Abstract
This is the first report of SARS-CoV-2 detection on field-collected Musca domestica housefly surface and tissue samples using the high-sensitive PCR assay which suggests the possible insect-borne transmission. The study was conducted in Shiraz city, southern Iran, in May and Jun 2020. Adult flies were sampled at the outdoor areas of two hospitals treating COVID-19 patients. Fly samples were first washed twice to remove the insect surface attached to SARS-CoV-2 virions. After that, the disinfected fly samples were homogenized. Fly surface washout and homogenate samples were tested using Taq Man real-time PCR assay for the SARS-CoV-2 virus. In a total of 156 houseflies, 75% of samples from the body washout samples were positive for SARS-CoV-2. Strikingly, 37% of the homogenized specimens were positive for the SARS-CoV-2, suggesting the possible infection of the insects or uptake of the virion to the insect metabolism. The other possibility is the houseflies up took the blood or blood fluids of the patients and the RNA of the SARS-CoV-2 survived in the insect body without replicating. Our preliminary findings suggest that the houseflies could transmit SARS-CoV-2 as a mechanical or biological vector especially during the warm seasons while increasing the population and activity of houseflies.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory illness reported from Wuhan City, Hubei Province, China in late 20191.
Two main mechanisms for SARS-CoV-2 virus transmission have been introduced, including transmission through air droplets and fomites2,3. The delivery of the viruses with the houseflies could be a new way to add to the previously described ways by researchers.
There are various studies in the world on the transmission of pathogens by insects. Biological and mechanical transmission methods are the two main reasons for the transmission of communicable diseases or epidermis. The possibility of transmitting viral diseases that cause acute respiratory infections in humans and animals has been studied in different parts of the world and the possibility of mechanical transmission of these viruses by some species of insects has been proven4,5,6,7,8,9,10,11,12,13,14.
The housefly (Musca domestica) is a non-blood feeding insect of the order Diptera that can be a mechanical vector of many diseases, especially nosocomial infections, due to its unique behavioral and nutritional mechanisms. This insect can transmit pathogens mechanically to humans in four main ways: (1) adherence of pathogens to its body through the legs, wings, and surface of the abdomen, etc. (2) By its diverse diet, which is omnivorous and can feed on anything such as household and hospital waste, mucus secretions such as sputum, as well as feces, etc. (3) Regurgitation behavior on foods, and (4) defecation behavior on surfaces and foods. The combination of these behaviors causes the mechanical transmission of many pathogens15. We also know from previous studies that, the housefly might suck the liquids such as sweat, or fecal that contains the SARS-CoV-2 and the insect might contain the RNA only.
Also, it has been proven that the SARS-CoV-2 could enter the host cells using cell surface components16. Some of these components have existed in the insects16. SARS-CoV-2 spike could hook up the insect body using these receptors17.
Given that Covid-19 is a new, deadly, and highly contagious disease worldwide, most countries are now more focused on preventing, controlling, and treating patients. Therefore, many different epidemiological aspects of this new virus and its transmission mechanisms are unknown to humans.
Some countries affected by the disease face with increasing temperature in the warm seasons of the year, leading to an increase in the population of houseflies and other synanthropic flies. If these insects can enter the disease transmission cycle and play a role in the mechanical transmission of the disease, it will make the disease control operation very difficult and the health system will face a very serious challenge18.
Therefore, in this study, we surveyed the existence of the SARS-CoV-2 in the field-collected houseflies by the identification molecular method for the first time in the world. Such investigations are very important because of the high abundance of houseflies and the possibility of its effect on infection transmission.
Methods
Study area
This cross‐sectional study conducted in Shiraz (29° 40′ N, 52° 33′ E), the capital city of Fars province, southern Iran. It is located at about 1500 m above sea level. It has a subtropical, hot, semi‐arid climate with a mean annual temperature of 18 °C, relative humidity of 41%, and precipitation of 337.8 mm. Its hilly landscape is corrugated with the Zagros Mountain range, which runs northwest-southeast across the country. Two main hospitals (Ali-Asghar hospital with coordinates 29.625959 N 52.541238 E and Chamran hospital with coordinates 29.662890 N 52.490799 E) were the spot point of this work which were two main referral centers of COVID-19 treatment in Shiraz (Fig. 1). Both of these referral hospitals are located in the center of the city with a clean environment and without any abnormal source of pollution, accumulation of garbage, or sewage around its location.
Collection and identification of flies
In this study, in coordination with the health department of Shiraz University of Medical Sciences, Muscid flies sampling operations were performed from outdoor environments of the two target hospitals (Ali-Asghar hospital and Chamran hospital) dealing with Covid-19 patients for 8 weeks from May to July 2020.
Sampling methods including net collection and baited traps (Sabz Avar Co. and a man-made type) were used to catch adult flies (Fig. 2)19. After collection, the flies were transferred live to the Medical Entomology Laboratory of Shiraz health school. All causations were made to prevent any secondary viral pollution during sample delivery to the laboratory or sample preparation. All collected specimens were killed by placing them in a freezer and were identified morphologically using valid systematic keys20,21,22. The samples were then transferred to the molecular laboratory under standard conditions for doing further studies to detect and isolate the SARS-CoV-2 from the collected specimens.
Molecular experiments
Samples preparation
To prepare the collected samples, 1 mL of sterile phosphate-buffered saline (PBS; 135 mmol/L NaCl, 3 mmol/L Na2HPO4·12H2O, and 13 mmol/L NaH2PO4·2H2O, pH 7.2) was added for every five mosquitoes placed into 2 mL microtubes to washout the external surface of flies. The supernatant was transferred into a new 1.5 mL collection microtubes (S1). In the next step, the external body of flies was washed by 1 mL sterile PBS followed by 70% ethanol washing. The second PBS supernatant was transferred into a new 1.5 mL collection microtubes (S2). The washed bodies were homogenized together with sterile mortar and pestle while 1 mL of PBS was added gently. The homogenate was transferred to new 1.5 mL collection microtubes and centrifuged at 5000 rpm for 5 min at 4 °C. The collected supernatant (H1), as well as S1 and S2, were stored at − 80 °C until RNA extraction.
Nucleic acid extraction
Viral RNA extraction was performed using SinaPure Viral kit (SinaClon BioScience, Iran). 200 µL of each S1, S2, and H1 specimens were subjected to extraction using a column-based kit according to the kit instruction.
Taq Man real-time PCR assay
The SARS-CoV-2 test Kit was a molecular in vitro diagnostic technique that uses Taq Man probe-based technology for the qualitative detection of SARS-CoV-2. The "COVID-19 One-Step RT-PCR" kit (COVITECH, Iran) was utilized to perform quantitative real-time PCR tests. The SARS-CoV-2 PCR assay consists of a primer/probe set that amplifies E-gene (labeled with FAM) and S-gene (labeled with ROX) and detects RNaseP (labeled with HEX) gene as an internal control.
A negative (no template) control was used to eliminate the possibility of sample contamination on the assay run. Also, a positive template control was used to verify that the assay run was performing as intended on every tested assay plate. The positive control must be positive at a Ct value of ct:35.
An internal control targeting RNase P was used to verify that nucleic acid was present in every sample and was used for every sample processed. The tests were performed using the StepOnePlus Real-Time instrument (Thermo Fischer Scientific, Applied Biosystems).
Ethical considerations
All experiments were conducted following the Declaration of Helsinki. This study was approved by the ethics committee of Shiraz University of Medical Sciences (SUMS) and is registered with ID number IR.SUMS.REC.1399.003.
Results
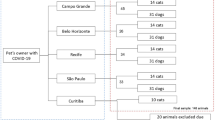
From the total of 156 adult Musca domestica L., forty specimens were considered for detection of the SARS-CoV-2 using Taq Man real-time PCR assay. External body samples, S1 and S2, and homogenate specimens for every 5 flies were utilized to assess for the presence of the virus (Table 1).
A Real-time PCR amplification plot for SARS-CoV-2 detection was obtained. From all the investigated flies, 75% of external body samples were positive in terms of contamination to the SARS-CoV-2 virus with a different load of virus (Table 1). All second-washed samples were negative for the SARS-CoV-2. Also, 37% of the homogenized specimens were positive for the new coronavirus, but with less load than body surface samples (Table 1, Fig. 3).
Discussion
Air droplets and fomites are considered as two main mechanisms for SARS-CoV-2 virus transmission. Insects can also be considered as a new tool for transmitting emerging diseases due to their body structure as well as their high abundance and activity in the environment.
Although the World Health Organization (WHO) has not yet confirmed the role of insects in the transmission of Covid-19 infection, scattered studies have been conducted in various parts of the world on the transmission of viral respiratory infections through insects. These studies have proven the biological and mechanical transmission of some viruses that cause respiratory infections in humans and animals by files4,5,6,7,8,9,10,11,12,13,14.
In 2000, the laboratory transmission of turkey Coronavirus was evaluated by a species of beetle called Alphitobius diaperinus (Coleoptera: Tenebrionidae). The results showed that turkey coronavirus can be transmitted mechanically, in a limited way, by this insect13.
Another study was performed in 2005 to evaluate the possibility of biological and mechanical transmission of the Reticuloendotheliosis virus by two insects (Culex pipiens mosquito and Musca domestica) in vitro and in the field. The results showed that the mosquitoes that were fed the virus had the virus in their bodies for up to 5 h. Houseflies, on the other hand, survived the virus for up to 72 h. Regarding the samples collected from the field, all samples were negative in terms of virus infection. In general, this study also introduced houseflies as a possible mechanical vector of the virus23.
Another study was conducted in 2002 on the housefly (Musca domestica L.) and the possibility of transmission of the turkey coronavirus (NC95 strain) strain by this insect. In this study, the larvae of flies were fed with virus-infected food and after their adult release in a turkey breeding environment, it was found that the disease rate was significantly associated with an increase in flies. In this study, flies have been suggested as a potential mechanical transmitter5.
In 2010, researchers examined the possible role of houseflies in transmitting Newcastle disease (Paramyxoviridae) in vitro and in the field. In this study, 2000 flies were collected from the field and evaluated for viruses. In the immature laboratory, flies were also exposed to the La Sota strain virus, which showed that the virus could be detected in the gastrointestinal tract of flies for up to 72 h. Although the virus was not isolated in the field samples, flies were suggested as a possible mechanical vector for this virus24.
Also, the persistence of Avulavirus in two fly species of Musca domestica and Fannia canicularis was evaluated. Both species had the infective virus in their bodies for up to one day6. Similar studies were conducted in 2007 by researchers in other countries on the transmission of the Newcastle virus by houseflies14.
In a 2013 study in Thailand, the possibility of in vitro transmission of the avian influenza virus (HPAI) subtype H5N1 virus was evaluated. In this study, 1500 adult flies were used in three different groups. Group one had no contact with the virus at all, but groups 2 and 3 were exposed to the virus with food for 15 min. Group 2 was immediately killed, homogenized, and given to sensitive birds. Group 3 was homogenized after 24 h. The results showed that all chickens in groups 2 and 3 died, whereas the load of the virus was more in group 2. No mortality was observed in the control group. This study identified houseflies as potential mechanical vectors of this viral disease among birds12. Another article in Japan identifies blowflies as a possible and mechanical vector of H5N1 bird flu11.
In one of the most recent studies conducted in Iran in 2020, the in vitro acquisition and maintenance of the avian influenza virus (AIV) H9N2 by houseflies was examined. In this study, which was conducted at the laboratory level, adult flies were fed by the virus and the results showed that the virus can survive up to 24 h on the body surface and up to 96 h in internal organs. This study identifies houseflies as a possible and mechanical vector of avian influenza virus10.
Recently, the potential of house flies to mechanically transmit SARS-CoV-2 was investigated by Balaraman et al. The results showed that, under laboratory conditions, house flies acquired and harbored SARS-CoV-2 for up to 24 h post-exposure. They mentioned that house flies were able to mechanically transmit SARS-CoV-2 genomic RNA to the surrounding environment up to 24 h25.
The role of other blood-sucking insects such as mosquitoes in transmitting the COVID-19 has also been discussed9. Some articles have also suggested that flies may be involved in the mechanical transmission of the Covid-19 virus4,8.
Based on the WHO report, there is no certainty about the survival duration of the SARS-CoV-2 on surfaces, but this virus seems to act like other coronaviruses. Previous studies show that coronaviruses, including the SARS-CoV-2, can survive on surfaces for 2 h to 9 days. This may vary under different conditions (e.g., type of surfaces, temperature, relative humidity, and characteristics of the virus strain)8,26,27.
Therefore, due to the relatively high persistence of the virus, there is a possibility of flies coming into contact with the virus and up taking them through contact and feeding in high-risk environments such as hospitals and their waste disposal sites. In this case, due to the high locomotor activity of these insects and also their synanthropic behavior, the possibility of mechanical transmission of the SARS-CoV-2 will not be far from the mind.
It has been proven that fomites (infected surfaces) play an important role in the spread of viruses, including SARS-CoV-22. Based on the results of this study, the possibility of transmission of the SARS-CoV-2 by houseflies through the fomites is quite probable.
The possibility of binding of SARS-CoV-2 to the insect epithelium surface molecules should be significantly considered. SARS-CoV-2 has a superior binding capacity to the surface sialic acids with the spike protein flat N-terminal domain. Therefore, the virus spike protein is capable of faster interaction with the surface glycans which is likely to be the core mode of action of its faster infection with the lower amount of virion, leading to the pandemic grade contagious capacity16,28,29.
Similar entry receptors are available in the insects' surface bodies. C-type lectin receptors were detected from the housefly epithelium surface30. Moreover, some glycoproteins have been identified from the epithelium surface of a housefly that could be bound to SARS-CoV-2 molecules17. Therefore, the possibility of binding the virus with surface molecules of the housefly is very probable and can strengthen the theory of the mechanical transmission of the SARS-CoV-2 by these vectors.
Since fecal transmission of the virus has been confirmed in patients, fecal feeding and mechanical transmission can play an important role in disease transmission31. If the environment is not properly managed, SARS-CoV-2 can be transmitted by mechanical transmission by insects32.
In our study, the SARS-CoV-2 was isolated from a high percentage of field-collected flies (75%). Surprisingly, the rate of infection of the external surface of the flies with the virus was high in both surveyed hospitals. This high rate of contamination indicates that these insects with high abundance and mobility are most likely involved in the mechanical transmission of this mysterious virus in crowded and high-risk places such as hospitals and pose a serious threat to human health and preventive measures should be taken to control such insects and their mobility pathways between infection intensive care units and normal parts of a hospital as an example.
In addition, detecting the virus in one-third of homogenized specimens could warn us that flies may be able to support the virus in their bodies and transmit it biologically to humans. However, expressing a detailed opinion on this issue requires additional and more accurate research in the future based on Koch's postulates for the infection of the insects and animal model for the transmission33.
Another interesting and warning point obtained in this study was that, in total, the virus load was higher in the samples taken from Ali-Asghar Hospital than in the samples collected from Chamran Hospital. This is because Ali-Asghar Hospital is the main referral center for Covid-19 patients in the province and the number of patients referred to this medical center is much higher.
These results can also confirm that the more people with symptoms as well as asymptomatic carriers are present in the community, the greater load of the virus will be present in the environment. As a result, some active and synanthropic insects such as house flies can play an effective role in the transmission of this disease in the community by infecting the external surface of their body as well as their internal organs.
Perhaps one of the reasons for the transmission of the disease in the warm months of the year can be attributed to the role of these medically important insects in the epidemiological cycle of the disease. Therefore, it is required to consider the role of insects in disease transmission, especially in overcrowded areas with poor health conditions and also high-risk areas (such as hospitals and health care centers).
Conclusion
Mechanical or biological transmission of the virus by flies can greatly complicate the epidemiology of the diseases34. In this case, the role of vector control along with other methods of prevention and control of the disease will be very important. Health policymakers should plan and implement an integrated vector management (IVM) program in high-risk environments during the warm seasons of the year in temperate regions as well as for all months of the year in tropical regions when the population and activity of these vectors increase18. Further in vivo studies on the laboratory and field are needed to examine the exact role of these insects in the transmission of this deadly universal virus.
Data availability
The data that support the findings of this study are available from the corresponding author, [AS], upon reasonable request.
References
Bogoch, I. I. et al. Potential for global spread of a novel coronavirus from China. J. Travel Med. 27, 1–3. https://doi.org/10.1093/jtm/taaa011 (2020).
Castaño, N. et al. Fomite transmission, physicochemical origin of virus-surface interactions, and disinfection strategies for enveloped viruses with applications to SARS-CoV-2. ACS Omega 6, 6509–6527 (2021).
April Si, X., Talaat, M. & Xi, J. SARS COV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry. Phys. Fluids 33, 023306 (2021).
Alfredo, M., Wilfrido, C. & Rosa, B. Can house flies mechanically carry and/or transport sars-cov-2?. Int. J. Clin. Virol. https://doi.org/10.29328/journal.ijcv.1001019 (2020).
Calibeo-Hayes, D. et al. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica Linnaeaus). Avian Dis. 47, 149–153. https://doi.org/10.1637/0005-2086(2003)047[0149:mtotcb]2.0.co;2 (2003).
Chakrabarti, S., King, D. J., Cardona, C. J. & Gerry, A. C. Persistence of exotic Newcastle disease virus (ENDV) in laboratory infected Musca domestica and Fannia canicularis. Avian Dis. 52, 375–379. https://doi.org/10.1637/8173-111407-reg (2008).
de Jonge, N. et al. Housefly (Musca domestica L.) associated microbiota across different life stages. Sci. Rep. 10, 7842–7842. https://doi.org/10.1038/s41598-020-64704-y (2020).
Dehghani, R. & Kassiri, H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19). Arch. Clin. Infect. Dis. https://doi.org/10.5812/archcid.102863 (2020).
Huang, Y.-J. S. et al. SARS-CoV-2 and mosquitoes: an extreme challenge. https://doi.org/10.21203/rs.3.rs-32261/v1 (Research Square, 2020).
Salamatian, I., Moshaverinia, A., Razmyar, J. & Ghaemi, M. In vitro acquisition and retention of low-pathogenic avian influenza H9N2 by Musca domestica (Diptera: Muscidae). J. Med. Entomol. 57, 563–567. https://doi.org/10.1093/jme/tjz175 (2020).
Sawabe, K. et al. Blow flies were one of the possible candidates for transmission of highly pathogenic H5N1 avian influenza virus during the 2004 outbreaks in Japan. Influenza Res. Treat. 2011, 652652–652652. https://doi.org/10.1155/2011/652652 (2011).
Wanaratana, S. et al. Experimental assessment of houseflies as vectors in avian influenza subtype H5N1 transmission in chickens. Avian Dis. 57, 266–272. https://doi.org/10.1637/10347-090412-reg.1 (2013).
Watson, D. W., Guy, J. S. & Stringham, S. M. Limited transmission of turkey coronavirus in young turkeys by adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 37, 480–483. https://doi.org/10.1603/0022-2585(2000)037[0480:ltotci]2.0.co;2 (2000).
Watson, D. W. et al. Experimental evaluation of Musca domestica (Diptera: Muscidae) as a vector of Newcastle disease virus. J. Med. Entomol. 44, 666–671. https://doi.org/10.1603/0022-2585(2007)44[666:eeomdd]2.0.co;2 (2007).
Service, M. Medical Entomology for Students 5th edn. (Cambridge University Press, 2012).
Seyran, M. et al. The structural basis of accelerated host cell entry by SARS-CoV-2. FEBS J. 20, 20 (2020).
Marchal, I., Jarvis, D. L., Cacan, R. & Verbert, A. Glycoproteins from insect cells: Sialylated or not? Biol. Chem. 382, 2. https://doi.org/10.1515/bc.2001.023 (2001).
WH Organization. Vector-Borne Diseases (WHO Regional Office for South-East Asia, 2014).
Geden, C. J., Szumlas, D. E. & Walker, T. W. Evaluation of commercial and field-expedient baited traps for house flies, Musca domestica L. (Diptera: Muscidae). J. Vector Ecol. 34, 99–103. https://doi.org/10.1111/j.1948-7134.2009.00012.x (2009).
Barnard, P. C. Order Diptera: The True Flies (Wiley, 2011).
Andersson, H. et al. Manual of Nearctic Diptera, vol. 1. 1981—Agriculture Canada Monograph No. 27, Cat. No. A54+3/27E.- 674 sid., 2026 fig., Can. doll. 48:-. (Canadian Government Publishing Centre, Supply and Services Canada, Hull, Quebec K1A OS9, Canada). Insect Syst. Evol. 14, 195. https://doi.org/10.1163/187631283x00074 (1983).
Pont, A. C., Werner, D. & Kachvoryan, E. A. A preliminary list of the Fanniidae and Muscidae (Diptera) of Armenia. Zool. Middle East 36, 73–86. https://doi.org/10.1080/09397140.2005.10638130 (2005).
Davidson, I. & Braverman, Y. Insect contribution to horizontal transmission of Reticuloendotheliosis virus. J. Med. Entomol. 42, 128–133. https://doi.org/10.1603/0022-2585(2005)042[0128:icthto]2.0.co;2 (2005).
Barin, A., Arabkhazaeli, F., Rahbari, S. & Madani, S. A. The housefly, Musca domestica, as a possible mechanical vector of Newcastle disease virus in the laboratory and field. Med. Vet. Entomol. 24, 88–90. https://doi.org/10.1111/j.1365-2915.2009.00859.x (2010).
Balaraman, V. et al. Mechanical transmission of SARS-CoV-2 by house flies. Parasit. Vectors 14, 1–9 (2021).
Kampf, G., Todt, D., Pfaender, S. & Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 104, 246–251. https://doi.org/10.1016/j.jhin.2020.01.022 (2020).
van Doremalen, N. et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567. https://doi.org/10.1056/NEJMc2004973 (2020).
Barrantes, F. J. The contribution of biophysics and structural biology to current advances in COVID-19. Annu. Rev. Biophys. 50, 493–523 (2021).
Baker, A. N. et al. The SARS-COV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. ACS Cent. Sci. 6, 2046–2052 (2020).
Zhou, J. et al. Identification and characterization of two novel C-type lectins from the larvae of housefly, Musca domestica L.. Arch. Insect Biochem. Physiol. 98, E21467 (2018).
Eslami, H. & Jalili, M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19). AMB Express 10, 92–92. https://doi.org/10.1186/s13568-020-01028-0 (2020).
Center for Disease, C & Prevention, C. Technical guidelines for COVID-19 laboratory testing. China CDC Wkly. 2, 332–336. https://doi.org/10.46234/ccdcw2020.085 (2020).
Byrd, A. L. & Segre, J. A. Adapting Koch’s postulates. Science 351, 224–226 (2016).
Kuno, G. & Chang, G.-J.J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 18, 608 (2005).
Acknowledgements
The authors are thankful for the assistance of the vice-chancellorship for research and technology at Shiraz University of Medical Sciences (SUMS). This study was an approved research project (Grant no.: 98-01-106-22012) funded by the SUMS.
Funding
This research was funded by the SUMS.
Author information
Authors and Affiliations
Contributions
A.B. and A.H.; designed, analyzed data, and co-wrote the paper. M.J., A.B., A.H., H.A., S.Y. and M.V.; performed experiments and co-wrote the paper. A.B.; supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soltani, A., Jamalidoust, M., Hosseinpour, A. et al. First molecular-based detection of SARS-CoV-2 virus in the field-collected houseflies. Sci Rep 11, 13884 (2021). https://doi.org/10.1038/s41598-021-93439-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93439-7
This article is cited by
-
COVID 19 infection clinical features in pediatric patients in Southwestern Iran: a cross-sectional, multi-center study
BMC Infectious Diseases (2023)
-
First molecular detection of SARS-CoV-2 virus in cockroaches
Biologia (2023)
-
Municipal solid waste, an overlooked route of transmission for the severe acute respiratory syndrome coronavirus 2: a review
Environmental Chemistry Letters (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.