Abstract
Giant cell tumor of bone (GCTB) is an intermediate malignant bone tumor that is locally aggressive and rarely metastasizes. Denosumab, which is a receptor activator of nuclear factor kappa B ligand (RANKL) inhibitor, can be used to treat GCTB. We focused on potential immunotherapy for GCTB and investigated the tumor microenvironment of GCTB. Programmed death-ligand 1 (PD-L1) and indoleamine 2,3-dioxygenase 1 (IDO1) expression and signal-regulatory protein alpha (SIRPα), forkhead box P3 (FOXP3), and cluster of differentiation 8 (CD8) infiltration were assessed by immunohistochemical studies of 137 tumor tissues from 96 patients. Of the naive primary specimens, 28% exhibited PD-L1 expression and 39% exhibited IDO1 expression. There was significantly more SIRPα+, FOXP3+, and CD8+ cell infiltration in PD-L1- and IDO1-positive tumors than in PD-L1- and IDO1-negative tumors. The frequency of PD-L1 expression and SIRPα+ cell infiltration in recurrent lesions treated with denosumab was significantly higher than in primary lesions and recurrent lesions not treated with denosumab. PD-L1 expression and higher SIRPα+ cell infiltration were significantly correlated with shorter recurrence-free survival. PD-L1 and SIRPα immune checkpoint inhibitors may provide clinical benefit in GCTB patients with recurrent lesions after denosumab therapy.
Similar content being viewed by others
Introduction
Giant cell tumor of bone (GCTB) is an intermediate malignant bone tumor with frequent local recurrence and rare metastasis1. GCTB typically arises in the metaphysis and epiphysis of long bones and in the spine between the ages of 20 and 40 years2,3,4,5. Histologically, GCTB is composed of oval- and short spindle-shaped tumor cells, mesenchymal stromal cells, mononuclear monocytes, and osteoclast-like multinucleated giant cells1,4,6. Mesenchymal stromal tumor cells and osteoclast-like multinucleated giant cells overexpress receptor activator of nuclear factor kappa B (RANK) and receptor activator of nuclear factor kappa B ligand (RANKL), respectively. H3F3A mutations occur in 90% of GCTB cases, and conventional GCTB generally harbors p.G34W mutations4,7,8,9,10,11. Denosumab is a human monoclonal antibody that inhibits the receptor activation of RANKL and the RANKL pathway11,12,13. In Japan in 2014, denosumab was approved for the treatment of unresectable and recurrent GCTB14. However, denosumab-treated GCTB has recurrence potential. Healey et al. suggested that the risk of malignant transformation with denosumab is increased15. The main objective of recent studies has been to investigate potential immunotherapy against GCTB. Programmed death-ligand 1 (PD-L1) is the ligand of programmed cell death protein 1 (PD-1), and it is thought to promote evasion of the antitumor immune response by suppressing T-cell function. Several investigations and clinical trials concerning the PD-L1/PD-1 axis have been developed for various cancers, including malignant bone tumors16,17,18,19,20,21. Metovic et al. investigated PD-L1 expression in GCTB patients and reported that PD-L1 expression was correlated with shorter disease-free survival22.
Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme of tryptophan metabolism, and it is associated with poor prognosis by enabling malignant tumors to avoid immune surveillance12. The expression of PD-L1 and IDO1 and the clinicopathological impact of PD-L1 and IDO1 co-expression have recently been investigated in several malignant tumors, such as lung cancer23,24,25,26,27,28, renal cell carcinoma29, thyroid cancer30, and osteosarcoma20.
Cluster of differentiation 47 (CD47) and signal-regulatory protein alpha (SIRPα) are “don’t eat me” signals, and they promote escape from phagocytosis in malignant tumors31,32,33, enabling lymphoma cells to evade phagocytosis and thereby promoting tumor growth32. Dancsok et al. investigated SIRPα expression in various bone and soft tissue sarcomas and reported that some sarcomas showed shorter progression-free survival with PD-L1 expression31.
We retrospectively analyzed PD-L1 and IDO1 expression and SIRPα, cluster of differentiation 8 (CD8), and forkhead box P3 (FOXP3) immune cell infiltration, and we examined their effects on the clinicopathological parameters of GCTB and their prognostic value in GCTB.
Results
Clinical results
The clinicopathological features of the subjects are shown in Table 1. The median age at initial diagnosis was 33 (17–84) years. The subjects included 54 females and 42 males. The tumors were mainly located in the femur or tibia (Table 1). Four patients did not undergo surgery and were treated only with denosumab. Ten patients received denosumab (six for neoadjuvant therapy and four for recurrence). Recurrence- and metastasis-free survival data were available for 78 patients, with follow-up ranging from 1 to 332 months (median: 58 months). In this study, 18 patients (23%) had local recurrence and eight (10%) had distant metastasis. All patients with metastasis developed pulmonary metastases. There were no patients with tumor-related death.
Radiological features
Radiological images, including plain radiographs, computed tomography scans, and/or magnetic resonance imaging scans, or medical records were available for 76 patients. Maximum diameters (N = 68) and pathological fractures (N = 76) were investigated. The radiological characteristics are shown in Table 1. Radiologically, the median of the maximum diameters was 4.3 cm (range: 0.6–7.9 cm). A pathological fracture was apparent in six of 76 cases (8%).
Immunohistochemistry
Immunoexpression in primary tumors treated without denosumab
Association of PD-L1 and IDO1 expression and SIRPα+ macrophage and FOXP3+ and CD8+ lymphocyte infiltration with clinicopathological features
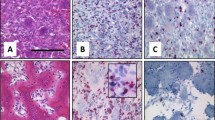
Representative images of the PD-L1, IDO1, SIRPα, FOXP3, and CD8 immunohistochemical studies are shown in Fig. 1a–e. Among the 92 non-denosumab-treated primary specimens, 26 (28%) exhibited PD-L1 expression and 36 (39%) exhibited IDO1 expression (Table 2). PD-L1 expression was more frequent in the cases that underwent neoadjuvant denosumab therapy than in the cases that did not (P = 0.0064) (Table 2). IDO1 expression was more frequent in cases with GCTB in the extremity than in cases with GCTB in the trunk (P = 0.0247) (Table 2). Frequent mitotic figures (≥ 10/10 HPF) showed significant correlation with FOXP3 infiltration. Histologically, spindle cell features were correlated with SIRPα infiltration (Table 2).
Representative images of the immunoexpression of PD-L1 (a), IDO1 (b), SIRPα (c), FOXP3 (d) and CD8 (e) in GCTB. PD-L1, SIRPα and CD8: Membranous staining, IDO1: Cytoplasmic and membranous staining. FOXP3: Nuclear staining Scale bars: 100 µm. PD-L1: programmed death ligand 1, IDO1: indoleamine 2,3-dioxygenase 1, SIRPα: Signal-regulatory protein alpha, FOXP3: forkhead box P3, CD8: cluster of differentiation 8.
Association of PD-L1 and IDO1 expression with SIRPα+ macrophage and FOXP3+ and CD8+ lymphocyte infiltration
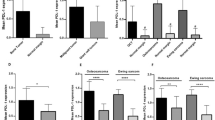
We evaluated the correlations between PD-L1 and IDO1 expression and SIRPα+, FOXP3+, and CD8+ infiltration. There was significantly more SIRPα+, FOXP3+, and CD8+ infiltration in all specimens from PD-L1-positive patients than from PD-L1-negative patients (P < 0.0001, P = 0.0143, and P = 0.0062, respectively) (Fig. 2a–c). In all specimens with IDO1 positivity, the infiltration of SIRPα+, FOXP3+, and CD8+ cells was significantly higher than in equivalent IDO1-negative cases (P < 0.0001, P < 0.0001, and P = 0.0016, respectively) (Fig. 2a-c).
Alteration of PD-L1 and IDO1 expression and SIRPα+ macrophage and FOXP3+ and CD8+ lymphocyte infiltration after denosumab treatment
Differences in PD-L1 and IDO1 expression between primary, ND-Rec, and D-Rec lesions
We evaluated PD-L1 and IDO1 expression in primary lesions, recurrent lesions after denosumab treatment (D-Rec), and recurrent lesions not treated with denosumab (ND-Rec). The frequency of PD-L1 expression in D-Rec lesions was significantly higher than in primary and ND-Rec lesions (P = 0.0243) (Fig. 3a). There were no significant differences in IDO1 expression between primary, ND-Rec, and D-Rec lesions (Fig. 3b).
Differences in SIRPα, FOXP3, and CD8 infiltration between primary, ND-Rec, and D-Rec lesions
We compared SIRPα+, FOXP3+, and CD8+ cell counts between primary, ND-Rec, and D-Rec lesions. SIRPα+ cell infiltration in D-Rec lesions was significantly increased compared with that in primary lesions and ND-Rec lesions (P = 0.0074 and P = 0.0188, respectively) (Fig. 4a). Representative figures of PD-L1 and SIRPα that compared pre- and post- denosumab treatment belong to same patient were shown in Fig. 5. There was no significant difference in SIRPα+ cell infiltration between primary tumors and ND-Rec lesions. FOXP3 and CD8 infiltration in primary, ND-Rec, and D-Rec lesions did not reach statistical significance (Fig. 4b,c).
Prognostic significance of PD-L1 and IDO1 expression, tumor-infiltrating lymphocytes (FOXP3 and CD8), SIRPα infiltration, and clinico-radio-pathological features in primary naive lesions
We assessed the prognostic significance of clinico-radio-pathological characteristics, PD-L1 and IDO1 expression, and SIRPα+, FOXP3+ and CD8+ cell infiltration by using Kaplan–Meier survival analysis (Table 3). PD-L1 expression and a high number of SIRPα+ cells were correlated with shorter recurrence-free survival (P = 0.0355 and P = 0.0243, respectively) (Table 3, Fig. 6a,b). There was no significant correlation between recurrence-free survival and preoperative denosumab treatment. The results of multivariate analysis of clinicopathological features and immunohistochemical studies suggested that high SIRPα+ and FOXP3+ infiltration were associated with shorter recurrence-free survival (Table 3).
Immunofluorescence double staining
Localization of PD-L1 expression in the tumor microenvironment of GCTB
We performed double immunofluorescence (n = 10) to evaluate whether tumor cells with H3F3A mutations or mononuclear histiocytoid cells express PD-L1. Representative images are shown in Fig. 7. Both tumor cells with H3F3A mutations and mononuclear histiocytoid cells were found to express PD-L1.
Localization of SIRPα on mononuclear cells of GCTB
Moreover, to verify which cells express SIRPα, double immunofluorescence was performed for SIRPα and CD14 (n = 10). Representative images are shown jn Fig. 8. SIRPα positive cells were diffusely found and some of these positive cells also expressed CD14 (monocyte marker).
Discussion
Classically, GCTB is treated with chemical adjuvant and/or surgery to reduce local recurrence. However, the rate of local recurrence after chemical adjuvant therapy is 15–50%2,14. Local recurrence significantly debases patients’ activities of daily living and quality of life. Thus, prevention of local recurrence is an important part of GCTB treatment. Denosumab, which is a human monoclonal anti-RANKL antibody that inhibits the RANK/RANKL signaling pathway, has been introduced to treat GCTB12,13,34. Pathological findings have previously revealed osteoclast-like multinucleated giant cell and mononuclear stromal cell depletion and new bone formation after denosumab treatment11,35,36. GCTB sometimes shows dramatic histological changes and mimics primary malignant bone tumors after denosumab treatment35,36. Kato et al. reported that after denosumab therapy, mononuclear tumor cells with H3F3A mutations could survive, but osteoclast cells could not survive without RANK/RANKL signaling6. Some reports have recently suggested that preoperative denosumab administration might increase the recurrence rate after operation34,37. New bone formation may be difficult to distinguish from preexisting bone trabeculae and make it difficult to identify true surgical margins34. No consensus has been reached about whether preoperative denosumab treatment might be useful to prevent local recurrence. Some GCTB cases are refractory to denosumab and have frequent recurrence. Thus, novel therapeutic targets, such as immunotherapy against GCTB, are required.
There have been few studies concerning tumor immunity in GCTB and immune microenvironment alterations after denosumab therapy. To understand the tumor microenvironment of GCTB, we established this study to focus on the tumor immune microenvironment of primary lesions and recurrent lesions with and without denosumab therapy in GCTB patients, and to investigate an immune checkpoint inhibitor treatment strategy for GCTB patients. A prior immunohistochemical study showed PD-L1 expression by tumor cells and multinucleated giant cells in 28.3% of GCTB specimens, and this was associated with shortened disease-free survival and high Ki-67 positivity22. Moreover, this previous study focused on two immune-related genes, TLR8 and LCK, which are related to innate immunity activation and CD4+ and CD8+ lymphocyte development. Lower TLR8 and LCK expression were correlated with PD-L1 immunoexpression positivity, but after denosumab treatment, TLR8 and LCK expression increased22. Our results showed PD-L1 immunoexpression in about 28% of primary specimens without denosumab treatment, and this was significantly related to shortened recurrence-free survival. Our double immunofluorescence staining study showed that PD-L1 expression in both neoplastic cells with H3F3A mutations and in mononuclear histiocytoid cells were seen. Moreover, the frequency of PD-L1 in D-Rec lesions was significantly higher than in primary and ND-Rec lesions. These results suggested possible interactions between tumor cells and mononuclear histiocytoid cells via the PD-1/PD-L1 axis in addition to RANK/RANKL signaling. It is well known that in GCTB, RANK/RANKL axis between neoplastic cells and mononuclear histiocytoid cells mediates osteoclastic pathways6. On the other hand, in tumor microenvironment, PD-1/PD-L1 axis enable tumor cells to avoid immune surveillance16,17,18,19,20,21. Our results suggested that tumor cells with H3F3A mutation and mononuclear histiocytoid cells might cooperate to express PD-L1 and escape tumor immunity because neoplastic cells and non-neoplastic cells expressed PD-L1. Moreover, our double immunofluorescence staining showed that SIRPα positive cells were diffusely found and some of these positive cells also expressed CD14 (monocyte marker). In GCTB, histologically mononuclear cells are tumor cells or monocytes11. Therefore, we suggested that tumor cells also would express SIRPα, although double staining for H3G34W and SIRPα was not possible in our study. There was the possibility of immune alterations associated with recurrence and denosumab treatment. More specifically, PD-L1 and SIRPα were more upregulated in recurrent tumors than in primary tumors due to resistance to treatment. It is suggested that tumor cells and/or non-neoplastic cells escaped tumor immunity by expressing PD-L1 and SIRPα.
Thus, patients with uncontrollable recurrent lesions may be treated with anti-PD-L1 or anti-PD-1 inhibitors after denosumab therapy.
On the other hand, the use of widely variable combinations of immune checkpoint inhibitors has gained great interest to improve immunotherapy outcomes31. For instance, macrophage-related immune checkpoints and CD47/SIRPα interactions have been focused on as new therapeutic targets of immunotherapy31,32,38,39. We showed that high SIRPα+ infiltration in primary specimens was associated with shorter recurrence-free survival by using univariate and Cox multivariate analyses. SIRPα+ macrophages were frequently seen in recurrent specimens treated with denosumab rather than in primary specimens with frequent PD-L1 immunopositivity. Previous studies have reported that SIRPα+ macrophage infiltrates were correlated with shorter overall survival and progression-free survival in other malignancies, such as diffuse large B-cell lymphoma32 and melanoma and renal cell carcinoma39. However, other reports have also shown a positive correlation between SIRPα expression and good prognosis33. In our immunohistochemical studies, it was suggested that SIRPα enabled tumor cells to evade phagocytosis, leading to promoted tumor growth and progression, similar to PD-L1 expression in GCTB patients. Treatment inhibiting CD47 and SIRP interactions has been investigated in previous studies, with clinical trials in hematopoietic and solid cancers40,41,42. We suggest that anti-SIRPα inhibitors will become a treatment for GCTB patients, especially patients with frequent recurrence.
Several studies have reported that RANK/RANKL inhibitors have the potential to increase the effectiveness of immune checkpoint inhibitors43,44,45,46,47,48. In a mouse study, Ahern et al. showed that combination therapy with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and anti-RANKL inhibitors increased CD8-positive T-cell infiltration compared to anti-CTLA-4 inhibitors or anti-RANKL inhibitors alone46. Our immunohistochemical study results showed no significant differences in CD8 infiltration between primary, ND-Rec, and D-Rec specimens. However, it was suggested that in GCTB, CD8+ lymphocyte infiltration would increase with combined immunotherapy and denosumab therapy. In a previous study, patients with lung cancer or melanoma who received both anti-CTLA-4 and/or anti-PD-1 inhibitors and denosumab had good disease control rates compared to patients not receiving denosumab48. Moreover, the authors of this previous study claimed that longer use of combined therapy was preferable to control tumor progression48. Although denosumab is widely used for GCTB treatment, the potential of recurrence remains. In our study, PD-L1 and SIRP expression in recurrent GCTB with denosumab treatment was higher than in primary GCTB and recurrent GCTB without denosumab treatment. Our findings suggested that the combination of PD-L1/PD-1 and/or SIRP inhibitors and denosumab might be effective for controlling recurrent GCTB. Further studies using a larger number of cases are required to confirm the effectiveness of these treatments. To the best of our knowledge, this study is the first to report SIRP expression in GCTB and investigate alterations in primary specimens and recurrent specimens with and without denosumab treatment. From the immunohistochemical and immunofluorescence results in this study, it can be concluded that PD-L1 and SIRPα immune checkpoint inhibitors may provide clinical benefits in GCTB patients with uncontrollable recurrent lesions after denosumab therapy.
Materials and methods
Patients and tissue samples
We used samples of GCTB patients registered from 1984 to 2019 in the database of the Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan. All cases were reviewed based on histological examinations with hematoxylin and eosin staining and immunohistochemical studies using antibodies specific to GCTB (anti-H3.3G34W, anti-H3.3G34R, and anti-H3.3G34V)4,7,11.
This study included 137 formalin-fixed, paraffin-embedded samples from 96 patients. The samples included primary conventional GCTB, recurrent conventional GCTB, post-denosumab GCTB, and lung metastasis of conventional GCTB. The lesions were collected by biopsy or resection. Clinical data, including age at diagnosis, sex, and tumor site, were collected. The presence of pathological fractures was investigated via plain radiographs, computed tomography scans, and/or magnetic resonance imaging scans that were examined by a radiologist (J.M.). Morphological features, mitotic figures, osteoclastic giant cells, foamy macrophages, bone formation, spindle cell features, and secondary aneurysmal bone cystic changes were also evaluated and investigated by three pathologists (Y.T., K.K., and Y.Y.). The institutional review board at Kyushu University approved this study (approval codes: 29-625 and 29-429). Written informed consent was obtained from the patients and their parents/guardians prior to tissue collection. All experiments were performed in accordance with guidelines provided by the Ethics Committees and Institutional Review Boards.
Immunohistochemical staining
For the immunohistochemical and immunofluorescence studies, formalin-fixed, paraffin-embedded tissues were sliced into 4-μm sections. The immunohistochemical studies were performed as previously described20,29. The following rabbit and mouse monoclonal antibodies were used as the primary antibodies: anti-PD-L1, anti-IDO1, anti- SIRPα, anti-FOXP3, anti-CD8, anti-H3.3G34W, anti-H3.3G34R, and anti-H3.3G34V (Supplementary Table S1). Appropriate controls were used throughout. Three pathologists (Y.T., K.K., and Y.Y.) independently evaluated the immunohistochemical staining results for each sample. For the immunohistochemical evaluation of PD-L1 and IDO1, the membrane PD-L1 expression and cytoplasmic IDO1 expression were defined by combined proportion scores, which evaluates on tumor cells and tumor-associated immune cells. Cases with a combined proportion score ≥ 1% were defined as positive. Moreover, SIRPα+, CD8+, and FOXP3+ cells were counted per high power field in five dependent fields for each case. This process was based on previous studies, with modifications32,33,49,50,51. Statistically, the median numbers of SIRPα+, CD8+, and FOXP3+ cells were determined as the cut-off points.
Immunofluorescence double staining
To identify PD-L1 and H3.3G34W localization in mononuclear stromal cells and tumor cells, double immunofluorescence was performed for H3.3G34W and PD-L1 (n = 10). Moreover, to verify which cells express SIRPα, double immunofluorescence was performed for SIRPα and CD14 (n = 10). The following antibodies were used as the primary antibodies: anti-PD-L1, anti-H3.3G34W, anti-SIRPα and anti-CD14 (Supplementary Table S1).
Statistical analysis
Fisher’s exact or Wilcoxon tests were used to analyze correlations between two dichotomous variables, such as clinicopathological findings and immunohistochemical results. Survival curves were calculated by using the Kaplan–Meier method, and significant differences were calculated by using the log-rank test. Statistical significance was defined as P < 0.05. In the multivariate analysis, a Cox proportional hazards model was used. Data analysis was performed by using the JMP statistical software package (version JMP version 14.2.0; SAS Institute Inc., Cary, NC, USA).
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
WHO Classification of Tumours, 5th ed, Vol 3, Soft Tissue and Bone Tumours (ed by WHO Classification of Tumours Editorial Board), IARC, Lyon, (2020).
Algawahmed, H., Turcotte, R., Farrokhyar, F. & Ghert, M. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: a systematic review and meta-analysis. Sarcoma 2010, 586090 (2010).
Balke, M. et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 134, 969–978 (2008).
Amary, F. et al. H3F3A (Histone 3.3) G34W immunohistochemistry: a reliadle marker defining benign and malignant giant cell tumor of bone. Am. J. Surg. Pathol. 41, 1059–1068 (2017).
Kivioja, A. H. et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 79, 86–93 (2008).
Kato, I. et al. Giant cell tumours of bone treated with denosumab: histological, immunohistochemical and H3F3A mutation analyses. Histopathology 72, 914–922 (2018).
Yamamoto, H. et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum. Pathol. 73, 41–50 (2018).
Presneau, N. et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J. Pathol. Clin. Res. 1, 113–123 (2015).
Behjati, S. et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 45, 1479–1482 (2013).
Arjen, H. G. et al. Mutataion analysis of H3F3A and H3F3B as a diagnostic tool for giant cell tumor of bone and chondeoblastoma. Am. J. Surg. Pathol. 39, 1576–1583 (2015).
Yamamoto, H., Ishihara, S., Toda, Y. & Oda, Y. Histone H3.3 mutation in giant cell tumor of bone: an update in pathology. Med. Mol. Morphol. 53, 1–6 (2020).
Chawla, S. et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 20, 1719–1729 (2019).
Ueda, T. et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann. Oncol. 26, 2149–2154 (2015).
Urakawa, H. et al. Clinical outcome of primary giant cell tumor of bone after curettage with or without perioperative denosumab in Japan: from a questionnaire for JCOG 1610 study. World J. Surg. Oncol. 16, 160 (2018).
Healey, J. H. Denosumab for giant cell tumour of bone: success and limitations. Lancet Oncol. 20, 1627–1628 (2019).
Koirala, P. et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 6, 30093 (2016).
Liao, Y. C. L. et al. Targeting programmed cell death ligand 1 by CRISPR Cas9 in osteosarcoma cells. Oncotarget. 8, 30276–30287 (2017).
Lussier, D. M. et al. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 38, 96–106 (2015).
Sundara, Y. T. et al. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol. Immunother. 66, 119–128 (2017).
Toda, Y. et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J. Cancer Res. Clin. Oncol. 146, 2607–2620 (2020).
Torabi, A., Amaya, C. N., Wians, F. H. Jr. & Bryan, B. A. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology 49, 506–513 (2017).
Metovic, J. et al. Prognostic role of PD-L1 and immune-related gene expression profiles in giant cell tumors of bone. Cancer Immunol. Immunother. 69, 1905–1916 (2020).
Kozuma, Y. et al. Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1 co-expression correlates with aggressive features in lung adenocarcinoma. Eur. J. Cancer 101, 20–29 (2018).
Takada, K. et al. Co-expression of IDO1 and PD-L1 in lung squamous cell carcinoma: Potential targets of novel combination therapy. Lung Cancer 128, 26–32 (2019).
Takada, K. et al. Expression of PD-L1, PD-L2, and IDO1 on tumor cells and density of CD8-positive tumor-infiltrating lymphocytes in early-stage lung adenocarcinoma according to histological subtype. J. Cancer Res. Clin. Oncol. 146, 2639–2650 (2020).
Zhang, M. L. et al. Differential expression of PD-L1 and IDO1 in association with the immune microenvironment in resected lung adenocarcinomas. Mod. Pathol. 32, 511–523 (2019).
Parra, E. R. et al. Immunohistochemical and image analysis-based study shows that several immune checkpoints are co-expressed in non-small cell lung carcinoma tumors. J. Thorac. Oncol. 13, 779–791 (2018).
Schalper, K. A. et al. Differential expression and significance of PD-L1, IDO-1, and B7–H4 in human lung cancer. Clin. Cancer Res. 23, 370–378 (2017).
Kiyozawa, D. et al. Programmed death ligand 1/indoleamine 2,3-dioxygenase 1 expression and tumor-infiltrating lymphocyte status in renal cell carcinoma with sarcomatoid changes and rhabdoid features. Hum. Pathol. 101, 31–39 (2020).
Rosenbaum, M. W. et al. PD-L1 and IDO1 are expressed in poorly differentiated thyroid carcinoma. Endocr. Pathol. 29, 59–67 (2018).
Dancsok, A. R. et al. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology 9, 1747340 (2020).
Kazama, R. et al. Combination of CD47 and signal-regulatory protein-alpha constituting the “don’t eat me signal” is a prognostic factor in diffuse large B-cell lymphoma. Cancer Sci. 2, 17 (2020).
Yanagida, E. et al. Clinicopathological analysis of immunohistochemical expression of CD47 and SIRPalpha in adult T-cell leukemia/lymphoma. Hematol. Oncol. 3, 19865 (2020).
Chen, X., Li, H., Zhu, S., Wang, Y. & Qian, W. Pre-operative denosumab is associated with higher risk of local recurrence in giant cell tumor of bone: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 21, 256 (2020).
Wojcik, J. et al. Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone. Am J Surg Pathol. 40, 72–80 (2019).
Roitman, P. D., Jauk, F., Farfalli, G. L., Albergo, J. I. & Aponte-Tinao, L. A. Denosumab-treated giant cell tumor of bone. Its histologic spectrum and potential diagnostic pitfalls. Hum. Pathol. 63, 89–97 (2017).
Errani, C. et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J. Bone Joint Surg. Am. 100, 496–504 (2018).
Murata, Y., Saito, Y., Kotani, T. & Matozaki, T. Blockade of CD47 or SIRPalpha: a new cancer immunotherapy. Expert Opin. Ther. Targets. 24, 945–951 (2020).
Yanagita, T. et al. Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2, e89140 (2017).
Sikic, B. I. et al. First-in-human, first-in-class phase I trial of the anti-CD47 antiobody Hu5F9-G4 in patients with advanced cancers. Science 37, 946–953 (2019).
Shi, R. et al. The identification of a CD47-blocking “hotspot” and design of a CD47/PD-L1 dual-specific antibody with limited hemagglutination. Signal Transduct Target Ther 5, 16 (2020).
Liu, J. et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 10, e0137345 (2015).
van Dam, P. A. et al. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit. Rev. Oncol. Hematol. 133, 85–91 (2019).
Smyth, M. J., Yagita, H. & McArthur, G. A. Combination anti-CTLA-4 and anti-RANKL in metastatic melanoma. J. Clin. Oncol. 34, e104-106 (2013).
Bostwick, A. D., Salama, A. K. & Hanks, B. A. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J. Immunother. Cancer 3, 19 (2015).
Ahern, E. et al. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin. Cancer Res. 23, 5789–5801 (2017).
Ahern, E. et al. Pharmacodynamics of Pre-Operative PD1 checkpoint blockade and receptor activator of NFkB ligand (RANKL) inhibition in non-small cell lung cancer (NSCLC): study protocol for a multicentre, open-label, phase 1B/2, translational trial (POPCORN). Trials 20, 753 (2019).
Liede, A. et al. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology 7, e1480301 (2018).
Li, Z. et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J. Cancer 7, 784–793 (2016).
Kinoshita, F. et al. Combined evaluation of tumor-infiltrating CD8+ and FoxP3+ lymphocytes provides accurate prognosis in stage IA lung adenocarcinoma. Ann. Surg. Oncol. 27, 2102–2109 (2020).
Witte, H. M. et al. Prognostic impact of PD-L1 expression in malignant salivary gland tumors as assessed by established scoring criteria: tumor proportion score (TPS), combined positivity score (CPS), and immune cell (IC) infiltrate. Cancers (Basel) 12, 873 (2020).
Author information
Authors and Affiliations
Contributions
Y.T., K.K., Y.N., M.M., and Y.O. were involved in the conception and design of the study. Y.T., K.K., and Y.Y. performed the slide review. Y.T., S.I., Y.I., Y.S., K.K., A.K., S.T., K. T., T.M., and Y.M. collected the clinical data. Y.T., S.I., Y.I., Y.S., K.K., D.K., D.T., and K.I. performed the immunohistochemical studies. Radiological data were collected by J.M. Data analysis was done by Y.T., K.K. and H.Y. The manuscript was drafted by Y.T. and revised by K.K., Y.N., M.M., and Y.O. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toda, Y., Kohashi, K., Yamamoto, H. et al. Tumor microenvironment in giant cell tumor of bone: evaluation of PD-L1 expression and SIRPα infiltration after denosumab treatment. Sci Rep 11, 14821 (2021). https://doi.org/10.1038/s41598-021-94022-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94022-w
This article is cited by
-
Denosumab for giant cell tumors of bone from 2010 to 2022: a bibliometric analysis
Clinical and Experimental Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.