Abstract
There is little data describing trends in the use of hydroxychloroquine for COVID-19 following publication of randomized trials that failed to demonstrate a benefit of this therapy. We identified 13,957 patients admitted for active COVID-19 at 85 U.S. hospitals participating in a national registry between March 1 and August 31, 2020. The overall proportion of patients receiving hydroxychloroquine peaked at 55.2% in March and April and decreased to 4.8% in May and June and 0.8% in July and August. At the hospital-level, median use was 59.4% in March and April (IQR 48.5–71.5%, range 0–100%) and decreased to 0.3% (IQR 0–5.4%, range 0–100%) by May and June and 0% (IQR 0–1.3%, range 0–36.4%) by July and August. The rate and hospital-level uniformity in deimplementation of this ineffective therapy for COVID-19 reflects a rapid response to evolving clinical information and further study may offer strategies to inform deimplementation of ineffective clinical care.
Similar content being viewed by others
Introduction
In the care of patients with COVID-19, anecdotal reports and in vitro data were made available in March of 2020 that suggested the potential for hydroxychloroquine to reduce disease severity1,2. Subsequent observational and randomized studies of hydroxycholorquine for COVID-19 have failed to demonstrate benefit, with the first peer-reviewed randomized trial published on May 14, 20203,4. The threshold at which evidence is considered sufficient to merit practice change may vary between hospitals, particularly in light of statements by high-ranking public officials in support of hydroxychloroquine for COVID-195. Furthermore, once practice patterns are established, they can be difficult to change6. We hypothesized that hospital rates of hydroxchloroquine use would vary over time with the potential for these findings to inform future studies of deimplementation strategies for ineffective care.
Methods
Study design, setting, and participants
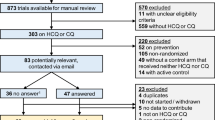
The American Heart Association (AHA) COVID-19 Cardiovascular Disease Registry captures data on all patients hospitalized for active COVID at participating hospitals, including patient demographics, comorbidities and risk factors, hospital treatments, and clinical outcomes. Additional details on the registry have been published7,8. As we sought to describe trends in hospital-level use of hydroxycholorquine, we restricted our analysis to hospitals with at least 10 cases of COVID-19 submitted to the registry between March 1 and August 31, 2020, to avoid inflating variation as a function of small sample size. Among patients with multiple admissions in the registry, we restricted our analysis to the first admission. We excluded patients with preexisting lupus, rheumatoid arthritis, or use of hydroxychloroquine prior to admission where it may reflect baseline therapy.
Statistical analysis
We describe the proportion of hospitalized patients who received hydroxychloroquine during the study period overall and by two-month calendar intervals. These intervals were selected to maintain sample size at the facility level for comparison of hydroxychloroquine use. We describe the median, interquartile range, and overall range of hydroxychloroquine use by hospital for the overall study period and by two-month calendar intervals.
Results
We identified 13,957 patients admitted for COVID-19 in the U.S. between March 1 and August 31, 2020, at 85 hospitals in 60 counties and 28 states. Patient characteristics by hydroxycholorquine are shown in Table 18. The overall proportion of patients receiving hydroxychloroquine was 37.6%, with a peak of 55.2% in March and April and decreasing to 4.8% in May and June and 0.8% in July and August. At the hospital-level, the median use of hydroxychloroquine for the period of study was 35.6% (IQR 14.2–37.7%, range 0–95.5%). In March and April, the hospital-level median use was 59.4% (IQR 48.5–71.5%, range 0–100%) and decreased to 0.3% (IQR 0–5.4%, range 0–100%) by May and June and 0% (IQR 0–1.3%, range 0–36.4%) by July and August (Fig. 1).
Discussion
In a large national study of patients hospitalized for COVID-19, we found use of hydroxychloroquine was common in March and April of 2020, but varied at the hospital-level. Use of hydroxychloroquine dropped precipitously after April and hospital-level variation decreased. The rate and hospital-level uniformity in deimplementation of this ineffective therapy for COVID-19 reflects a rapid response to evolving clinical information9,10.
Early in the COVID-19 pandemic, use of hydroxychloroquine was supported on the basis of in vitro data, small case series, approval of this therapy by governmental agencies, and statements from public leaders. In this early phase of the pandemic, the median hospital rate of hydroxychloroquine use was 59% with rates of use varying by facility. These findings mirror prior studies of the early phase of the pandemic11,12. Prior studies of uptake of new medical therapies and devices have demonstrated similar variation in rates of uptake with use influenced by the strength of scientific evidence, exposure to marketing of the new therapy, and support of the new device or therapy by national programs13,14. The observed variation in hydroxycholoroquine use early in the pandemic may reflect the lack of formal national programs related to the therapy in the U.S. and the lack of high quality data in the form of randomized controlled trials demonstrating benefit. Our study lacks data on the presence or absence of local clinical champions for the therapy and provider perception of statements by public leaders related to hydroxycholorquine use that may have also contributed to the observed variation. Similarly, the high degree of interest by non-medical personnel and patients may have also contributed to provider knowledge and attitudes in the use of hydroxychloroquine.
Following the publication of randomized trials that failed to demonstrate benefit for hydroxychloroquine in the care of COVID3,4, we observed a precipitous and uniform drop in the use of this therapy. Typically, as new evidence emerges that casts doubt on existing treatments, change to reduce or eliminate use of ineffective therapy is often slow and inconsistent and requires external support, active engagement with providers, and the development of training and education to impact a provider’s knowledge and attitude toward practice behaviors6,15,16,17. This slow pace of change in the use of ineffective and unnecessary care puts patients at risk and contributes to high costs of healthcare6. As a result, unnecessary care remains prevalent and a focus of efforts to improve healthcare value9,15.
The change in the use of hydroxychloroquine for COVID-19 is atypical in both the rapidity and uniformity of discontinuation of an ineffective practice. This may in part reflect that use of hydroxychloroquine was not an ingrained practice behavior such that typical processes needed to support deimplementation were not required6. Similarly, it may reflect that provider’s knowledge and attitudes on hydroxycholorquine were malleable17, given the evolving understanding of the pandemic and the high level of interest in COVID-19 from both medical and non-medical personnel. Finally, the lack of U.S. national programs related to the use of hydroxychloroquine may have facilitated a rapid transition, as change was not dependent on the dissolution of a national program. As health systems continue to look for strategies to reduce and eliminate ineffective care delivery, further study of adoption and deimplemenation of therapies during the pandemic may offer additional insights10.
References
Liu, J. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6, 16. https://doi.org/10.1038/s41421-020-0156-0 (2020).
Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 56(1), 105949. https://doi.org/10.1016/j.ijantimicag.2020.105949 (2020).
Self, W. H. et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial. JAMA 324(21), 2165–2176. https://doi.org/10.1001/jama.2020.22240 (2020).
RECOVERY Collaborative Group, Horby, P., Mafham, M., et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 383(21), 2030–2040. https://doi.org/10.1056/NEJMoa2022926 (2020).
Saag, M. S. Misguided use of hydroxychloroquine for COVID-19: The infusion of politics into science. JAMA 324(21), 2161–2162. https://doi.org/10.1001/jama.2020.22389 (2020).
Parchman, M. L. et al. Taking action on overuse: Creating the culture for change. Healthcare (Amst). 5(4), 199–203. https://doi.org/10.1016/j.hjdsi.2016.10.005 (2017).
Alger, H. M. et al. American Heart Association COVID-19 CVD registry powered by get with the guidelines. Circ. Cardiovasc. Qual. Outcomes. 13(8), e006967. https://doi.org/10.1161/CIRCOUTCOMES.120.006967 (2020).
Rodriguez, F., Solomon, N., de Lemos, J.A., et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.052278 (2017).
Reid, R. O., Rabideau, B. & Sood, N. Low-value health care services in a commercially insured population. JAMA Intern. Med. 176(10), 1567–1571. https://doi.org/10.1001/jamainternmed.2016.5031 (2016).
Anderson, T. S. & Lin, G. A. Testing cascades-A call to move from descriptive research to deimplementation science. JAMA Intern. Med. 180(7), 984–985. https://doi.org/10.1001/jamainternmed.2020.1588 (2020).
Bull-Otterson, L. et al. Hydroxychloroquine and chloroquine prescribing patterns by provider specialty following initial reports of potential benefit for COVID-19 treatment—United States, January-June 2020. MMWR Morb. Mortal Wkly. Rep. 69(35), 1210–1215. https://doi.org/10.15585/mmwr.mm6935a4 (2020).
Stroever, S.J., Ostapenko, D., Scatena, R., et al. Medication use among patients with COVID-19 in a large, national dataset: Cerner Real-World DataTM. Clin Ther. https://doi.org/10.1016/j.clinthera.2021.03.024 (2021).
Lublóy, Á. Factors affecting the uptake of new medicines: A systematic literature review. BMC Health Serv. Res. 14, 469. https://doi.org/10.1186/1472-6963-14-469 (2014).
Campbell, B. et al. A new health technology assessment system for devices: The first five years. Int. J. Technol. Assess. Health Care. 33(1), 19–24. https://doi.org/10.1017/S0266462317000253 (2017).
Kulkarni, S. A., Leykum, L. K. & Moriates, C. Deimplementation: Discontinuing low-value, potentially harmful hospital care. J. Hosp. Med. 16(1), 63. https://doi.org/10.12788/jhm.3563 (2021).
Lesley Davies, C. & Walley, P. Quality of care: Replacing or removing ineffective services. Int. J. Health Care Qual. Assur. 15(3), 124–129 (2002).
Rizki, S.A., Kurniawan, J., Budimulia, P., et al. Knowledge, attitude, and practice in Indonesian health care workers regarding COVID-19. Asia Pac. J. Public Health. https://doi.org/10.1177/10105395211011017 (2021).
Author information
Authors and Affiliations
Contributions
Study concept and design: S.M.B., G.R., S.D. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: S.M.B. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: S.E.-B.. Study supervision: S.M.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bradley, S.M., Emmons-Bell, S., Mutharasan, R.K. et al. Repeated cross-sectional analysis of hydroxychloroquine deimplementation in the AHA COVID-19 CVD Registry. Sci Rep 11, 15097 (2021). https://doi.org/10.1038/s41598-021-94203-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94203-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.