Abstract
The seven pyridine alkaloids 1–7, the flavonoid acacetin (8), and L-proline anhydride (9) have been isolated from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. The structures of the natural products 1–9 have been assigned by MS, as well as IR, 1D NMR (1H, 13C, DEPT), and 2D NMR (COSY, HSQC, HMBC, NOESY) spectroscopic methods. The compounds 2 and 4–7 represent new chemical structures. Acacetin (8) and L-proline anhydride (9) have been obtained from C. mongolica for the first time.
Similar content being viewed by others
Introduction
Caryopteris mongolica Bunge is a deciduous shrub and belongs to the Verbenaceae family. C. mongolica which is widely distributed in the mountainous and Gobi regions of Mongolia (Khentei, Khangai, Mongol-Daurian, Middle Khalkha, Mongolian Altai, East Mongolia Valley of Lakes, Govi-Altai, East Govi, Trans-Altai Gobi, Gobi and Alashan Gobi)1. In fact only this species of Caryopteris is growing in Mongolia, whereas about 16 species of this genus occur all over the world. In traditional Mongolian medicine, the aerial parts of this plant have been prepared as decoction and used for haemorrhage, increasing muscle strength, urinary excretion, pulmonary windy oedema and chronic bronchitis2. In Chinese folk medicine, Caryopteris terniflora has been used as antipyretic, detoxifying, expectorant, and anti-inflammatory agent and for the treatment of cold, tuberculosis and rheumatism3.
Previous chemical investigations of Caryopteris mongolica showed the presence of mono- and sesquiterpenoids4, hypolaetin-7-glucoside5, iridoid glucosides and steroids6. From the roots of this plant, the abietane ortho-quinone caryopteron A and derivatives were isolated7. In our previous phytochemical investigation of the aerial parts of C. mongolica, we have identified some phenolic compounds such as acacetin, apigenin, luteolin, and caffeic acid8. From other species of Caryopteris steroidal and iridoid glucosides, phenylethanoids, diterpenoids, α-caryopterone, a new pyranojuglone, clandonoside and its acetylated derivatives, flavonoids and sterols have been isolated9,10,11,12,13. In the present paper, we describe the isolation of pyridine monoterpene alkaloids, isoquinoline alkaloids, a dipyrrolopyrazine, and a flavonoid (acacetin) from the aerial parts of C. mongolica and their structure elucidation using MS, as well as IR, 1H NMR, 13C NMR, and 2D NMR spectroscopic methods.
Experimental section
General experimental procedures
Flash column chromatography (FCC) was performed using silica gel 200–300 mesh from Acros Organics occasionally on a Büchi Sepacore system equipped with a UV monitor. For thin layer chromatography (TLC), silica gel plates Merck 60 F254 were used. Spots detected on the TLC plates under the UV lamp at 254 or 365 nm were visualized by spraying Dragendorff’s reagent. The EI-MS were recorded on a Finnigan MAT-95 mass spectrometer (70 eV) and the ESI-MS on an Bruker Esquire LC with an ion trap detector. Positive and negative ions were detected. ESI-HRMS were recorded on a Waters Xevo G2-XS QTOF mass spectrometer. The 1D and 2D NMR spectra (600 MHz 1H NMR and 150 MHz 13C NMR) were measured on a Bruker AVANCE III 600 MHz spectrometer using CDCl3 or CD3OD as solvents. The chemical shifts δ are reported in ppm using either CHCl3/CDCl3 (δH = 7.25, δC = 77.0) or CH3OH/CD3OD (δH = 3.31, δC = 49.0 ppm) as internal standard relative to TMS. Coupling constants J are given in Hz. The complete assignment of 1H and 13C NMR signals was confirmed by 2D NMR spectroscopic methods (COSY, HSQC, HMBC and NOESY).
Plant material
The aerial parts of Caryopteris mongolica Bunge were collected at Baruun kharaa of Selenge province Bayantsogt Mountain, during the flowering period in July 2014. The plant was identified by Prof. E. Ganbold and Dr. B. Mandahk, Institute of Botany, MAS, and voucher specimen were deposited at the Herbarium of Laboratory of Natural Products Chemistry, ICCT, Research Institute of the Mongolian Academy of Sciences, in Ulaanbaatar (voucher numbers: UBA-0004020 and UBA-0004021). The authors confirm that the present study complies with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Extraction and isolation of compounds
The air-dried and powdered aerial parts (12.5 kg) of Caryopteris mongolica were extracted three times at room temperature with 95% aqueous ethanol. The ethanol was removed and the residue treated with 2.5% HCl (pH = 1–2). The aqueous acidic solution was extracted successively with n-hexane and chloroform. Then, the residual aqueous solution was adjusted to pH = 9–10 by addition of aqueous NH4OH (25%), extracted with CHCl3 and dried over Na2SO4. Finally, CHCl3 was evaporated under reduced pressure to give 12.02 g of crude total alkaloid extract (0.096%).
The crude total alkaloid extract (12.02 g) isolated from C. mongolica was purified by FCC (flash column chromatography on silica gel, 200–300 mesh from Acros Organics) (175 g silica gel). The elution with CHCl3, CHCl3–CH3OH mixtures ranging from 90:10, 85:15, 80:20, 75:25, and 50:50 (1000–1500 mL), and pure CH3OH afforded 11 sub-fractions from A to K: A (108 mg), B (69.6 mg), C (1.50 g), D (851 mg), E and F (218 mg), G (399 mg), H (1.34 g), I (161 mg), J (274 mg), and K (85.0 mg). The fractions were monitored by TLC, compounds detected as described by Dragendorff’s reagent, and checked by ESI-MS.
Fraction C (1.50 g) was subjected to FCC (25 g silica gel), and eluted with CHCl3–CH3OH mixtures ranging from 99:1 to 95:5 to afford seven fractions from A1 to G1: A1 (8.7 mg), B1 (46.0 mg), C1 (55.0 mg), D1 (46.9 mg), E1/F1 (234 mg), and G1 (183 mg). From fraction B1, compound 3 (aucubinine B)14, 15 (13.2 mg) was isolated (Fig. 1). The compounds 1 (7-methylene-6,7-dihydro-5H-cyclopenta[c]pyridin-5-ol)13 (20.1 mg) and 2 (10.1 mg, ESI-MS: m/z = 194.2 [M + H]+) have been obtained from fraction G1. Fraction C1 provided by crystallization from CHCl3 colorless needles which were identified as compound 8 (acacetin)8, 16,17,18,19 (23.6 mg). The purified fraction E1/F1 afforded a mixture of the compounds 4 and 5 (30.8 mg, ESI-MS: m/z = 296.2 [M + H]+). Finally, we have combined all fractions (139 mg) exhibiting in their ESI–MS a peak at m/z = 296.2 [M + H]+, subjected them to FCC (25 g silica gel), and eluted with CHCl3–CH3OH mixtures ranging from 99:1 to 95:5 to provide compound 4 (2.7 mg) and compound 5 (7.7 mg) as shown by their NMR spectra (Figure S51).
Fraction D (851 mg) was subjected to FCC (25 g of silica gel), eluted with CHCl3–CH3OH mixtures ranging from 99:1 to 95:5 to afford 5 fractions from A2 to E2: A2 (27.2 mg), B2 (137 mg), C2 (172 mg), D2 (84.5 mg), and E2 (37.8 mg). After evaporation of the solvent, fraction B2 afforded a colorless residue which has been purified to provide a mixture of compounds 6 and 7 (137 mg) showing in the ESI-MS a peak at m/z = 293.2 [M + H]+. Finally, we further purified a fraction of this mixture (20.3 mg) by preparative thin layer chromatography (PTLC, preparative silica gel plates 60 F254 Merck, Germany) using the solvent system CHCl3–CH3OH (9:1). The PTLC afforded a mixture of the compounds 6 and 7 (3.2 mg) and compound 7 (17.2 mg) along with compounds 1 and 2 as impurities.
Results and discussion
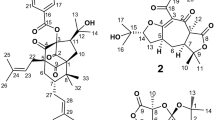
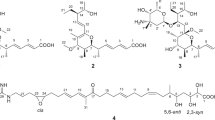
After isolation from the aerial parts of Caryopteris mongolica Bunge, the structures of the compounds 1–9 (Fig. 1) were elucidated by a combination of mass spectrometry and 2D NMR spectroscopy (1H and 13C NMR data, see: Tables 1, 2, 3). The assignment of the NMR is based on the 1D and 2D NMR spectra (Supplementary Information).
Compound 1: 7-Methylene-6,7-dihydro-5H-cyclopenta[c]pyrindin-5-ol.
Compound 1 (20.1 mg) was isolated as colorless needles (mp 81.3–90 °C). The ESI-MS (m/z = 148.1 [M + H]+) and EI-MS (m/z = 147 [M+]) correspond to the molecular formula C9H9NO. The 1H NMR spectrum of 1 exhibits nine proton signals (Table 1): three signals for the aromatic pyridine protons, two broad singlets for the exocyclic methylene group, a doublet of doublets for CH–O, two signals for the two methylene protons at C-6, and a broad line at 3.2 ppm for the OH group. In the 13C NMR spectrum, nine resonances appeared: five signals for the carbon atoms of the pyridine ring and four signals for the CH–O, methylene, and C=CH2 moieties. Based on the HSQC and HMBC spectra, an unambiguous assignment of the NMR signals was achieved. Particularly, the long-range HMBC correlations of C-4a with H-1, H-3, H-5, and H-6, as well as of C-8 with H-1, H-4, H-5, H-6, and olefinic H-9 confirmed the bicyclic pyridine framework of 1. Compound 1 has been isolated previously from Caryopteris tangutica13. Based on the spectroscopic data which are in agreement with those reported in the literature20, 21, compound 1 was assigned as 7-methylene-6,7-dihydro-5H-cyclopenta[c]pyridin-5-ol.
Compound 2: (5S*,7R*)-7-Ethoxy-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridin-5-ol.
Compound 2 (10.1 mg) was obtained as a brown residue. The ESI-MS (m/z = 194.2 [M + H]+) corresponds to the molecular formula C11H15NO2. The 1H NMR spectrum of 2 exhibits nine proton signals (Table 1): three signals for the pyridine protons, a doublet of doublets for CH–O, two doublets of doublets for the two methylene protons at C-6, a singlet at 1.53 ppm for the isolated methyl group, and the signals characteristic for the ethoxy group. Based on all NMR data and supported by long-range HMBC correlations in analogy to those of 1, the framework was confirmed. The strong NOE correlation between the protons H-5 and H-9 (Me) indicated a cis-arrangement for the hydroxy and the ethoxy group. The 2D NMR correlations supporting the stereochemical assignment for compound 2 are shown in the Supplementary Information (Figures S12–S17-1). Compound 2 has been identified as (5S*,7R*)-7-ethoxy-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridin-5-ol and represents a new compound obtained from C. mongolica for the first time. However, it cannot be excluded that compound 2 has been formed by electrophilic addition at 1 on treatment with ethanol/HCl during the isolation process.
Compound 3: 6,7-Dihydro-7-methylcyclopenta[c]pyridine-5-one (aucubinine B).
Compound 3 (10.1 mg) was obtained as a brown residue. The ESI-MS (m/z = 148.1 [M + H]+) corresponds to the molecular formula C9H9NO. The 1H NMR spectrum of compound 3 exhibits three signals for the pyridine protons, a quartet of doublet of doublets for the methine group (H-7), two doublets of doublets for the two methylene protons at C-6, and one doublet at 1.46 ppm for the methyl group (Table 1). The 13C NMR spectrum of 3 displays a characteristic peak at 206.0 ppm for the C=O group, five carbon signals for the pyridine ring, one signal for the methylene group, and the signals for the CH–Me moiety at 31.5 and 21.2 ppm (Table 1). The assignment of the signals was confirmed by the 2D NMR spectra. The NMR data of compound 3 are in agreement with those previously reported in the literature14, 15. Thus, 3 has been assigned as 7-methyl-6,7-dihydro-5H-cyclopenta[c]pyridin-5-one (aucubinine B) (3). Compound 3 was obtained from C. mongolica for the first time.
The polycyclic pyridine alkaloids 4 and 5 were obtained as pure compounds and as a mixture (Figure S51). They show the same ESI-MS-peak at m/z = 296.2 [M + H]+ corresponding to the molecular formula C18H17NO3. The 1H NMR and 13C NMR signals of 4 and 5 are clearly different from each other (Table 2). Their structures have been assigned based on the 2D NMR spectra.
Compound 4: (5aR*,6S*,10S*,11R*,11aR*)-10,11a-Dimethyl-6,7,9,10,11,11a-hexahydro-5H-6,11-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5,8(5aH)-dione.
Compound 4 (30.8 mg) was isolated as a colorless solid. The ESI-MS (m/z = 296.2 [M + H]+) and EI-MS (m/z = 295 [M+]) correspond to the molecular formula C18H17NO3; ESI-HRMS calcd. for C18H18NO3+ ([M + H]+): 296.1281, found: 296.1286. The 1H NMR spectrum exhibits 13 signals for 17 protons (Table 2): three pyridine protons, four methine protons, two methylene groups, as well as one singlet and one doublet for the two methyl groups. The 13C NMR spectrum shows signals for 18 carbon atoms (Table 2): five aromatic carbon atoms for the pyridine ring, two olefinic C atoms, four methine groups, two methylene groups, two methyl groups, one quaternary atom, and two signals at δ = 206.42 and 205.50 ppm for two carbonyl groups.
A series of longe-range HMBC correlations has been used to confirm the connectivity of the structural moieties in compound 4 (Fig. 2). The signals of the pyridine protons showed interactions with the neighboring carbon atoms: the doublet of H-4 with the carbonyl C-5 signal at δ = 205.50 ppm and the singlet of H-1 with the quaternary C-11a at δ = 58.90 ppm. Moreover, cross peaks of H-5a and H-6 with C-5; of H-13 (Me) and H-6 with C-11a; of H-11 with C-11a, C-5a, C-6, C-7a, C-10, C-10a, and C-11b; of H-7, H-6, and H-9 with C-7a; and of H-7, H-9, and H-14 (Me) with C-10a suggest the polycyclic skeleton for compound 4 with a C-6–O–C-11 bridge. The stereochemical arrangement of cyclic moieties in 4 has been determined by NOESY measurements. A positive NOE correlation between the protons H-5a and H-13 of the methyl group confirms the cis-configuration of the annulated five-membered ring (Fig. 3). Based on the extensive NMR analysis, compound 4 was identified as (5aR*,6S*,10S*,11R*,11aR*)-10,11a-dimethyl-6,7,9,10,11,11a-hexahydro-5H-6,11-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5,8(5aH)-dione. Compound 4 is a new pyridine derivative that has been isolated from C. mongolica for the first time.
Compound 5: (5aR*,6S*,7aR*,8S*,11aR*)-10-Hydroxy-7a,11a-dimethyl-5a,6,7,7a,8,11a-hexahydro-5H-6,8-epoxycyclopenta[6,7]azuleno[1,2-c] pyridin-5-one.
Compound 5 has been obtained as a mixture with compound 9 as a colorless solid. The ESI-MS (m/z = 296.2 [M + H]+) and EI-MS (m/z = 295 [M+]) correspond to the molecular formula C18H17NO3; ESI-HRMS calcd. for C18H18NO3+ ([M + H]+): 296.1281, found: 296.1287. Thus, it is isomeric to compound 4. The 1H NMR spectrum of 5 displayed 12 proton signals (Table 2): one singlet and two doublets for the pyridine protons, two olefinic protons, three methine groups, one methylene group, and two singlets for the two methyl groups. Due to the H–D exchange, the hydroxyl group gives no signal. The 13C spectrum exhibits signals for 18 carbon atoms (Table 2): five pyridine carbon atoms, four olefinic carbon atoms, two methyl groups, three methine groups, one methylene group, two quaternary carbon atoms, and one signal for a carbonyl group at δ = 204.76 ppm. The molecular structure of compound 5 has been secured by COSY, HSQC, HMBC, and NOESY measurements in order to confirm the stereochemistry (Fig. 3).
The assignment of the NMR signals and the elucidation of the framework of compound 5 are based on the COSY interactions of H-5a with H-6 and of H-6 with the methylene protons H-7, and by the following long-range HMBC correlations: H-4 and H-5a with the low-field signal for the carbonyl group (C-5); H-5a with C-10a, C-11b, and C-13; H-13 (Me) with C-1, C-5a, C-11, and C-11b; H-6 with C-5a and C-10a; H-11 with C-5a, C-7a, C-9, C-10a, and C-11a; H-7, H-8, and H-14 (Me) with C-10a (Fig. 3). The NOE correlation of the methine proton H-5a with the protons of the methyl group (H-13) confirms the cis-arrangement of both. The stereochemistry for the two methyl groups H-13 and H-14 is supported by NOE interactions of H-13 with H-1, H-5a, H-7a, and H-11, as well as of H-14 with the methylene protons H-7a and H-7b (Fig. 3). Based on the extensive NMR analysis, compound 5 was identified as (5aR*,6S*,7aR*,8S*,11aR*)-10-hydroxy-7a,11a-dimethyl-5a,6,7,7a,8,11a-hexahydro-5H-6,8-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5-one. Compound 5 is a new pyridine derivative that has been obtained from C. mongolica for the first time.
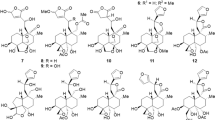
We also performed detailed NMR studies of the compounds 6 and 7. While compound 6 was isolated as a pure substance, compound 7 was obtained only as a mixture, either together with the compounds 6, 1, and 2, or together with the compounds 1 and 2 (Supplementary Information). All isolated fractions of either pure 6 or mixtures containing 7 showed the same ESI-MS peak at m/z = 293.2 ([M + H]+) that corresponds to a molecular formula of C18H16N2O2. However, we obtained two different sets for the 1H NMR and 13C NMR signals of both compounds (Table 3). Thus, we were able to assign the structure for 6 and for the by-product 7 which has the same molecular formula but a different constitution (see below).
Compound 6: (5R*,5aR*,10bS*,11R*)-5-Hydroxy-10b,11-dimethyl-5,5a,10b,11-tetrahydro-6H-5,11-methanopyrido[3',4':3,4]cyclopenta[1,2-g]isoquinolin-6-one.
Compound 6 (3.2 mg) was isolated as a colorless solid. The ESI-MS (m/z = 293.2 [M + H]+) and EI-MS (m/z = 292 [M+]) correspond to the molecular formula C18H16N2O2. The 1H NMR spectrum exhibits 11 signals for 16 protons (Table 3): four doublets and two singlets for six pyridine protons, one singlet for a methine proton, two doublets for the methylene bridge, and two singlets for the two methyl groups. The 13C NMR spectrum displays signals for 18 carbon atoms (Table 3): ten carbon atoms of the two pyridine rings, one carbonyl group (at 203.76 ppm in CD3OD), one methine group (at 66.89 ppm in CD3OD), three quaternary carbon atoms, one methylene group (64.12 ppm in CD3OD), and two methyl groups.
The assignments of the NMR signals and the framework of compound 6 were confirmed by the following characteristic long-range HMBC correlations: H-4 with C-5; H-5a with C-5, C-6 (carbonyl group), C-10b, C-6a, C-10a, C-4a, and C-13; H-7 with C-6 (carbonyl group); H-10 with C-10a; H-12 with C-5, C-11, C-5a, C-4a, C-10b, and C-11a; H-13 with C-5a, C-10a, C-10b, and C-11; and H-14 with C-10b, C-11, C-11a, and C-12 (Fig. 4). The configuration of 6 with a syn-orientation of the pyridine rings is suggested by the NOE correlations between H-13 (Me) with H-10, H-5a, and one of the protons from the methylene group (H-12a); H-12a with H-5a; and H-14 (Me) with H-1 and H-10 (Fig. 4). In conclusion, compound 6 has been assigned as (5R*,5aR*,10bS*,11R*)-5-hydroxy-10b,11-dimethyl-5,5a,10b,11-tetrahydro-6H-5,11-methanopyrido[3',4':3,4]cyclopenta[1,2-g]isoquinolin-6-one. Compound 6 is a new compound that has been obtained from C. mongolica for the first time.
Compound 7: (6S*,6aR*,11R*,11aS*)-6a-Hydroxy-11,11a-dimethyl-6,6a,11,11a-tetrahydro-5H-6,11-methanopyrido[3',4':4,5]cyclopenta[1,2-h]isoquinolin-5-one.
Compound 7 is a colorless solid and was obtained as a small by-product during the extraction of compound 6. The ESI-MS (m/z = 293.2 [M + H]+) corresponds to the molecular formula C18H16N2O2. The 1H NMR spectrum of 7 exhibited 11 signals for 16 protons (Table 3): four doublets and two singlets for the six pyridine protons, one doublet for the methine proton, two doublets of methylene bridge, and two singlets for the two methyl groups. The 13C NMR spectrum showed signals for 18 carbon atoms (Table 3): ten carbon atoms of the two pyridine rings, one carbonyl group (202.52 ppm), one methine group (64.12 ppm), three quaternary carbon atoms, one methylene group (57.36 ppm), and two methyl groups.
The assignment of the NMR signals and the structural elucidation of compound 7 are based on the COSY, HSQC, and HMBC spectra (Fig. 5). The COSY spectrum shows a cross peak between the protons of the methylene bridge (H-12) and the proton of the methine group (H-6). The structural assignment is supported by the following series of HMBC correlations: H-4 with C-5; H-6 with C-5, C-6a, C-6b, and C-11b; H-12 with C-6, C-11, C-6a, C-11a, C-6b, C-10a, C-7, and C-10; H-13 (Me) with C-11b, C-11a, C-11, and C-6; H-14 (Me) with C-10a, C-11, C-11a, and C-12. Compound 7 has been assigned as (6S*,6aR*,11R*,11aS*)-6a-hydroxy-11,11a-dimethyl-6,6a,11,11a-tetrahydro-5H-6,11-methanopyrido[3',4':4,5]cyclopenta[1,2-h]isoquinolin-5-one. Compound 7 is a new compound that has been obtained from C. mongolica for the first time.
Compound 8: 5,7-Dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (5,7-dihydroxy-4'-methoxyflavone, acacetin).
Compound 8 (23.6 mg) was obtained as colorless needles. The ESI-MS (m/z = 285.1 [M + H]+) corresponds to the molecular formula C16H12O5. The 1H NMR spectrum shows signals for 10 protons including seven aromatic protons and a methoxy group at δ 3.88 ppm. The 13C NMR spectrum exhibits signals for 16 carbon atoms: seven aromatic methine (CH) groups, six aromatic carbon atoms, one olefinic carbon atom, a methoxy group at 55.94 ppm, and a carbonyl group at 181.20 ppm. The assignment was additionally supported by 2D NMR spectroscopy. Thus, the structure of compound 8 was confirmed as 5,7-dihydroxy-4'-methoxyflavone (acacetin)8, 13, 16, 17. This natural product was isolated for the first time from the aerial parts of C. mongolica.
1H NMR (CD3OD, 600 MHz): δ 7.90 (d, J = 12.0 Hz, H-2', 2 H), 7.07 (d, J = 12.0 Hz, H-3', 2 H), 6.45 (s, H-3, 1 H), 6.16 (d, J = 1.9 Hz, H-8, 1 H), 5.98 (d, J = 1.9 Hz, H-6, 1 H), 3.88 (s, OCH3, 3 H).
13C NMR, (CD3OD, 151 MHz): δ 181.20 (C-4), 163.73 (C-2), 162.75 (C-7), 161.76 (C-4'), 160.91 (C-5), 156.79 (C-9), 129.02 (C-2', 2 C), 122.30 (C-1'), 115.51 (C-3', 2 C), 104.05 (C-6), 103.19 (C-10), 102.99 (C-3), 97.97 (C-8), 55.94 (OCH3).
Compound 9: Octahydro-5H,10H-dipyrrolo[1,2-a:1',2'-d]pyrazin-5,10-dione (L-proline anhydride).
Compound 9 (2.4 mg) was obtained as a colorless amorphous solid. The ESI-MS (m/z = 195.2 [M + H]+) and EI-MS (m/z = 194 [M+]) correspond to the molecular formula C10H14N2O2. The 1H NMR spectrum displays six proton signals for six methylene groups and two methine protons. The 13C NMR spectrum exhibits five signals for six methylene groups, two methine groups and two carbonyl groups. Therefore, compound 9 has been assigned as octahydro-5H,10H-dipyrrolo[1,2-a:1',2'-d]pyrazine-5,10-dione (L-proline anhydride). Compound 9 was isolated for the first time from the aerial parts of C. mongolica.
1H NMR (CDCl3, 600 MHz): δ 4.18 (t, J = 18.0 Hz, H-4, 2 H), 3.53 (m, H-1, 4 H), 2.31 (m, H-3a, 2 H), 2.18 (m, H-3b, 2 H), 2.02 (m, H-2a, 2 H), 1.92 (m, H-2b, 2 H).
13C NMR (CDCl3, 151 MHz): δ 166.2 (C-5), 60.54 (C-4), 45.21 (C-1), 27.69 (C-3), 23.35 (C-2).
Conclusion
We have isolated seven pyridine alkaloids (1–7), the flavonoid acacetin (8), and L-proline anhydride (9) from the aerial parts of Caryopteris mongolica Bunge. The chemical structures of these compounds were elucidated by MS as well as by 1H NMR, 13C NMR and 2D NMR (COSY, HSQC, HMBC, and NOESY) spectroscopic methods. (5S*,7R*)-7-Ethoxy-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridin-5-ol (2), (5aR*,6S*,10S*,11R*,11aR*)-10,11a-dimethyl-6,7,9,10,11,11a-hexahydro-5H-6,11-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5,8(5aH)-dione (4), (5aR*,6S*,7aR*,8S*,11aR*)-10-hydroxy-7a,11a-dimethyl-5a,6,7,7a,8,11a-hexahydro-5H-6,8-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5-one (5), (5R*,5aR*,10bS*,11R*)-5-hydroxy-10b,11-dimethyl-5,5a,10b,11-tetrahydro-6H-5,11-methanopyrido[3',4':3,4]cyclopenta[1,2-g]isoquinolin-6-one (6), (6S*,6aR*,11R*,11aS*)-6a-hydroxy-11,11a-dimethyl-6,6a,11,11a-tetrahydro-5H-6,11-methanopyrido[3',4':4,5]cyclopenta[1,2-h]isoquinolin-5-one (7), 5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (5,7-dihydroxy-4'-methoxyflavone, acacetin) (8), and octahydro-5H,10H-dipyrrolo[1,2-a:1ʹ,2ʹ-d]pyrazine-5,10-dione (L-proline anhydride) (9) have been obtained from C. mongolica for the first time.
Most likely, the cyclopenta[c]pyridine framework of the compounds 1–3 derives from an 11-nor-iridoid compound by condensation with ammonia14, 15. The novel compounds 4–7 would be generated by subsequent condensations of the cyclopenta[c]pyridine. Compounds 4 and 5 could be formed by fusion of the cyclopenta[c]pyridine with an 11-nor-iridoid, while compounds 6 and 7 would be generated by dimerization of two cyclopenta[c]pyridines. However, a formation of the cyclopenta[c]pyridine skeleton on treatment with ammonia during the extraction process cannot be ruled out completely. Compound 2 might have been formed by electrophilic addition of ethanol at compound 1 during the extraction. According to database search (SciFinder and Reaxys), the compounds 2, 4, 5, 6, and 7 (Fig. 6) represent new chemical structures.
Supplementary Information
600 MHz 1H NMR, 150 MHz 13C NMR, COSY, HSQC, HMBC, and NOESY spectra of the compounds 1–9.
References
Grubov, V. I. Key to the Vascular Plants of Mongolia. Ulaanbaatar, p. 254 (2008).
Ligaa, U. Medicinal Plants of Mongolia Used in Mongolian Traditional Medicine. Ulaanbaatar, pp. 210–211 (2006).
Zhang, Y.-H., Wei, Q.-Y., Liu, Z.-L., Yang, L. & Cheng, D.-L. Two new steroidal glycosides from Caryopteris terniflora. Chin. Chem. Lett. 15, 1445–1447 (2004).
Shatar, S. & Adams, R. P. The essential oil of Caryopteris mongolica Bunge from Mongolia. J. Essent. Oil Bear. Plants 2, 25–28 (1999).
Zapesochnaya G. G., Pangarova T. T. Hypolactin-7-glucosiode from Caryopteris mongolica. Khimiya Prirodnych Soedinenii, No 4, p. 554 (1973).
Zhang, Y.-H., Yang, L. & Cheng, D.-L. Iridoid glucosides from Caryopteris mongholica. Pharmazie 55, 845–847 (2000).
Saruul, E. et al. An antibacterial ortho-quinone diterpenoid and its derivatives from Caryopteris mongolica. Bioorg. Med. Chem. Lett. 25, 2555–2558 (2015).
Dumaa, M., Chunsriimyatav, G., Gruner, M., Knölker, H.-J., Bolortuya, M. & Regdel, D. Determination of phenolic compounds from aerial parts of Caryopteris mongolica Bunge grown in Mongolia. In 4th International Conference on Chemical Investigation and Utilization of Natural Resources, Ulaanbaatar (Mongolia), Abstract Book, p. 48 (2016).
Zhao, D. P., Matsunami, K. & Otsuka, H. Iridoid glucoside, (3R)-oct-1-en-3-ol glycosides, and phenylethanoid from the aerial parts of Caryopteris incana. J. Nat. Med. 63, 241–247 (2009).
Zhang, Y.-H. et al. Diterpenoids from the Chinese herb Caryopteris terniflora and their antibacterial and antitumor activity. Pharmazie 60, 551–553 (2005).
Gao, J. J., Han, G. Q. & Yang, L. Two new phenylpropanoid glucosides from Caryopteris incana (Thumb.) Miq. Chin. Chem. Lett. 7, 445–448 (1996).
Luo, G. et al. Iridoid glucosides and diterpenoids from Caryopteris glutinosa. J. Nat. Prod. 79, 886–893 (2016).
Dai, Y., Zhang, B.-B. & Liao, Z.-X. Chemical constituents of Caryopteris tangutica. Nat. Prod. Res. 26, 643–647 (2012).
Baghdikian, B. et al. Two new pyridine monoterpene alkaloids by chemical conversion of a commercial extract of Harpagophytum procumbens. J. Nat. Prod. 62, 211–213 (1999).
Hattori, M. et al. Transformation of aucubine to new pyridine monoterpene alkaloids, aucubines A and B, by human intestinal bacteria. Phytother. Res. 4, 66–70 (1990).
Perkin, A. G. Die gelben Farbstoffe verschiedener Tanninarten. Chem. Zentralbl. 71, 669 (1900).
Ma, Z. K., Niu, B. J., Zhang, B. B. & Liao, Z. X. Chemical constituents from Pedicularis longiflora var. tubiformis. Chin. Tradit. Herb. Drugs 44, 403–407 (2013).
Wang, X., Perumalsamy, H., Kwon, H. W., Na, Y.-E. & Ahn, Y.-J. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 5, 16127 (2015).
Singh, S., Gupta, P., Meena, A. & Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 145, 111708 (2020).
Jones, K., Fiumana, A. & Escudero-Hernandez, M. L. Pyridine radicals in synthesis. Part 3: cyclopentannulation of pyridine via the 3-pyridyl radical and a formal synthesis of (±)-oxerine. Tetrahedron 56, 397–406 (2000).
Zhao, J., Yang, X., Jia, X., Luo, S. & Zhai, H. Novel total syntheses of (±)-oxerine by intramolecular Heck reaction. Tetrahedron 59, 9379–9382 (2003).
Acknowledgements
We are grateful to the DAAD (Deutscher Akademischer Austauschdienst) for fellowships to D.M. and C.G. We also thank the GFF TU (Gesellschaft von Freunden und Förderern der Technischen Universität Dresden) for a fellowship to C.G.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
D.M. and C.G. extracted the plants and isolated the compounds. D.R. supported the project. M.G. and T.L. performed the NMR spectroscopic studies. H.-J.K. supervised the research project. M.G. and H.-J.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mishig, D., Gruner, M., Lübken, T. et al. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. Sci Rep 11, 13740 (2021). https://doi.org/10.1038/s41598-021-93010-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93010-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.



 ), HMBC (2,3JH-C H
), HMBC (2,3JH-C H
 C) and NOE correlations (
C) and NOE correlations ( ) of 4.
) of 4.


