Abstract
This article addresses the issue of estimating Pom—the probability of misclassifying the chemical status confidence of a water body status assessment. The main concerns of the authors were chemical quality elements with concentrations in water bodies which are close to or even smaller than the limit of quantification (LOQ). Their values must be set to half of this limit to calculate the mean value. This procedure leads to very low standard deviation values and unrealistic values of Pom for chemical indicators. In turn, this may lead to the false conclusion that not only is the chemical status good but also that this status assessment is perfect. Therefore, for a more reliable calculation of Pom, the authors suggested a modified calculation in which the value of half the LOQ for calculating the mean value was kept, but zero as the concentration value for the standard deviation calculation was adopted. The proposed modification has been applied to the Hierarchical Approach procedure for Pom estimation of the chemical status of Polish rivers and lakes. The crucial finding is that current chemical status assessments may be incorrect in the case of approximately 25% of river water bodies and 30% of lake water bodies categorised as good, and 20% of both types of water bodies classified as below good.
Similar content being viewed by others
Introduction
The assessment of a water body status according to the Water Framework Directive (WFD)1 is a set of procedures in which water quality monitoring data gathered for all European water bodies are processed resulting in assigning to each water body (wb) its status class. The overall wb status is determined as the poorer one from its ecological and chemical status. The assessment of chemical status can only be twofold: good or below good. Whereas ecological status assessment is mostly based on five biological quality elements, chemical status assessment was originally based on 33 chemical substances, listed in Annex X to Directive 2000/60/EC1, called priority substances as they elimination from the environment is a priority. The detailed lists of priority substances and their threshold values separating good and below good statuses in the classification of water status were established in Directive 2008/105 /EC2. Both directives were amended by Directive 2013/39/EU3, where the list of priority substances was extended to 45 substances. Appropriate Environmental Quality Standards (EQS) were introduced for all these priority substances and some of them were selected as priority hazardous substances. The introduction of EQS aims to protect the most sensitive species from direct toxicity as well as humans via secondary poisoning. A smaller group of priority hazardous substances were identified as uPBT (ubiquitous, persistent, bioaccumulative and toxic)4 to which belong mercury, brominated diphenyl ethers (pBDE), tributyltin and certain polyaromatic hydrocarbons (PAHs).
Chemical status assessment is based on at least twelve measurements as stated in the Annex V of the WFD so it requires monthly measurements of each priority substance and other pollutants. Chemical status assessment is based not only on the annual average concentrations (AA-EQS) of the substances for which EQS are established, but also on the maxima expressed as maximum allowable concentration environmental quality standards (MAC-EQS). Good chemical status is defined as one in which no concentrations of priority substances in a wb exceed the relevant EQSs. As both threshold values: AA-EQS and MAC-EQS are set by Directive 2013/39/EU3, the chemical status assessment in all EU countries is performed in the same way.
The European Environmental Agency (EEA) in a water assessment report5 concluded that 40% of surface waters are in good ecological status or potential but only 38% are in good chemical status. This is why the ambitious goal of reaching good status by the year 2015 has been shifted to 2027 in order to give additional time to water managers to launch appropriate remediation actions.
Due to the fluctuation of river flows superimposed with random dynamics of point and non-point sources of pollution, concentrations of chemical pollutants measured over a period of one year can vary considerably6,7.The calculated annual mean and maximum values of concentrations from time series, treated as indices for chemical status assessment in the classification procedure, can lead to the overestimation or underestimation of the ‘true value’ of the index, or an incidental adoption of a result equal to the ‘true value’.
The resultant value of the indicator, i.e. the mean and/or maximum value as compared to legally set thresholds, can be responsible for misclassification of a wb, and may lead to essentially undesired consequences:
-
(a)
false positive assessment (good status of a wb when its true status is below good). This can prevent implementation of corrective or remedial actions by water authorities;
-
(b)
false negative assessment (below good status of a wb when the true status is good). This can trigger a decision of implementing unnecessary, difficult and costly remedial measures.
The details concerning estimation of probability in these two cases are presented in more detail in the “Data and methods” section.
Although it is defined in the WFD in a rather vague way, a certain measure of uncertainty has become a mandatory element of the reporting process.
The problem of status misclassification has been discussed in several papers8,9,10 but mostly in the context of ecological status. The literature includes numerous papers regarding the uncertainty of biological indicators concerning various water body types (rivers, lakes, reservoirs, transitional, etc.). Quantification issues related to errors in water sampling procedures, errors in chemical and biological analyses, estimates of uncertainty of water quality indicators, and river status assessment uncertainty have been addressed in many research reports and papers discussing monitoring of lakes and rivers11,12,13,14,15,16,17,18.
All these sources of uncertainty are inextricably linked to field and laboratory measurements. Errors concerning laboratory procedures19,20, however, are considered much smaller than those related to sampling in non-homogenous environments, transport of water samples, etc.21. From the point of view of status assessment, the standard deviation of the measured values plays an important role in the assessment of the mean value against thresholds defining classes.
In the case of considerable annual dynamics of pollutant concentrations, or bias of the mean value of a water quality indicator by the spatial heterogeneity of the waterbody (wb), accurate performance of sampling and further analytical measurements may still not result in an assessment of wb chemical status representing its actual chemical status.
Chemical status as it is introduced by WFD and the problems of its assessment have not been thoroughly discussed in literature22,23. Only recently have there been reports of mixtures of chemicals24,25,26 as well as studies focusing on diagnostic models of chemical pressures on ecological status27,28. In the first 15 years after the adoption of the WFD, most efforts have focused solely on ecological status29.
Quantification of the uncertainty of the ecological, chemical, and overall status of rivers was attempted30 but with relative precision applied as the measure of uncertainty instead of probability of misclassification. Regarding the chemical status assessment, it was concluded30 that assessments were highly uncertain as a result of logistic and economic conditions of the water monitoring system where the number of measured indicators of chemical status was low due to the cost-intensity of measurements.
Many water bodies show no occurrence of substances considered indicators of chemical status, as expected by the environmental legislation of the WFD1. If such substances do occur, they are often in very low concentrations. The values of monitored concentrations are frequently lower than the limit of quantification (LOQ), which is a concentration of the substance that can reasonably be determined with an acceptable level of accuracy and precision, or even lower than the detection limit of the method which is many times lower than the limit of quantification31.
The problem of dealing with data sets of concentrations below LOQ, i.e. censored data, particularly in the context of quality of environment, has been discussed by many authors32,33,34. The recently published, method35 is based on spatial modelling of concentrations close to LOQ but it cannot be applied to data, routinely collected within water monitoring. The adoption of a method involving arbitrary assigning of a numerical value to censored values leads to biased estimates of the mean value. At least three simple alternative options are available, namely the median, trimmed mean, and Winsorized mean36. With the exception of adoption of the median value instead of the mean value, the other methods consist of symmetrical changes on both sides of the data distribution values. For example, the trimmed mean is computed from a set of observations by eliminating the smallest np values (np is the number of values which are below LOQ) and the np largest values. Both methods are advised to be used when not more that 25% of the data are censored37.
Calculation of the mean value from a set of measured monitoring data with a share of censored data is regulated by Directive 2009/90/EC31, establishing technical specifications for the analysis and monitoring of chemical status of waters and it is further discussed in guidance38. Pursuant to the Directive 2009/90/EC31, measurement results which are below the LOQ should be set to half of the value of the relevant LOQ. The application of this rule increases the mean value from the measurement data when the substance is not actually present in the wb and its concentration is equal to zero, or when its true concentration is greater than zero but still below half the LOQ. On the other hand, such an approach decreases the true mean value when the actual concentration is below the LOQ but higher than half the limit. The Directive 2009/90/EC31 also provides a definition of ‘uncertainty of measurement’ as a parameter characterising the dispersion of quantity values attributed to a measurement. Unfortunately, it offers no suggestion on how to calculate the value of this uncertainty when data are censored.
It seems that the only attempt to present some estimations of confidence in assessing chemical status is available through the European Environmental Agency (EEA) web pages4. Unfortunately, the scale adopted to report the confidence of the chemical status assessment within the 2nd River Basin Management Plan by Member States is purely qualitative with three levels only. For Poland, 56% water bodies are characterized by low confidence, 30% by high confidence and the remaining 15% are unknown. The best picture appears for the Netherlands with 89% of water bodies with high confidence, 9% of medium, and 10% of unknown uncertainty. The highest percentage of water bodies with low confidence of chemical status was reported for Lithuania and Finland. Note, however, that there is a warning, added to these results pointing out that they are affected by the methods used in Member States and that they cannot be compared directly.
As for Poland, the method applied for estimation of confidence was based on the completeness of data only39. The highest level of confidence was assigned in case of chemical status assessment based on the full set of chemical indicators (at that time 33 substances) monitored at least 12 times per year. The lowest confidence was assigned when less than 50% of substances were monitored and the number of measurement data were lower than 10 in a year. Unfortunately, the description of the methods applied both to extrapolation and to uncertainty assessments for other countries were missing. There is also no information concerning the application of those confidence results for any practical water management issues.
This paper shows how to meet the obligation introduced by WFD and Guidance document No. 740, i.e. how to estimate chemical status confidence defined as the probability that the indicator value estimated from the data points does in fact lie within some specified limits, or desired interval. The method presented in this paper of calculating probability of misclassification (Pom) for chemical status fills the gap in reporting of chemical status uncertainty estimation. Together with estimated Pom for ecological status, it permits fulfilling the obligation of reporting the overall status assessment, together with the uncertainty measure, expected in the reporting procedure by the WFD. The study also focuses on the problem of dealing with censored data.
This paper presents a general overview of the bias existing in chemical status assessment. The sampling and chemical analysis procedures applied in chemical monitoring programmes carried out under Directive 2000/60/EC are validated and documented in accordance with EN ISO/IEC-1702541 standard or other equivalent standards accepted at international level42. Therefore, the presented results can be assumed representative of chemical status assessment not only in Poland, but also in other European countries with similar laboratory facilities.
Data and methods
Water quality monitoring data
This study is based on water quality monitoring data collected in the scope of the Polish National Monitoring System during 2016–2018, in both riverine and lacustrine water bodies throughout the territory of Poland. The number of monitored water bodies in particular categories and years is presented in Table 1.
The monitoring of chemical parameters included 45 chemical status indicators in accordance with the regulation concerning the classification used in the analysed period43.
Water bodies in Poland include 26 abiotic types of river waters, most of which are lowland rivers; rivers with organic substrate; and lowland sandy clay and gravel rivers44 as well as 13 types of lakes.
Classification of chemical indicators
According to the regulation43, classifying a water body’s chemical status as good requires meeting both of the criteria tested with annual average (AA-EQS) and maximum allowable concentration (MAC-EQS) for all indicators. The exception is the group of pesticides: aldrin, dieldrin, endrin, and isodrin since the criterion for them is formulated as the threshold value for the sum of concentrations of these substances.
There are generally four cases of assigning classes to a chemical indicator:
-
(i)
indicator is classified as good status class when both criteria for good status are met;
-
(ii)
indicator is classified as below good status class when the mean concentration exceeds AA-EQS, whereas the maximum measured concentration is below the threshold;
-
(iii)
indicator is classified as below good status class when the maximum concentration exceeds MAC-EQS, whereas the mean value from measurements is below the threshold for the mean; and
-
(iv)
indicator is classified as below good status class when the annual mean and the maximum concentrations are above thresholds.
Any case of the assessment carries a possibility of misclassification, i.e. ascribing a class different than the actual class.
As in the works by Clarke and Herring8 and Kelly45, also in this paper, Pom is understood as the probability of committing a type II error, i.e. accepting the null hypothesis H0 consisting of accepting a below good class when the true class is good or vice versa.
Probability of misclassification calculation method
Due to the difficulty of determining the actual value of the index (i.e. representing the true status of a wb) and thus to which class a water body status does belong, the class indicated by the average value of measurement data for the chemical quality element, at a given measurement location within a certain period of time, is considered as the resulting status assessment. This quantity, commonly used in the monitoring of surface waters to indicate the class is, however, a random variable—the random realization of a variable of which does not necessarily indicate the class to which the estimated true value belongs.
When only the criterion based on annual average is considered, Pom can be calculated from the following formula:
where:
l(i) , u(i) are the specified lower and upper limits of the true class ‘i’ of the indicator mean value. \(g\left(\stackrel{-}{\mathrm{x}}\right)\) is the distribution function for the indicator mean value.
This study assumes that the distribution function g \(\left(\stackrel{-}{\mathrm{x}}\right)\) of the average value of the chemical element \(\stackrel{-}{\mathrm{x}}\) can be approximated by the Student's t-distribution function, where two parameters of this distribution—the expected value E(x) and the standard deviation σ(x), result directly from the statistical assumptions: E \(\left(\stackrel{-}{\mathrm{x}}\right)\) = E (x) and σ \(\left(\stackrel{-}{\mathrm{x}}\right)\) = σ(x)/√n, where n is the numerical size of a finite sample of measurements of the chemical element x. This means that, in practice, the values of the statistical parameters of the distribution g \(\left(\stackrel{-}{\mathrm{x}}\right)\) are calculated directly from the values of statistical parameters of the Student's t-distribution estimated on the basis of the measurement data of the chemical element x. Developing this method, a conscious assumption was made that the measurement bias was negligible, as biases values were simply missing from the whole data base. It was noted that this assumption may have an effect on the results, but taking into account that measurements which results were stored in the data base were performed by many people, in three different years, using different devices with different precision, it was concluded that this approach was the only reasonable one.
The Pom value for an indicator depends on the distance between the mean or maximum value and the boundary of the class, and on the standard deviation characterising the width of measurement distribution.
Due to the heterogeneity of the water environment in both horizontal and vertical directions, temporal evolution of biochemical processes, and temporal variability of pollution discharges, mean values of concentrations of dissolved substances frequently show considerable variance46,47,48.
In the presented methodology authors have not taken into account the impact of possible correlations between chemical parameters on Pom value. Correlation among chemical indicators might be significant and could be dealt with by using for example PCA as shown in49. Unfortunately in case of existing data base of monitoring results, there was a different number of measured parameters for different water bodies and all the sample sizes were relatively small. Including the possible effect of correlation between parameters, would make the task of uncertainty assessment very challenging. It is very likely that the whole procedure would complicate the calculations to the point when they cease to be applicable and thus useful for wider community consisting not only from researchers but also water managers and water administration.
The calculation of Pom, taking into consideration the criterion of maximum concentration, employed the right-hand side Gumbel distribution type I. For both Student's t-distribution and Gumbel distribution, functions from the Python package Scipy50 were applied to fit the distribution models.
In line with the definition given above, the probability of misclassification (Pom) is the probability of committing the type II error. The H0 hypothesis is defined in agreement with the result of the chemical class accessed either on the basis of the mean value or on the maximum value. In case of false positive assessment of the class, Pom means the probability of the rejection hypothesis H0 stating ‘chemical status class is below good’ while the true class is below good. On the other hand in case of false negative assessment, Pom expresses the probability of the rejection of hypothesis H0 stating ‘chemical status class is good’ while it is correct. It means that, for each indicator, Pom means the probability that the class is assessed incorrectly. A similar situation occurs in the case of the calculation of the probability of misclassification for the maximum value, i.e., either the good status class is determined when below good is the actual class, or vice versa.
The entire data processing was performed in Python 3 using the Numpy51, Scipy50, Pandas52 libraries for analysis purposes and the Matplotlib53 and Seaborn libraries54 for visualization purposes.
Hierarchical approach of chemical status assessment
The assessment of chemical status misclassification requires taking into consideration uncertainties in all chemical indicators. Following the Hierarchical Approach introduced by Loga9, but mainly for ecological status55 the Hierarchical Approach presented in this paper was tailored to estimate the uncertainty of chemical status assessment. In this study, the assessment of chemical status was based on monitoring data separately for each year. When all chemical indicators were not available (not all substances included in the chemical status assessment procedure were measured), the chemical status was assessed from the available chemical indicator values by applying One-Out-All-Out rule (OOAO) and then its uncertainty measure with the aid of the Hierarchical Approach.
The general idea of the Hierarchical Approach is to adopt a procedure similar to that in the OOAO rule, where the indicator classified in the worst class is the decisive indicator as it determines the result of classification. Unlike for ecological status assessment where there are five classes, for chemical status there are no intermediate classes between good and below good so in case of just one chemical indicator classified in the below good class the chemical status of water body is downgraded to the below good. Taking the same approach for the chemical status uncertainty assessment, the probability of misclassification of the indicator decisive for the chemical status is assumed to be the probability of misclassification of this status.
Because chemical status assessment is based on two criteria, namely, annual mean and annual maximum value, errors are possible for both criteria independently. Therefore, two Pom values are assessed for each chemical indicator.
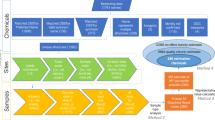
The modified version of the Hierarchical Approach for assessing misclassification of chemical status is presented in Fig. 1. It has to be noted that there are differences in following the hierarchical process for water bodies classified in good and below good chemical status.
Hierarchical Approach for assessing Probability of Misclassification for the chemical status for a water body. Level 1 consists of classified chemical quality indicators (good or below good status class); Level 2 consists of Poms for the class of each indicator; there are separate Poms for the mean and max criterion; Level 3 represents eventual Pom values for all chemical indicators; and Level 4 is the final Pom for chemical status.
When water bodies are classified as good chemical status, the lowest level of hierarchy (Level 1) depicted in Fig. 1 represents all indicators classified as good chemical class, as both criteria of good chemical status are fulfilled. For every indicator on the second level (Level 2), both probabilities are calculated: for the mean value criterion Pom mean and maximum value criterion Pom max. On the third level (Level 3) of hierarchy, Pom values for each indicator are assigned by choosing the larger of the two values calculated on the lower level. On the highest level of hierarchy—Level 4, the largest value of Pom for indicators is chosen as the final Pom of the chemical status for a given water body.
In the case of a water body assessed as below good chemical status, at least one of its indicators of chemical status was ascribed to the class below good. In such a case, only indicators ascribed to this class are taken into account on the first level of the hierarchy (Level 1), because indicators assessed as good are not decisive for the status class.
The second level (Level 2) consists of Pom values for mean and maximum values of indicators classified in below good class.
The third level of hierarchy (Level 3) is the most complex one and it depends on the reason why indicators were classified to the below good class (one of three cases listed earlier (ii)–(iv) in “Classification of chemical indicators” in “Data and methods” section).
In case (ii) and (iii) when an indicator is classified in the below good status class i.e. when the mean concentration exceeds AA-EQS or maximum concentration exceeds MAC-EQS, the Pom for this indicator takes the value of Pom estimated for the criterion decisive for the below good status (mean ii) or maximum iii)).
The fourth possibility (iv) applies when both annual mean and maximum values are higher than the corresponding AA -EQS and MAC-EQS. Then, the probability of inaccurate class assessment involves committing two errors simultaneously, i.e. inaccurate classification of both the annual mean and the maximum. Therefore, the Pom for this chemical indicator is calculated as a product of the two corresponding Poms.
The third level of hierarchy (Level 3) consist of Pom values for all chemical indicators used for chemical status assessment.
At the Level 4 of the Hierarchical Approach the highest value among Level 3 Pom’s is adopted as the Pom for chemical status.
The description above corresponds to the uncertainty assessment for classification of every substance included in the chemical status assessment procedure.
Modification of standard deviation calculation
Because chemical status is based on substances which either are not present in water bodies or occur at very low concentrations, the measured values are frequently very close to the LOQ or even the detection limits.
When all the measured concentrations are smaller than the LOQ, and are assigned half of this limit (EC, 2009/90/EC), the standard deviation from the set of identical values is equal to zero. From the statistical point of view, the zero value of standard deviation suggests the most efficient estimator of the expected value. It gives a false impression that the class assessed based on this indicator value (the mean value of the measured concentration) was assessed with high precision. The more measurement results are below the limit of quantification, the smaller the value of the standard deviation becomes, resulting in a decrease in uncertainty whereas it is not true .The true value of the measurement reported as ‘below limit of quantification’ can be any value from zero (including zero) to the value of the LOQ. Therefore, the conventional standard deviation formulae lead to wrong conclusions when applied to a series of measurements below the LOQ.
To find a way out of this erroneous approach which distorts the estimation of confidence in the wb status, particularly when the good chemical status was adopted with relatively high values of detection limits in laboratory chemical procedures, it is useful to introduce a modified approach for calculating the standard deviation. When using the definition formula for calculating the standard deviation whenever the real measurement (xi) value is below the limit of quantification, it is beneficial to set the value to zero (instead of half of the LOQ) but keep the value of half the LOQ for calculating the mean value. Therefore a modified standard deviation proposed in this approach can be calculated from the following formulas:
and
Taking the value zero instead of half the limit of quantification results in a bigger standard deviation value when measurement results are below the LOQ. In fact, the same result could have been reached using the LOQ value instead of zero as, in both cases, the distance from LOQ/2 was maximized.
Results
The study results are divided into three subchapters in order to emphasise three evident outcomes: the results of introducing a modified way of taking into account censored data in the assessment of uncertainty; the results of the uncertainty analysis of particular chemical indicators, i.e. priority substances; and the results of the uncertainty of chemical status based on the example of water bodies of Polish rivers and lakes.
Comparison of uncertainty for the conventional and modified approach to taking into account censored data for calculation of standard deviation
The differences of application to the assessment of Pom of the standard deviation calculated for below the LOQ concentrations with the conventional formula, versus the modified approach are shown in Table 2.
These differences are relevant for Level 2 of the Hierarchical Approach (Fig. 1). Three examples of data are provided in the upper part of Table 2, i.e. censored concentrations of three chemical substances in different lacustrine water bodies and data transformed for the purpose of calculation of the mean value. Standard deviations and probabilities of the class (P), together with probabilities of misclassification (Pom) calculated in line with the conventional (Excel) formulas are contrasted with the P and Pom resulting from the modified formula for st. dev for censored data.
A detailed calculation of the modified standard deviation for benzo(a)pyrene, as in the Table 2—example (a) is as follows:
In the example (a) of Table 2, only two data points of benzo(a)pyrene are greater than the LOQ. The good class is assigned to this indicator because neither the AA-EQS nor MAC-EQS value was exceeded. The Pom of the good class for benzo(a)pyrene when calculated by means of the conventional method is a high value, i.e. 0.80 but with the standard deviation calculated in the modified way it is slightly higher. For fluoranthene, the corresponding Pom values are generally lower than in case (a), but higher than those based on the conventional st. dev. formula, and still almost reaching the value of 0.5.
A comparison of Pom values for endosulfan is hardly possible because the zero value of standard deviation does not permit reporting any uncertainty related to the classification for this indicator. This is clearly not acceptable as there is always uncertainty in class assessment for any indicator.
The uncertainty of chemical status based on Pom as a measure of this uncertainty was assessed for all the analysed riverine and lacustrine wbs. For better presentation of the results, the range of Pom values was divided arbitrarily into four intervals, namely < 0, 0.1 > , (0.1, 0.3 > , (0.3, 0.5 > , and (0.5, 1 > .
The comparison of Poms for chemical indicators, i.e. the results from the third level of hierarchy (Fig. 1) assessed based on the conventional formula for standard deviation with Pom values based on the modified formula, shows no differences for indicators classified in the below good class However, there are significant differences in percentages in the Pom intervals, between results reached with conventional and modified formulas, for indicators assessed in the good class. These differences in distribution among four intervals of Poms are presented in Fig. 2 based on the example of wbs in 2017, representative both for river and lake waterbodies over three years.
Comparison of Pom values calculated with the conventional (stacked bar plot on the left) and modified (stacked bar plot on the right) st. dev in the case of measurements below the LOQ. The comparison is based on all chemical indicators in riverine water bodies classified in the good class in 2017. The range of Pom values is divided into four intervals: < 0, 0.1 > , (0.1, 0.3 > , (0.3, 0.5 > , and (0.5, 1 > . For each interval, the percentage of indicators with Pom belonging to this interval is presented.
The bar plots in Fig. 2 evidently show that the greatest difference in abundance occurs between the percentage of indicators belonging to the first two intervals of Pom s, i.e. < 0, 0.1 > and (0.1, 0.3 > . The interval characterised by the lowest Pom decreased two and a half times when the modified st. dev was applied to Pom estimation, whereas the group with Pom values within a range of (0.1, 0.3 > increased in almost the same proportion. The percentage of the fourth interval, i.e. the highest Pom, was unaffected by the change in the formula of standard deviation. It should be emphasised that the modified way of calculating standard deviation is only important for estimating Pom for chemical indicators characterised by very low concentrations. On the other hand, it permits improved performing of the complete assessment of chemical status uncertainty.
Probability of misclassification for chemical indicators for Polish riverine and lacustrine water bodies
The modified formula for calculating the standard deviation for censored data and Pom assessment was applied to all chemical indicators and to all riverine and lacustrine water bodies. The results of this assessment, corresponding to Level 3 of the hierarchy (Fig. 1), are presented in Fig. 3.
Probability of misclassification (Pom ) of all chemical indicators (Level 3) for riverine and lacustrine water bodies in the years 2016–2018. The range of Pom values is divided into four intervals: < 0, 0.1 > , (0.1, 0.3 > , (0.3, 0.5 > , and (0.5, 1 > . For each interval, the percentage of indicators with Pom belonging to this interval is presented.
The monitored riverine water bodies are several times as numerous as the lacustrine ones (Table 1). Nonetheless, a striking similarity is observed in the percentage of chemical indicators belonging to each of the four intervals, defined by the range of the probability of misclassification presented in Fig. 3. The group characterised by Pom greater than 0.5 contains no more than 3.5% of indicators. The interval characterised by the smallest values of Pom consist of 20–25% of the indicators of chemical status. The greatest number of indicators is characterised by Pom within a range of (0.3,0.5 >.
Probability of misclassification of chemical status
Results of the probability of misclassification of the chemical status of water bodies, i.e. Pom values corresponding to Level 4 of the hierarchy, are presented in Table 3. The results for all years are lumped together but presented separately for good and below good status. The two main columns on the left of Table 3 display values of Pom resulting from conventional and modified approach to st. dev. for censored data. Chemical substances, on the basis of which below good status was assessed, occurred in waters in concentrations much higher than LOQ. This is the reason that assessment of Pom values for wbs in below good chemical status was not affected by the method of calculation of st. dev. and that is why there are single columns for both types of wbs in below good status in Table 3. Similarly to the way of presenting Pom for indicators, Pom values for chemical status were discretized into four intervals depending on the Pom value.
There are differences in percentage in the first three intervals in the columns for good chemical status but the values for the fourth interval i.e. (0.5, 1 > are unchanged. For both types of wbs, this comparison of intervals for Pom shows a clear decrease in the percentage within the lowest value of Pom and an increased percentage of Pom values belonging to the (0.3, 0.5 > interval. Changes within the (0.1, 0.3 > interval are rather insignificant. The overall results of uncertainty of good chemical status for lakes and rivers when the modified st. dev. was applied are more pessimistic than that of the conventional approach, as only around 12% of wbs have a certain good status. Intervals of Pom values for chemical status smaller than 0.1 are considerably more numerous for good status than for below good.
From the above table (Table 3) it can be concluded that, for about 40% of riverine and lacustrine water bodies, their good chemical status was assessed with a probability of misclassification below 0.3. The most difficult to accept, especially for water managers, is that an incorrect assessment of status has been made in approximately 25–30% of lake and river water bodies previously assigned the good chemical status and about 20% of those with below good status. According to the authors, this is the interpretation of the probability of misclassification higher than 0.5.
Discussion
There are many publications and reports criticizing the whole concept of chemical status assessments as introduced by WFD1 and, through this failure, the attempts of status remediation however apart from the draft report EEA5 and web EEA pages4, the issue of the uncertainty of the assessed chemical status does not appear in any document relating to water management.
There was an earlier attempt to apply the hierarchical approach to the chemical status of water bodies in three voivodships in Poland55, but due to the scarcity of measurement data of many priority substances and thus not representative character of assessments, the results were mostly focus on ecological status. The additional constraint encountered was the problem with dealing with left-side censored values of chemicals concentration. It was difficult to accept zero value of Pom as shown on the example of endosulfane in Table 2.
When assessing chemical status of a water body as below good, it is not reported to the European Commission how many and which substances for wbs categorized below good violated the threshold values, nor how big the discrepancies were56.The same authors suggest that the chemical status assessment is also ill-defined due to excluding many substances from the list obligatory for chemical monitoring. They suggest that if the list of chemicals is enlarged, there would be no water body in the EU territory in good chemical status. However, now with 45 priority substances, as many as 62% of wb have been classified as failing to reach good chemical status5. There was a First Watch List for pollutants including emerging ones initiated in 201557 for which the available monitoring information was considered as insufficient.
It has been concluded that EQS established as set by Directive 2008/105/EC2 aims to protect the most sensitive species from direct toxicity and humans via secondary poisoning5 but are not significantly protective against mixture effects. Moreover, there is an urgent need to revise the tools used to assess the safety of chemical pollution24,58.
The most important problems, together with recommendations aiming at reaching effective methods of assessments and management concerning chemical pollutants, were presented by Brack59. These are the conclusions of the NORMAN network60 and FP7 Collaborative Project SOLUTION61. There are altogether ten main problems grouped around three basic recommendations as “improve monitoring, and strengthen comprehensive prioritization, foster consistent assessment and support solution-oriented management”. The issue of uncertainty of chemical status assessment presented in this paper is strictly connected with the first recommendation put forward by Brack59 i.e. modification and improvement of the monitoring system. The results of measurements below LOQ of substances such as tributyltin, benzopyrene, benzofluoranthene or endosulfan as well as many others, caused by their hydrophobic nature resulting in very low solubility in water, can be overcome by introduction of passive sampling methods and biota monitoring to monitoring programmes instead of their measurements in water42,62.Since a high uncertainty of biological indices may be found for measurements performed just once in the six-year River Basin Management Plan, it can be expected that measurement values of substances accumulated in the biomass, if performed also only once per the six-year period, similarly will result in high uncertainty in the assessment of the chemical status. However, before these new methods will be operational, the currently run monitoring gives results which are highly uncertain. It is also important to notice that, for this passive and biota monitoring, there is also a necessity to prepare an uncertainty assessment method. The Hierarchical Approach presented here seems feasible for this purpose. This kind of approach has been already put into practice in assessing water status uncertainty for ecological, chemical and overall status of water bodies in Poland by water administration.
Concerning changes which are required in future water quality monitoring in order to upgrade protection from chemical mixtures risks and improve management of water bodies that are at greatest risk, several sound recommendations are formulated as results of European Collaborative Project SOLUTIONS63,64. These are inclusion of mixture effects of substances below experimental no observed adverse levels, extend the impact assessment of multiple receptors, replace traditional surveys, by effect-based monitoring and assessment methods. Definitely these recommendations cannot be introduced at short notice and without changes in legislation. Therefore, it is necessary to amend the WFD and build feedback links between the WFD and other EU legislation on industrial chemicals, pesticides, biocides and pharmaceuticals63.
As for the needs expressed occasionally by water managers concerning monitoring, these are typically requests for cost-efficient methods of monitoring future threats to water quality38,59, not demands for narrower uncertainty intervals for water status. It seems that the more appropriate approach to improve existing monitoring programs would be to ask water managers about the satisfactory confidence from their point of view and, according to the accepted width of this uncertainty interval, the necessary frequency of sampling and methods of monitoring can be prepared.
Conclusions
Adopting the value of half the LOQ in the case of left-side censored concentrations of substances can increase the mean value in comparison to the case where data points below LOQ are set as zero, when the true concentration is higher than half the LOQ but still below it. It can decrease the mean value in the opposite situation. When chemical quality elements are classified in the good class, more measurement results below the limit of quantification show a smaller value of the standard deviation. It is in fact the reverse, because the true measurement value can be any value between zero (including zero) and the limit of quantification. The proposed method of calculating the standard deviation in the case of censored data improves the results of the estimation of uncertainty for chemical indicators. It also permits reliable and complete assessment of chemical status uncertainty.
The Hierarchical Approach to the assessment of probability of misclassification of chemical status applied in this study fills the gap in reporting the chemical status together with its uncertainty measure.
Less than half of all water bodies in Poland monitored in the period 2016–2018 assessed in good chemical status are characterised by relatively low (lower than 0.3) probability of misclassification. Therefore, their good chemical status is rather certain and no remedial measures should be undertaken within water management plans for these water bodies. The most crucial finding is that current chemical status assessments may be incorrect in the case of approximately 25% of river water bodies and 30% of lake water bodies categorised as good, and 20% of both types of water bodies classified as below good.
It is very likely that in countries such as Poland, where the obligation to test an increasing number of substances included in the EQS list and to extend monitoring onto biota if operational, will lead to attempts to economize on the monitoring programs, which is already the case for the ecological status. A principle of inheritance of the assessment from previous years currently operates for those indicators whose measurements are not performed in the current monitoring cycle. This means that the assessments of chemical indicators and their uncertainties obtained from the previous monitoring periods can be used for some time. That is why the uncertainty issues of chemical status presented in this paper should be known to water managers, which it is not the case at present.
Unfortunately, the question whether the presented range of status uncertainty is satisfactory to water managers remains open. Due to the high cost of measurements of priority substances and other hazardous substances, another question arises: Is there any possibility to decrease the sampling frequency, inevitably leading to an increase in uncertainty?
References
Directive 2000/60/EC (2000) of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (2000).
Directive 2008/105/EC (2008) of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council (2008).
Directive 2013/39/EU (2013) of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy (2013).
Kristensen, P., Whalley, C., & Klančnik, K. European Waters: Assessment of Status and Pressures 2018. (European Environment Agency, 2018).
Chin, D. A. Water-Quality Engineering in Natural Systems: Fate and Transport Processes in the Water Environment (Wiley, 2012).
CIS. Best Practice for Establishing Nutrient Concentrations to Support Good Ecological Status. Guidance Document WG ECOSTAT (2018).
Clarke, R. T. & Herring, D. Errors and uncertainty in bioassessment methods-major results and conclusions from STAR project and their application using STARBUGS. Hydrobiologia 566, 433–439 (2006).
Loga, M. Hierarchical Approach to Water Body Status Misclassification 97–109 (WIT Press, 2012).
Loga, M. & Wierzchołowska-Dziedzic, A. Probability of misclassifying biological elements in surface waters. Environ. Monitor. Assess. 12 (2017).
Clarke, R. &Jones, I. Deliverable D6.1–1, WISER FP7 Project, Report on a Workshop to Bring Together Experts Experienced with Development and Uncertainy Estimation (2009).
Naddeo, V., Scannapieco, D., Zarra, T. & Belgiorno, V. River water quality assessment: Implementation of non-parametric tests for sampling frequency optimization. Land Use Policy 30(1), 197–205 (2013).
Moe, S. J. et al. Integrated assessment of ecological status and misclassification of lakes: The role of uncertainty and index combination rules. Ecol. Ind. 48, 605–615 (2015).
Kotamäki, N., Pätynen, A., Taskinen, A., Huttula, T. & Malve, O. Statistical dimensioning of nutrient loading reduction: LLR assessment tool for lake managers. Environ. Manag. 56(2), 480–491 (2015).
Carstensen, J. & Lindegarth, M. Confidence in ecological indicators: A framework for quantifying uncertainty components from monitoring data. Ecol. Ind. 67, 306–317 (2016).
Guigues, N. et al. Estimating sampling and analysis uncertainties to assess the fitness for purpose of a water quality monitoring network. Accred. Qual. Assur. 21(2), 101–112 (2016).
Wach, M. et al. Probability of misclassifying river ecological status: A large-scale approach to assign uncertainty in macrophyte and diatom-based biomonitoring. Ecol. Ind. 101, 285–295 (2019).
Borges, C., Palma, C. & da Silva, R. B. Optimization of river sampling: Application to nutrients distribution in Tagus river estuary. Anal. Chem. 91(9), 5698–5705 (2019).
Eurachem/CITAC (2012). Quantifying Uncertainty in Analytical Measurement. s.l.:CITAC. https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf (2020).
Eurachem/CITAC (2019). Measurement Uncertainty Arising from Sampling A Guide to Methods and Approaches. https://www.eurachem.org/images/stories/Guides/pdf/UfS_2019_EN_P2.pdf (2020).
Ingersoll, W.S. Environmental Analytical Measurement Uncertainty Estimation: Nested Hierarchical Approach Naval Sea Systems Command Washington DC (2001).
von der Ohe, P. C. et al. Toward an integrated assessment of the ecological and chemical status of European river basins. Integr. Environ. Assess. Manag. 5(1), 50–61 (2009).
Tueros, I. et al. Integrating long-term water and sediment pollution data, in assessing chemical status within the European Water Framework Directive. Mar. Pollut. Bull. 58(9), 1389–1400 (2009).
Posthuma, L., de Zwart, D. & Dyer, S. D. Chemical mixtures affect freshwater species assemblages: From problems to solutions. Curr. Opin. Environ. Sci. Health 11, 78–89 (2019).
Drakvik, E., Altenburger, R., Aoki, Y., Backhaus, T., Bahadori, T., Barouki, R., & van Klaveren, J. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 134, 105267 (2020).
Escher, B. I., Stapleton, H. M. & Schymanski, E. L. Tracking complex mixtures of chemicals in our changing environment. Science 367(6476), 388–392 (2020).
van Gils, J. et al. The European Collaborative Project SOLUTIONS developed models to provide diagnostic and prognostic capacity and fill data gaps for chemicals of emerging concern. Environ. Sci. Eur. 31(1), 72 (2019).
Van Gils, J., Posthuma, L., Cousins, I. T., Brack, W., Altenburger, R., Baveco, H. & Lindim, C. Computational material flow analysis for thousands of chemicals of emerging concern in European waters. J. Hazard. Mater. 122655 (2020).
SWD. Commission Staff Working Document. Fitness check of the Water Framework Directive, Groundwater Directive, Environmental Quality Standards Directive and the Floods Directive (2019).
Loga, M. Estimation of certainty and presision—The basic measures of uncertainty of surface water status [in Polish]. Ochrona Środowiska 38, 15–23 (2016).
Directive 2009/90/EC (2009) Laying Down, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, Technical Specifications for Chemical Analysis and Monitoring of Water Status (2009).
Sharaf, M. A., Illman, D. L., & Kowalski, B. R. Chemometrics Vol. 117. (Wiley, 1986).
Olivieri, A. C. et al. Uncertainty estimation and figures of merit for multivariate calibration (IUPAC technical report). Pure Appl. Chem. 78(3), 633–661 (2006).
Antweiler, R. C. & Taylor, H. E. Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environ. Sci. Technol. 42(10), 3732–3738 (2008).
Borges, C., Palma, C., Dadamos, T., & da Silva, R. J. B. Evaluation of seawater composition in a vast area from the Monte Carlo simulation of georeferenced information in a Bayesian framework. Chemosphere 263, 128036 (2021).
Berthouex, P. M. & Brown, L. C. Statistics for Environmental Engineers (Lewis Publishers, 1994).
Hoaglin, D. C., Moesteller, F. & Tukey, W. J. Understanding Robust and Exploratory Data Analysis (Wiley, 1983).
CIS. Guidance Document No. 19. On Surface Water Chemical Monitoring Under the Water Framework Directive, s.l. (Common Implementation Strategy, 2009).
GIOŚ. Internal Document on Polish State Monitoring System, Chief Inspectorate of Environment Protection (2012).
CIS. Guidance Document No. 7 Monitoring under the Water Framework Directive, Luxembourg: Office for Official Publications of the European Communities (2003).
ISO/IEC 17025. General Requirements for the Competence of Testing and Calibration Laboratories.
CIS. Guidance Document No. 25. On Chemical Monitoring of Sediment and Biota Under the Water Framework Directive (2010).
Laws, J. Item. 2149. The Act on the Classification of Ecological Status, Ecological Potential and Chemical Status as Well as the Method of Classifying the Status of Surface Water Bodies (in Polish) (2019).
Laws, J. No. 122, Item 1018. The Act on the Classification of Ecological Status, Ecological Potential and Chemical Status (In Polish) (2009).
Kelly, M. et al. Uncertainty in ecological status assessments of lakes and rivers using diatoms. Hydrobiologia 633, 5–15 (2009).
Hildrew, A. G. Whole river ecology: spatial scale and heterogeneity in the ecology of running waters. Large Rivers 10(1–4), 25–43 (1996).
Yoshida, S., Ohtani, M., Nishida, S., & Linden, P. F. Mixing processes in a highly stratified river. Coastal Estuarine Stud. 389–400 (1998).
Zohary, T. & Ostrovsky, I. Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Waters 1(1), 47–59 (2011).
Kuselman, I., Pennecchi, F. R., da Silva, R. J. & Hibbert, D. B. Risk of false decision on conformity of a multicomponent material when test results of the components’ content are correlated. Talanta 174, 789–796 (2017).
https://www.scipy.org/ (2019)
NumPy Developers. NumPy [WWW Document]. NumPy. https://numpy.org/index.html. Accessed 27 Apr 2020 (2005).
https://pandas.pydata.org/ (2019).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95. https://doi.org/10.1109/MCSE.2007.55 (2007).
Waskom, M. 2012. Seaborn: Statistical data visualization. https://seaborn.pydata.org/ (2020).
Loga, M., Wierzchołowska-Dziedzic, A. & Martyszunis, A. The problem of water body status misclassification—A hierarchical approach. Environ. Monitor. Assess. (2018).
Posthuma, L. et al. Chemical pollution imposes limitations to the ecological status of European surface waters. Sci. Rep. 10(1), 1–12 (2020).
https://www.ec.europa.eu/jrc/en/news/first-watch-list-emerging-water-pollutants (2020).
Carvalho, R. N. et al. Mixtures of chemical pollutants at European legislation safety concentrations: How safe are they?. Toxicol. Sci. 141(1), 218–233 (2014).
Brack, W. et al. Towards the review of the European Union Water Framework management of chemical contamination in European surface water resources. Sci. Total Environ. 576, 720–737 (2017).
Brack, W. et al. The SOLUTIONS project: challenges and responses for present and future emerging pollutants in land and water resources management. Sci. Total Environ. 503, 22–31 (2015).
CIS. Guidance Document No. 32. On Biota Monitoring (the Implementation of EQS Biota) Under the Water Framework Directive (2014).
Kortenkamp, A. et al. Mixture risks threaten water quality: The European Collaborative Project SOLUTIONS recommends changes to the WFD and better coordination across all pieces of European chemicals legislation to improve protection from exposure of the aquatic environment to multiple pollutants. Environ. Sci. Eur. 31(1), 1–4 (2019).
Altenburger, R. et al. Future water quality monitoring: Improving the balance between exposure and toxicity assessments of real-world pollutant mixtures. Environ. Sci. Eur. 31(1), 1–17 (2019).
Author information
Authors and Affiliations
Contributions
M.L. wrote the manuscript and prepared tables. K.P. performed all computations and prepared figures. Both authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loga, M., Przeździecki, K. Uncertainty of chemical status in surface waters. Sci Rep 11, 13644 (2021). https://doi.org/10.1038/s41598-021-93051-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93051-9
This article is cited by
-
Bayesian decision tables for estimation of risk of water management decisions based on uncertain surface water status: a case study of a Polish catchment
Environmental Sciences Europe (2022)
-
Seasonal variation in metal concentration in various tissues of the European chub (Squalius cephalus L.)
Environmental Science and Pollution Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.