EMJ 32, 916–920. https://doi.org/10.1136/emermed-2014-203959 (2015).
Banks, S. E. & Lewis, M. C. Trauma in the elderly: Considerations for anesthetic management. Anesthesiol. Clin. 31, 127–139. https://doi.org/10.1016/j.anclin.2012.11.004 (2013).
Salottolo, K. M., Mains, C. W., Offner, P. J., Bourg, P. W. & Bar-Or, D. A retrospective analysis of geriatric trauma patients: Venous lactate is a better predictor of mortality than traditional vital signs. Scand. J. Trauma Resuscit. Emerg. Med. 21, 7. https://doi.org/10.1186/1757-7241-21-7 (2013).
Lehmann, R., Beekley, A., Casey, L., Salim, A. & Martin, M. The impact of advanced age on trauma triage decisions and outcomes: A statewide analysis. Am. J. Surg. 197, 571–574. https://doi.org/10.1016/j.amjsurg.2008.12.037 (2009).
TraumaRegister, D. G. U. 20 years TraumaRegister DGU((R)): Development, aims and structure. Injury 45(Suppl 3), S6–S13. https://doi.org/10.1016/j.injury.2014.08.011 (2014).
Bouillon, B. & Marzi, I. The updated German “Polytrauma—Guideline”: An extensive literature evaluation and treatment recommendation for the care of the critically injured patient. Eur. J. Trauma Emerg. Surg. 44, 1. https://doi.org/10.1007/s00068-018-0949-0 (2018).
Baker, S. P., O’Neill, B., Haddon, W. Jr. & Long, W. B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 14, 187–196 (1974).
Jennett, B. & Bond, M. Assessment of outcome after severe brain damage. Lancet 1, 480–484. https://doi.org/10.1016/s0140-6736(75)92830-5 (1975).
Pisciotta, A. V. Concepts of haemostasis and thrombosis: A study of the coagulation defect in hemophilia and in jaundice (Quick, Stanley-Brown and Bancroft 1935). Armand J. Quick (1894–1978)—a short biography. Thrombosis and haemostasis 44, 1–5 (1980).
Lefering, R., Huber-Wagner, S., Nienaber, U., Maegele, M. & Bouillon, B. Update of the trauma risk adjustment model of the TraumaRegister DGU: The revised injury severity classification, version II. Crit. Care 18, 476. https://doi.org/10.1186/s13054-014-0476-2 (2014).
Ciurus, T., Sobczak, S., Cichocka-Radwan, A. & Lelonek, M. New oral anticoagulants—a practical guide. Kardiochirurgia i torakochirurgia polska 12, 111–118. https://doi.org/10.5114/kitp.2015.52851 (2015).
Zirlik, A. & Bode, C. Vitamin K antagonists: relative strengths and weaknesses vs. direct oral anticoagulants for stroke prevention in patients with atrial fibrillation. J. Thromb. Thrombol. 43, 365–379. https://doi.org/10.1007/s11239-016-1446-0 (2017).
Bereznicki, L. R. et al. Improving the management of warfarin in aged-care facilities utilising innovative technology: A proof-of-concept study. Int. J. Pharm. Pract. 22, 84–91. https://doi.org/10.1111/ijpp.12035 (2014).
Shea, M. K. & Holden, R. M. Vitamin K status and vascular calcification: Evidence from observational and clinical studies. Adv. Nutr. 3, 158–165. https://doi.org/10.3945/an.111.001644 (2012).
Luo, G. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386, 78–81. https://doi.org/10.1038/386078a0 (1997).
Price, P. A., Faus, S. A. & Williamson, M. K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler. Thromb. Vasc. Biol. 18, 1400–1407. https://doi.org/10.1161/01.atv.18.9.1400 (1998).
Schurgers, L. J. et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 109, 2823–2831. https://doi.org/10.1182/blood-2006-07-035345 (2007).
Ballegooijen, A. J. et al. Joint association of low vitamin D and vitamin K status with blood pressure and hypertension. Hypertension 69, 1165–1172. https://doi.org/10.1161/HYPERTENSIONAHA.116.08869 (2017).
Bouillon, B. & Marzi, I. The updated German “Polytrauma – Guideline”: An extensive literature evaluation and treatment recommendation for the care of the critically injured patient. Eur. J. Trauma Emerg. Surg. 44, 1–1. https://doi.org/10.1007/s00068-018-0949-0 (2018).
Gaines, R. J., Randall, C. J., Browne, K. L., Carr, D. R. & Enad, J. G. Delayed presentation of compartment syndrome of the proximal lower extremity after low-energy trauma in patients taking warfarin. Am. J. Orthop. (Belle Mead NJ) 37, E201-204 (2008).
Byrne, A. M., Kearns, S. R. & Kelly, E. P. Posterior compartment syndrome associated with clopidogrel therapy following trivial trauma. Emerg. Med. J. EMJ 23, 697–698. https://doi.org/10.1136/emj.2006.037150 (2006).
Hassan, K. et al. Bleeding complications after use of novel oral anticoagulants in patients undergoing cardiac surgery. Ann. Thorac. Surg. 105, 702–708. https://doi.org/10.1016/j.athoracsur.2017.11.066 (2018).
Wang, Z., Anderson, F. A. Jr., Ward, M. & Bhattacharyya, T. Surgical site infections and other postoperative complications following prophylactic anticoagulation in total joint arthroplasty. PLoS ONE 9, e91755. https://doi.org/10.1371/journal.pone.0091755 (2014).
Steffel, J. et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 39, 1330–1393. https://doi.org/10.1093/eurheartj/ehy136 (2018).
Sorensen, B., Spahn, D. R., Innerhofer, P., Spannagl, M. & Rossaint, R. Clinical review: Prothrombin complex concentrates–evaluation of safety and thrombogenicity. Crit. Care 15, 201. https://doi.org/10.1186/cc9311 (2011).
Majeed, A., Eelde, A., Agren, A., Schulman, S. & Holmstrom, M. Thromboembolic safety and efficacy of prothrombin complex concentrates in the emergency reversal of warfarin coagulopathy. Thromb. Res. 129, 146–151. https://doi.org/10.1016/j.thromres.2011.07.024 (2012).
Ghadimi, K., Levy, J. H. & Welsby, I. J. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth. Analg. 122, 1287–1300. https://doi.org/10.1213/ane.0000000000001188 (2016).
Pfeifer, R. & Pape, H. C. Missed injuries in trauma patients: A literature review. Patient Saf. Surg. 2, 20. https://doi.org/10.1186/1754-9493-2-20 (2008).
Enderson, B. L. et al. The tertiary trauma survey: A prospective study of missed injury. J. Trauma 30, 666–669 (1990).
Beck, B. et al. Differences in the epidemiology of out-of-hospital and in-hospital trauma deaths. PLoS ONE 14, e0217158. https://doi.org/10.1371/journal.pone.0217158 (2019).
Greco, E., Lupia, E., Bosco, O., Vizio, B. & Montrucchio, G. Platelets and multi-organ failure in sepsis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18102200 (2017).
Yadav, H. & Kor, D. J. Platelets in the pathogenesis of acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L915-923. https://doi.org/10.1152/ajplung.00266.2015 (2015).
Batchelor, J. S. & Grayson, A. A meta-analysis to determine the effect of preinjury antiplatelet agents on mortality in patients with blunt head trauma. Br. J. Neurosurg. 27, 12–18. https://doi.org/10.3109/02688697.2012.705361 (2013).
de Gea-Garcia, J. H. et al. Antiplatelet therapies are associated with hematoma enlargement and increased mortality in intracranial hemorrhage. Med. Intensiva 36, 548–555. https://doi.org/10.1016/j.medin.2012.01.004 (2012).
Tsivgoulis, G. et al. Direct oral anticoagulant- vs vitamin K antagonist-related nontraumatic intracerebral hemorrhage. Neurology 89, 1142–1151. https://doi.org/10.1212/wnl.0000000000004362 (2017).
Inohara, T. et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA 319, 463–473. https://doi.org/10.1001/jama.2017.21917 (2018).
Mullins, B., Akehurst, H., Slattery, D. & Chesser, T. Should surgery be delayed in patients taking direct oral anticoagulants who suffer a hip fracture? A retrospective, case-controlled observational study at a UK major trauma centre. BMJ Open 8, e020625. https://doi.org/10.1136/bmjopen-2017-020625 (2018).
Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ 363, k4413, doi:https://doi.org/10.1136/bmj.k4413 (2018).
Siegbahn, A. et al. D-dimer and factor VIIa in atrial fibrillation - prognostic values for cardiovascular events and effects of anticoagulation therapy A RE-LY substudy. Thromb. Haemost. 115, 921–930. https://doi.org/10.1160/th15-07-0529 (2016).
Maung, A. A., Bhattacharya, B., Schuster, K. M. & Davis, K. A. Trauma patients on new oral anticoagulation agents have lower mortality than those on warfarin. J. Trauma Acute Care Surg. 81, 652–657. https://doi.org/10.1097/ta.0000000000001189 (2016).
Hackam, D. G., Kopp, A. & Redelmeier, D. A. Prognostic implications of warfarin cessation after major trauma: A population-based cohort analysis. Circulation 111, 2250–2256. https://doi.org/10.1161/01.cir.0000163548.38396.e7 (2005).
Rubboli, A., Becattini, C. & Verheugt, F. W. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J. Cardiol. 3, 351–358. https://doi.org/10.4330/wjc.v3.i11.351 (2011).
Friberg, L. & Oldgren, J. Efficacy and safety of non-vitamin K antagonist oral anticoagulants compared with warfarin in patients with atrial fibrillation. Open Heart 4, e000682. https://doi.org/10.1136/openhrt-2017-000682 (2017).
Fallon, W. F. Jr. et al. Geriatric outcomes are improved by a geriatric trauma consultation service. J. Trauma 61, 1040–1046. https://doi.org/10.1097/01.ta.0000238652.48008.59 (2006).
Chao, T. F. et al. Age threshold for the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehy837 (2019).
Acknowledgements
We would like to thank all staff and volunteer colleagues of the Trauma Register DGU for their cooperation and support (traumaregister@auc-online.de).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.M.B., F.H. and K.H. designed the trial. F.M.B., H.A., M.L., C.L., P.L., F.H. and K.H. performed the data collection and reviewed the manuscript. R.L. provided statistical advice. F.M.B., R.L. and M.L. analysed the data. F.M.B., F.H. and K.H. drafted the manuscript. F.M.B. takes responsibility for the article as a whole. K.H. supervised the trial. All authors contributed substantially to the manuscript revision, read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: RL declares that his institution (IFOM) receives an ongoing financial support from AUC GmbH, the owner of TR-DGU, which includes statistical support of data analysis from registry data. The other authors declare no personal competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bläsius, F.M., Laubach, M., Andruszkow, H. et al. Impact of anticoagulation and antiplatelet drugs on surgery rates and mortality in trauma patients. Sci Rep 11, 15172 (2021). https://doi.org/10.1038/s41598-021-94675-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94675-7
This article is cited by
-
Bilateral simultaneous knee arthroplasty shows comparable early outcome and complication rate as staged bilateral knee arthroplasty for patients scored ASA 1–3 if performed by a high-volume surgeon: a retrospective cohort study of 127 cases
Archives of Orthopaedic and Trauma Surgery (2023)
-
Treatment and outcomes of anticoagulated geriatric trauma patients with traumatic intracranial hemorrhage after falls
European Journal of Trauma and Emergency Surgery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.