Abstract
Muscle growth of low birth weight (LBW) piglets may be improved with adapted nutrition. This study elucidated effects of glutamine (Gln) supplementation on the cellular muscle development of LBW and normal birth weight (NBW) piglets. Male piglets (n = 144) were either supplemented with 1 g Gln/kg body weight or an isonitrogeneous amount of alanine (Ala) between postnatal day 1 and 12 (dpn). Twelve piglets per group were slaughtered at 5, 12 and 26 dpn, one hour after injection with Bromodeoxyuridine (BrdU, 12 mg/kg). Muscle samples were collected and myogenic cells were isolated and cultivated. Expression of muscle growth related genes was quantified with qPCR. Proliferating, BrdU-positive cells in muscle sections were detected with immunohistochemistry indicating different cell types and decreasing proliferation with age. More proliferation was observed in muscle tissue of LBW-GLN than LBW-ALA piglets at 5 dpn, but there was no clear effect of supplementation on related gene expression. Cell culture experiments indicated that Gln could promote cell proliferation in a dose dependent manner, but expression of myogenesis regulatory genes was not altered. Overall, Gln supplementation stimulated cell proliferation in muscle tissue and in vitro in myogenic cell culture, whereas muscle growth regulatory genes were barely altered.
Similar content being viewed by others
Introduction
Low birth weight (LBW) piglets, often occurring in large litters, have a greater mortality rate, altered lipid deposition and retarded body growth in comparison with their normal birth weight (NBW) littermates1, 2. The delayed growth of LBW piglets is mainly due to a slower development of skeletal muscle as the myogenic activity is usually impaired in these animals during the fetal and early postnatal period3, 4. These disadvantages could cause long-term negative effects in LBW piglets1 and different adapted feeding strategies have been developed to compensate the growth delay and improve the body composition of LBW piglets5,6,7. Feeding with amino acids can stimulate protein synthesis in all tissues, especially in skeletal muscle as reviewed by Davis et al.3. This stimulation effect decreases with age8, thus it is reasonable to support muscle development with additional amino acids during the suckling period of piglets. In this project, we aimed to ameliorate the retarded growth of LBW piglets by oral glutamine (Gln) supplementation. Wu and Knabe9 reported that free Gln in porcine milk increased from 0.1 to 4 mM between day 1 and 28 of lactation indicating the potential importance of Gln for pig development in the early postnatal phase. Glutamine is regarded as the most abundant amino acid in the body, is mainly synthesized in skeletal muscle10, and muscle tissue is the most important site for Gln storage11. It promotes cell proliferation as a precursor for the synthesis of purine and pyrimidine nucleotides and provides energy, when metabolized to glutamate12, 13. Furthermore, Gln serves as a precursor for the synthesis of arginine, which is indispensable for the optimal growth of neonatal piglets14 and plays important roles in essential metabolic pathways15. However, LBW piglets might not receive enough Gln from milk to compensate the delayed body growth15. Our previous studies showed that Gln had the potential to increase muscle fiber size in piglets16, but the mechanism was not clear. We observed a temporarily increased intramuscular availability of free Gln that could have influenced cellular processes in skeletal muscle. Therefore, we complemented our investigations into the cellular development of the skeletal muscle of neonatal piglets with in vitro studies using a primary porcine myogenic cell culture model with Gln supplementation.
Protein deposition occurs when protein synthesis rate is greater than the protein degradation rate, which contributes to the postnatal muscle growth together with myonuclei accretion3, 17,18,19. In general, nuclei from myoblasts are not able to divide when myoblasts have been incorporated into myofibers20. Thus, during this process, muscle satellite cells (also named muscle stem cells) are the origin of the increasing number of myonuclei3, 20, 21, providing growth and regeneration of muscle cells22, 23. However, the proliferative satellite cells are most abundant in piglets during the first days of life, and then decrease with age24.
A number of key factors regulate muscle growth and regeneration. Paired box transcription factor 7 (PAX7) is always expressed in quiescent satellite cells25, regulating muscle growth in the early phase of cell proliferation26, and restricting the muscle stem cell differentiation27. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) is a possible regulator of satellite cell activation19. When satellite cells are activated, the myogenic regulatory factors (MRFs) including myoblast determination protein 1 (MYOD), myogenic factor 5 (MYF5), myogenic factor 6 (MYF6 or MRF4) and myogenin (MYOG) start to modulate the muscle cell differentiation28. It was shown that MYOD, MYF5 and MRF4 are regulators for satellite cell differentiation to myocytes in vivo and for early differentiation in vitro23, 29, whilst MYOG is indispensable for terminal differentiation29, 30. In contrast, myostatin (MSTN) is a negative regulator of muscle growth during the whole life31. Altogether, these genes are suited as biological markers when investigating muscle cell development.
Since Doumit and Merkel32 first isolated myogenic satellite cells from pig in 1992, porcine satellite cell culture has been considered as an ideal model for studying muscle cell growth and development including proliferation and differentiation33. Furthermore, the successful transplantation of myofibers generated from cultured satellite cells into mouse muscle34 underlined the suitability of this in vitro system.
Our hypothesis was that Gln supplementation could improve muscle growth in early postnatal piglets by stimulation of satellite cell proliferation, their differentiation to myoblasts and fusion with myofibers. The objective of the present study was to evaluate the impact of glutamine through two approaches, one in vivo and one in vitro. Therefore, we quantified cell proliferation and expression of involved myogenic regulator genes in the skeletal muscle of piglets differing in birth weight (BiW) and in a porcine myogenic cell culture model under the influence of a supplementation with Gln.
Results
Detection of cell proliferation within M. longissimus
Incorporation of Bromodeoxyuridine (BrdU) into newly synthesized DNA was used to identify proliferating cells in muscle tissue. Proliferating, BrdU-positive cells in Musculus longissimus dorsi (MLD) were detected with immunohistochemistry (nuclei appear green in Fig. 1) close to muscle fibers (MF), but also within connective tissue (CT), near developing adipocytes (AD), and in blood vessels (BV). The localization suggests that different cell types were involved, such as satellite cells, fibroblasts, preadipocytes, endothelial cells, etc., but the individual cell types with BrdU-positive nuclei could not be quantified in this analysis.
Incorporation of BrdU in proliferating cells within muscle tissue. Immunohistochemical detection of BrdU-positive nuclei (green) and total nuclei (red, stained with propidium iodide) in a longissimus muscle cross section of a 12-day-old piglet. Arrows indicate proliferating cells of different types. MF muscle fibers, AD adipocytes, BV blood vessel, CT connective tissue.
The number of BrdU-positive nuclei per area unit decreased with age (Fig. 2a–c). This observation was reflected by the area percentage of BrdU-positive nuclei that decreased from 5 days post-natum (dpn) to 26 dpn (p < 0.001, Fig. 2e). The total number of nuclei, measured as area percentage, decreased at the same time due to muscle fiber growth, but to a lesser extent (Fig. 2d). Thus, the ratio of BrdU-positive to total nuclei (Fig. 2f) also decreased from 5 to 26 dpn (p < 0.001). An influence of Gln supplementation was observed in LBW piglets at 5 dpn, indicating more total (p = 0.024) and more BrdU-positive nuclei (p = 0.009) in LBW-GLN than in LBW-ALA piglets. However, this difference was not observed in older piglets at 12 or 26 dpn (p > 0.1). The ratio of BrdU-positive nuclei to total nuclei was not influenced by BiW (p = 0.220) or Gln supplementation (p = 0.892, Fig. 2f). The numbers of total and BrdU-positive nuclei per mm2 in regions occupied exclusively by muscle fibers, without visible connective tissue, mainly represent satellite cell nuclei. Both were higher in piglets at 5 dpn than at 12 or 26 dpn (p < 0.001, Fig. 2g,h), without being influenced by BiW or Gln supplementation (p > 0.1). The ratio of BrdU-positive muscle nuclei to total muscle nuclei was not influenced by BiW (p = 0.583) or Gln supplementation (p = 0.820, Fig. 2i) as well.
Detection and quantification of proliferating cells in M. longissimus. (a–c) Immunohistochemical detection of BrdU-positive nuclei (green) and total nuclei (red) at 5, 12 and 26 dpn; (d) area percentage of total nuclei; (e) area percentage of BrdU-positive nuclei; (f) ratio of BrdU-positive nuclei to total nuclei; (g) number of total nuclei per mm2 in a region comprising muscle fibers exclusively; (h) number of BrdU-positive nuclei per mm2; (i) ratio of BrdU-positive nuclei to total nuclei in M. longissimus of glutamine (GLN) or alanine (ALA) supplemented low birth weight (LBW) and normal birth weight (NBW) piglets at 5, 12 and 26 dpn. Values are presented as LSmeans and SE (n = 12 per group). Different uppercase letters (A-C) indicate significant differences among ages and different lowercase letters (a, b) indicate significant differences among groups within the same age (p ≤ 0.05, Tukey–Kramer test).
Expression of muscle growth-related genes in M. longissimus
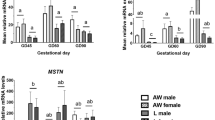
The expression of muscle growth-related genes was quantified in MLD and is presented in Fig. 3. Relative mRNA abundances of PAX7, MYOD and MYF5 were not affected by BiW, Gln supplementation or age (p > 0.1, Fig. 3a–c). The mRNA abundance of MYOG was higher in piglets at 26 dpn compared with animals at 5 dpn (p < 0.001, Fig. 3d). Moreover, MYOG tended to be less expressed in LBW-GLN than in NBW-GLN piglets (p = 0.067) at 26 dpn. The mRNA abundance of MSTN was greater in piglets at 26 dpn in comparison with piglets at 5 or 12 dpn (p < 0.05, Fig. 3e), but was not influenced by BiW or supplementation (p > 0.1). Expression of PPARGC1A tended to be lower in LBW-ALA piglets compared with NBW-ALA animals at 26 dpn (p = 0.080, Fig. 3f), although it was not altered by supplementation or age (p > 0.1).
Relative mRNA abundances of genes in M. longissimus. Expressions of PAX7 (a), MYOD (b), MYF5 (c), MYOG (d), MSTN (e) and PPARGC1A (f) were analyzed in MLD of glutamine (GLN) or alanine (ALA) supplemented low birth weight (LBW) and normal birth weight (NBW) piglets at 5, 12 and 26 dpn (n = 12 per group). Values were normalized to YHWAZ and PPIA expression and are presented as LSmeans and SE. Different uppercase letters (A, B) indicate significant differences among ages (p ≤ 0.05, Tukey–Kramer test), #trend among groups within the same age (0.05 < p ≤ 0.1).
Effects of supplementation on proliferation of cultured myogenic cells
A highly pure and vital population of satellite cells can be obtained with the established protocol developed in our lab35, 36. Those cells were used to conduct three independent xCELLigence assays, which demonstrated a high repeatability of the cell growth measurements. Cell indexes (CI) were continuously recorded every 15 min for each treatment over 96 h as exemplarily shown for one experiment with intervals of 1 h in Fig. 4. Low concentrations of supplements had no clear effect on recorded CI of myogenic cells. In NBW myogenic cells, CI curves were higher in cells with Gln supplementation (5 and 10 mM) compared with corresponding cells with alanine (Ala, 5 and 10 mM) or no supplementation. Of note, the basic medium (control) contained 0.28 mM Gln and Ala to ensure normal cell growth. Nevertheless, this condition was referred to as “0” (no supplementation) in the following tables and figures. Cells from LBW piglets had lower CI under the same supplementation than the cells from NBW piglets, but could catch up growth with NBW cells at final time points when supplemented with Gln at 5 or 10 mM. The CI curve of Ala (5 and 10 mM) supplemented LBW cells plateaued earlier than that of corresponding Gln supplemented cells.
Cell indexes of proliferating myogenic cells. Cell indexes were recorded for myogenic cells with the xCELLigence RTCA-DP device. Cells, isolated from M. longissimus of low birth weight (LBW) and normal birth weight (NBW) piglets at 4 dpn, were supplemented with 0.5 mM Gln or Ala (a), 5 mM Gln or Ala (b), 10 mM Gln or Ala (c) and compared with myogenic cells without supplementation (no suppl.). The basic medium contained 0.28 mM Gln and Ala. All values are presented as Means and SD (standard deviations) of three repetitive wells.
Maximal cell indexes (CIMax) in xCELLigence assays were reached by NBW cells about 7 h earlier then by LBW cells (p = 0.068, Table 1). The CI8–48 h (mean CI between 8 and 48 h) and the CI56–78 h (mean CI between 56 and 78 h) were calculated as measures for the proliferation of myogenic cells before and after medium change, respectively. Independent of supplementation, CI8–48 h or CI56–78 h of LBW cells was lower or tended to be lower compared with that of NBW cells at concentrations of 0 and 0.5 mM supplementation (Table 1). The CI56–78 h of 10 mM Gln supplemented cells was greater than that of 10 mM Ala supplemented cells (p = 0.038). Stimulation effects of different supplementations on myogenic cells were normalized to corresponding cells without supplementation from 8 to 48 h before medium change, or from 56 to 78 h before the curves reached plateau. The stimulation effect of supplements at 5 and 10 mM tended to be greater in LBW than NBW cells (p = 0.057 and p = 0.054, respectively) before medium change. Later, Gln supplementation stimulated the CI56–78 h in both cell types (Table 1). In particular in LBW cells, supplementation with 0.5, 5 and 10 mM Gln increased the CI56–78 h by 3.54%, 22.46% and 42.37%, respectively. The greater stimulation effect of 10 mM compared to 0.5 mM in LBW cells (p = 0.006) indicates dose dependency. In comparison, supplementation with the same concentrations of Ala increased the CI56–78 h by 1.98%, 15.95% and 20.83%, respectively. In NBW cells (Table 1), Gln supplementation with 0.5, 5 and 10 mM enhanced the CI56–78 h by 7.53%, 7.80% and 31.91%, whereas the same concentrations of Ala affected CI56–78 h by − 8.25%, − 2.80% and − 12.91%, respectively. The stimulation effect of supplements at 5 and 10 mM was greater in LBW than NBW cells (p = 0.028 and p = 0.044, respectively).
Expression of muscle growth-related genes in proliferating myogenic cells
To further investigate the effect of supplementation on the transcriptional level of muscle growth-related genes in proliferating cells, LBW or NBW myogenic cells were cultivated in growth medium supplemented with 0 or 10 mM Gln or Ala (Fig. 5a). The supplementation of 10 mM was chosen according to the xCELLigence assay results, where 10 mM Gln stimulated the CI to the highest degree. The mRNA abundances (Fig. 5b) of PAX7, MYOD, MYOG and PPARGC1A were quantified in proliferating LBW or NBW cells at day 2 and day 3 after seeding. However, no supplementation effects were observed on the mRNA level of these potential growth-related genes (p > 0.1).
Expression of muscle growth-related genes in proliferating porcine myogenic cells. (a) Representative microscopic images of cells of the same sample at day 2 and day 3 without supplementation or with 10 mM Gln or Ala. Scale bars represent 200 µm. (b) Relative mRNA abundances of PAX7, MYOD, MYOG and PPARGC1A in proliferating cells of low (LBW) or normal birth weight (NBW) piglets at day 2 and day 3 after seeding. Values were normalized to YHWAZ and PPIA expression and are presented as LSmeans and SE (n = 3 per group).
Expression of muscle growth-related genes in differentiating myogenic cells
Myogenic cells were induced to differentiate after reaching confluence and were harvested at day 0 (day of differentiation induction), day 3 or day 6 after induction (Fig. 6a). Relative mRNA abundances of PAX7, MYOD, MYOG and PPARGC1A were determined in differentiating LBW and NBW cells (Fig. 6b). The results indicated no influence of supplementation on relative mRNA abundances of PAX7, MYOD and MYOG at all three time points, neither in LBW nor in NBW myogenic cells (p > 0.1). Expression of MYOG in NBW cells tended to be higher (p = 0.052) at day 3 in comparison with day 0 with 10 mM Ala supplementation and was higher (p = 0.016) with 10 mM Gln supplementation. In differentiating LBW cells at day 3, relative mRNA abundance of PPARGC1A was higher in 10 mM Gln supplemented cells than that in 10 mM Ala supplemented cells (p = 0.044). This supplementation effect was not found in NBW differentiating myogenic cells (p = 0.160). Furthermore, PPARGC1A abundance tended to decrease from day 0 to day 6 in both LBW and NBW cells supplemented with 10 mM Ala (p = 0.065 and p = 0.081), and tended to decrease in LBW cells from day 0 to day 3 with 10 mM Ala (p = 0.085) or no supplementation (p = 0.051).
Expression of muscle growth-related genes in differentiating porcine myogenic cells. (a) Representative microscopic images of cells of the same sample at day 0 (day of differentiation induction), day 3 and day 6 without supplementation or with 10 mM Gln or Ala. Scale bars represent 200 µm. (b) Relative mRNA abundances of PAX7, MYOD, MYOG and PPARGC1A in myogenic cells from low (LBW) or normal birth weight (NBW) piglets at day 0, day 3 and day 6 after induction of differentiation. Values were normalized to YHWAZ and PPIA expression and are presented as LSmeans and SE (n = 3 per group). Different lowercase letters indicate significant differences among groups within the same time point, different uppercase letters indicate significant differences (p ≤ 0.05, Tukey–Kramer test) and #trend for differences (0.5 < p ≤ 0.1) among different time points.
Discussion
The current study aimed at clarifying whether oral Gln supplementation affected skeletal muscle growth in LBW and NBW piglets during the early postnatal period. Glutamine supplementation could improve muscle growth in two different ways, either through direct stimulation of muscle satellite cell differentiation and fusion with muscle fibers or indirectly through enhanced nutrient accretion and supply because of improved intestinal function. The current study focused on direct effects of Gln on skeletal muscle. In our previous study, we observed an influence of Gln supplementation on muscle fiber size in MLD of these piglets16. If the increase in muscle fiber size was caused by a stimulatory effect on myogenic precursor cells, this could further improve the muscle fiber growth in the long term. Therefore, the current investigation was focused on the muscle cell development in vivo and in vitro and involved muscle growth-related genes. Our study indicates that LBW piglets supplemented with Gln had more total and BrdU-positive nuclei compared with Ala supplemented animals at 5 dpn, but not at 12 or 26 dpn. However, this effect was not observed in NBW piglets. It could be speculated that compared with NBW piglets, LBW piglets might use more Gln to compensate the growth delay by increased cell proliferation and/or differentiation of precursor cells. Beside myogenic precursor cells, preadipocytes may also be stimulated leading to enhanced fat deposition in LBW animals later in life. In our previous study, we observed that the total muscle nuclei number decreased with age, whereas the muscle fiber size increased16. In the current study, we observed a stronger reduction of the number of proliferating cells in older piglets as indicated by much less BrdU-positive nuclei. Accordingly, studies of Cardasis and Cooper37 and Mesires et al.24 reported that the number of satellite cell nuclei is reduced with age in relation to the total nuclei number. Thus, stimulation of satellite cell proliferation could be more effective in younger animals. However, skeletal muscle cells represent a heterogeneous population of cells within muscle tissue, beside muscle fibers and satellite cells there are fibroblasts, preadipocytes, adipocytes, endothelial and immune cells, etc. Fully differentiated cells, such as adipocytes and muscle fibers, are no longer able to proliferate in contrast to precursor cells38. Muscle fibers occupy an increasing part of the muscle cross sectional area during growth leading to a decreasing number of nuclei per area unit39. Our previous study has shown that intramuscular Gln availability was only increased in piglets at 5 dpn upon supplementation in NBW piglets, but not in LBW piglets16.
Gene expression of PAX7, MYOD, MYF5, MYOG, PPARGC1A and MSTN was quantified in MLD to elucidate the effect of Gln supplementation on these regulators of muscle growth. The role of these genes in the process of muscle development is well established, as reviewed by Zammit et al.29, 40, Yin et al.41, Schmidt, et al.23, Aiello et al.42 and Liu et al.43. The results of our study indicated that BiW or supplementation did not influence mRNA abundance of PAX7, MYOD, MYF5 and MSTN. The expression of PAX7 suggests that the quiescent or activated satellite cells were not altered upon supplementation or BiW differences, because PAX7 was reported to be expressed in quiescent satellite cells, but it was co-expressed with MYOD when they were activated23, 41. Furthermore, MYF5 and MYOD are involved in satellite cell proliferation and differentiation41, and loss of the two genes led to failure of muscle regeneration44. Expression of both genes was not altered in the current study. This is consistent with the results of BrdU analysis indicating that the number of total nuclei and BrdU-positive nuclei in regions exclusively filled with muscle fibers was not altered by BiW, supplementation or age. In accordance with muscle growth, transcription levels of MYOG and MSTN were higher with greater protein deposition within MLD in piglets at 26 dpn than at 5 dpn3. Besides, the tendency of increased MYOG mRNA in NBW-GLN compared with LBW-GLN piglets at 26 dpn suggests that activated satellite cells from NBW piglets had more potential to differentiate and fuse with myofibers compared with those in LBW animals, but whether the effect was regulated by Gln supplementation could not be determined. Piglets of the NBW-ALA group tended to have more PPARGC1A mRNA in comparison with LBW-ALA counterparts at 26 dpn, suggesting that there might be more oxidative, slow muscle fibers formed in NBW-ALA piglets45. Expression data of PPARGC1A point to the same direction like MYH7 protein abundances in the same animals reported by Zhao et al.16. Higher protein abundances of MYH7, representing slow, oxidative or type I fibers, were observed in NBW-ALA compared with LBW-ALA piglets at 26 dpn within MST16. Furthermore, PPARGC1A is vital for mitochondria content46, indicating LBW piglets supplemented with Ala but not Gln tended to have lower oxidative capacity within skeletal muscle compared with their NBW littermates. A recent study reported that PAX7, MYOD, MYF5 and MYOG within M. semitendinosus were downregulated in female piglets suffering from intrauterine growth retardation (IUGR) compared with normal pigs from birth to 100 dpn47. The discrepancy with our study might result from gender, developmental stage of pigs, and the BiW definition of LBW pigs.
To verify the effects of different concentrations of Gln in cultured myogenic cells from skeletal muscle, an in vitro model was applied using cell pools generated from MLD of 2 LBW or 2 NBW piglets at 4 dpn, respectively. Cell pooling helped to ameliorate the biological variance among animals and was necessary to overcome the shortage of isolated satellite cells from single animals48. The proliferation capacity of cultured myogenic cells was investigated in three independent experiments using the xCELLigence device. Continuous real-time monitored CI reveal the adhesion and proliferation of the cells49. The results indicated a general effect of BiW independent of supplementation, indicating that the myogenic cells from LBW piglets had lower proliferation capacity. Nissen et al.50 likewise observed a lower number of viable cells after 3 days of cultivation of LBW porcine satellite cells isolated from M. semimembranosus compared with cells from normal or high birth weight animals. Additionally, fewer myogenic cells could be isolated per gram MLD from 4-day-old LBW piglets than from muscle of their NBW littermates51. The lower number and delayed growth of LBW myogenic cells might be the reason of retarded body growth and prolonged finishing time of LBW pigs2. Regarding the concentration effects of Gln supplementation on cultured muscle cells, Wu and colleagues reported a positive effect of Gln concentration applied in cultured chicken skeletal muscle and 15 mM Gln stimulated protein synthesis in cultured muscle cells by 58% or 19% in the presence or absence of tyrosine, respectively52. In line with their results, cultured myogenic cells from both NBW and LBW piglets, in the current study, exhibited the highest stimulation effects from 56 to 78 h with 10 mM Gln supplementation compared with 10 mM Ala, in the presence of 36 mg/L tyrosine. This confirms the positive dosage-dependent effects of Gln in cultured porcine primary muscle cells.
Since 10 mM Gln was most effective in promoting proliferation of cells in the xCELLigence assays, we applied the same supplementary concentration of Gln to the satellite cells during proliferation and differentiation in comparison to cells cultivated without supplementation or with 10 mM Ala. Expression of myogenic regulatory factors (MYOD, MYOG) as well as PAX7 and PPARGC1A was quantified in the proliferating and differentiating myogenic cells as indicators for growth. However, no significant effect of supplementation was observed in the expression of those genes during the proliferation period. This suggests that these regulators were not involved in the effects of Gln supplementation on proliferating cells observed in xCELLigence assays. Moreover, expression of MYOG in NBW cells supplemented with 10 mM Gln increased from day 0 to day 3 after induction of differentiation and then tended to decrease from day 3 to day 6, indicating more cells in terminal differentiation at day 3 after induction41. Similarly, there was a trend for higher MYOG mRNA abundance at day 3 compared with day 0 of differentiation induction in NBW cells with Ala supplementation. However, this effect was not observed in LBW cells. Thus, this suggests that Gln has only minor positive effects on cell differentiation. Fewer LBW cells were differentiated compared with NBW cells under the same conditions, indicating the general developmental delay of these cells that could partly explain the retarded growth of LBW piglets in concordance with Rehfeldt et al.2. Expression of PPARGC1A tended to decrease from day 0 to day 6 in LBW and NBW cells with 10 mM Ala and from day 0 to day 3 in LBW cells without supplementation, suggesting a trend for decreasing oxidative activities in cells without Gln supplementation according to Rowe et al.46. Notably, in differentiating myogenic cells at day 3 after induction, PPARGC1A expression was higher in LBW myogenic cells supplemented with 10 mM Gln compared with 10 mM Ala, indicating that Gln supplementation supported higher oxidative activities45, 46. This is consistent with the in vivo analysis of PPARGC1A expression in the current study.
We observed a dose dependent stimulation effect of Gln supplementation in xCELLigence assays with the largest effect at 10 mM Gln. However, this is much higher than the physiological concentration of plasma Gln in pigs (~ 0.5 mM) that could be increased by oral administration of 0.5 g/kg BW twice daily to ~ 0.7 mM53. Thus, we applied a supplemental concentration of 0.5 mM Gln (0.78 mM final concentration) in the cell culture similar to the reported plasma concentration after supplementation53. This Gln concentration, however, induced no clear effects. Given the fact, that even this concentration may not be maintained in plasma of piglets after supplementation once daily, it might explain why the Gln supplementation to the animals had only minor effects in promoting skeletal muscle development in vivo16. The intramuscular concentration of free Gln was determined between 1 and 9 mmol/kg fresh matter for piglets in our study16, with highest values upon Gln supplementation. The Gln concentration may be high enough to stimulate cell proliferation in muscle tissue in a short term, but it is not feasible to keep the intramuscular Gln concentration at such a high level in a live animal in a long term. This may be due to the short half-life of Gln in blood (0.65–0.7 h according to Wu et al.54). Consequently, the elevated intramuscular Gln concentration upon supplementation was only observed in piglets at 5 dpn, indicating that the oral supplementation might have only minor modulating effects for the intramuscular Gln concentration and thus for the stimulation of satellite cells. Furthermore, cultured satellite cells function in a different way as those in vivo because they lack regulation by niche environment including regulating niche factors and complex signaling pathways41, 55. Thus, the high proliferation possibility of satellite cells might be restricted to in vitro conditions, although supplementation in vivo was applied during the early postnatal period of piglets. Our study indicated that LBW myogenic cells caught up growth upon Gln supplementation to the level of NBW cells in the xCELLigence assays, but this could not be confirmed in vivo in LBW compared to NBW piglets.
Taken together, Gln supplementation stimulated proliferation of different, undefined cell types within MLD of early postnatal piglets. However, the mRNA abundances of muscle growth-related genes were not affected by Gln supplementation and were only slightly influenced by BiW. Glutamine supplementation promoted proliferation of cultured myogenic cells, isolated from M. longissimus of LBW and NBW piglets in a dose dependent manner. In conclusion, the oral Gln supplementation has some potential to improve muscle cell development, but the positive effects of applicable doses are not clear enough to justify the higher effort.
Methods
All experimental procedures and animal care were carried out in agreement with the instructions of European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (2010/63/EU) and were approved by the ethics committee of the State Office for Agriculture, Food Safety and Fisheries Mecklenburg-Western Pomerania, Germany (permission No. 7221.3-1-026/16). The study was carried out in compliance with the ARRIVE guidelines56.
Animals and sampling
The current study comprised 144 male German Landrace piglets, littermates with low or normal birth weight as described recently16. The piglets were assigned to four groups as LBW (n = 72) or NBW (n = 72) piglets, supplemented with Gln (1 g/kg body weight) or an isonitrogeneous amount (1.22 g/kg body weight) of Ala (group names: LBW-GLN, LBW-ALA, NBW-GLN and NBW-ALA). Twelve piglets per group were stunned by captive bolt and exsanguinated at 5, 12 and 26 dpn. One hour before slaughter, the piglets were injected intraperitoneally with BrdU (12 mg/kg body weight, Merck, Darmstadt, Germany). Tissue of MLD was sampled after slaughter from the left side of the carcass, cut into small pieces, snap frozen in liquid nitrogen, and stored in − 80 °C until further analysis.
Additionally, untreated male and female German Landrace piglets were used to isolate myogenic progenitor cells from MLD for primary cell culture as described below. Cells from 6 piglets with LBW (0.92 ± 0.03 kg) and their respective NBW littermates (1.38 ± 0.03 kg) at 4 or 5 dpn were used for proliferation assays with xCELLigence (RTCA-DP, ACEA Biosciences, San Diego, CA, USA) and for cell proliferation and differentiation assays.
Immunohistochemistry
Muscle sections from MLD were cut 8 µm thick with a cryostat microtome (CM3050 S, Leica, Bensheim, Germany). The sections were fixed in 4% paraformaldehyde solution for 20 min, washed 2 × 5 min with PBS (phosphate-buffered saline) and permeabilized with 0.1% TritonX-100 (Sigma-Aldrich, Munich, Germany) in PBS for 10 min. Then, the slides were incubated with 2 N HCl at 37 °C for 60 min to denature DNA. After washing 3 × 5 min with PBST, nonspecific bindings of the secondary antibody were blocked with 10% normal goat serum (NGS) in PBST for 15 min at room temperature. The slides were incubated overnight at 4 °C with the primary mouse anti-BrdU antibody (1:100 in PBST incl. 1% NGS) in a humidity chamber. After washing 3 × 10 min with PBST, slides were incubated with the secondary antibody (Alexa Fluor 488 goat anti-mouse IgG, 1:1000 in PBST, Thermo Fisher Scientific, Schwerte, Germany) for 45 min at room temperature in the dark and then washed 3 × 5 min with PBST. Nuclei were counterstained with propidium iodide (PI, 5 µg/mL, Sigma-Aldrich) in the dark for 10 min. Finally, slides were washed 1 × 5 min with PBS and 1 × 10 min with distilled water and covered with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific) and coverslips (Roth, Karlsruhe, Germany). Immunofluorescence of BrdU and PI stained nuclei was detected using a Nikon Microphot SA fluorescence microscope (Nikon, Duesseldorf, Germany) equipped with a CC-12 color camera (OSIS, Münster, Germany) and Cell^F image analysis software (OSIS). Two macro programs were developed to determine either the total nuclei area percentage (stained red) as well as the area percentage of BrdU-positive nuclei (stained green), or the number of muscle fiber nuclei (nuclei within muscle fibers and nuclei of satellite cells) and corresponding BrdU-positive nuclei. Eight randomly selected pictures, of approximately 2.1 mm2 in total, were analyzed for nuclei area percentages and five regions, of about 0.32 mm2 in total, for nuclei numbers from each piglet. The ratios between BrdU-labeled areas or nuclei numbers and total nuclei area or numbers, respectively, were determined as measures of intramuscular cell proliferation.
Isolation of myogenic progenitor cells
The cell isolation protocol was adapted from Mau et al.57 with moderate adjustments. Briefly, muscle tissue of MLD was collected immediately after slaughter from LBW or NBW piglets and kept in the isolation medium, which consisted of 80% PBS-D (144 mM NaCl, 5.4 mM KCl, 25 mM glucose, 14 mM sucrose, 5 mM Na2HPO4, 0.5% penicillin–streptomycin (10,000 U/mL penicillin, 10 mg/mL streptomycin, PAN-Biotech, Aidenbach, Germany), and 1 mg/L phenol red, pH 7.4), 10% penicillin–streptomycin and 10% amphotericin B (250 μg/mL). Then, the MLD tissue was rinsed with PBS-M (137 mM NaCl, 2.7 mM KCl, 3.2 mM Na2HPO4, pH 7.4) with antibiotics and 70% ethanol. After removal of visible extraneous tissue, the MLD tissue was minced into pieces, as small as possible, with scissors in a petri dish with 10 mL HBSS (PAN-Biotech). The muscle pulp was digested with 100 mL trypsin solution (100 mL 4232 U/mL trypsin, Sigma-Aldrich and 5.8 ml HBSS) per 50 g muscle tissue at 37 °C for 1 h with a sterilized magnetic stirrer at 250 rpm. Then, the digestion solution was transferred to falcon tubes, equal amounts of growth medium (77.5% Alpha MEM Eagle (Sigma-Aldrich), 20% fetal bovine serum (FBS, Gibco), 1% penicillin–streptomycin, 1% amphotericin B and 0.5% gentamicin) were added and the tubes were centrifuged at 800 × g for 10 min. After centrifugation, the pellet without supernatant was resuspended with growth medium and filtered with a funnel and sterilized gauze together with the resuspension from the last step. The filtrate was centrifuged at 1200 × g and 4 °C for 15 min. Myogenic precursor cells were isolated with percoll (GE Healthcare Life Sciences, Freiburg, Germany) density gradient centrifugation (100%, 70% and 40%). Cells at interface between 40 and 70% of the gradient layers were recovered, diluted with growth medium in a new tube and centrifuged. The pellet was resuspended with growth medium and the cell number was counted with an automated cell counter (Thermo Fisher Scientific). Previous studies demonstrated that the vast majority of cells isolated with this method are satellite cells that are able to differentiate. Abundance of myogenic markers were demonstrated with flow cytometry and the ability to terminally differentiate and generate myotubes was shown35, 36. For xCELLigence assays (see below), myogenic cells from single animals were seeded in 10 cm collagen-coated culture dishes (Greiner Bio-One, Kremsmünster, Germany) and incubated at 37 °C, 6% CO2 and 95.5% humidity. For proliferation and differentiation assays, cells from the same birthweight (BiW) group (4 donors per group) were pooled after isolation and cultivated under the same condition as aforementioned. Growth medium was replaced after 24 h. After 4 days, cells were collected using trypsin/EDTA solution in PBS (0.05%/0.02%, Biochrom, Berlin, Germany) and mixed with equal amounts of freezing solution containing 50% culture medium, 20% dimethyl sulfoxide (DMSO, SERVA, Heidelberg, Germany) and 30% FBS and stored in cryo tubes. The cells were immediately stored at − 80 °C for 24 h and then transferred to liquid nitrogen for long-term storage. Frozen cells were used for the subsequent cell culture experiments.
Monitoring of growth kinetics with xCELLigence
Growth medium without Gln including 77.5% Gln-free Alpha MEM Eagle (Sigma-Aldrich), 20% FBS, 1% penicillin–streptomycin, 1% amphotericin B and 0.5% gentamicin, was prepared for different concentrations of Gln and Ala growth medium. To encounter the same concentration of 0.28 mM Ala in the basic commercial Alpha MEM Eagle medium, Gln concentration was adjusted to 0.28 mM with 200 mM liquid Gln solution (Sigma-Aldrich) as well. Thus, the basic medium with no supplementation of Gln could still supply the cells with a certain small amount of Gln to sustain the cell growth and mimic the physiological environment of muscle cells. The different supplementation concentrations (0.5, 5, 10 mM) of Gln or Ala (Sigma Aldrich) were added to the original medium with 0.28 mM Gln and Ala. Frozen LBW or NBW myogenic cell suspensions were quickly thawed in a 37 °C water bath. Then, the cells from two donors with similar BiW were pooled and seeded in 10 cm collagen-coated culture dishes with growth medium, without Gln supplementation. After 3 days, the cells were harvested with trypsin/EDTA solution in PBS and prepared for xCELLigence experiments. In brief, 100 µl warm growth medium with different concentrations of Gln or Ala (0, 1, 10 and 20 mM, which was diluted later to 0, 0.5, 5 and 10 mM with resuspended cells) was added to the xCELLigence E-plate (OMNI Life Science, Bremen, Germany), and the E-plate was placed on the xCELLigence RTCA-DP instrument. The background data of the medium without cells was measured with a RTCA Software 2.0 (ACEA Biosciences). Then, the cells resuspended with 100 µl growth medium without Gln were seeded into the wells (1 × 104 cells per well) of the xCELLigence E-plate and monitored with the RTCA software under the same condition as cultivation after cell isolation. Each supplementation was performed in triplicates in three independent xCELLigence assays. The whole xCELLigence assay was continuously monitored and the CI was recorded every 15 min over a time period of 96 h. The growth medium with different concentrations of supplementation was changed after 2 days.
Cell proliferation and differentiation assays
Myogenic cells were cultivated and harvested at different time points during proliferation and after induction of differentiation to determine gene expression of myogenic genes. Cell pools from LBW and NBW piglets, respectively, were thawed and seeded on Matrigel (Corning, New York, USA) coated 24-well plates (Sarstedt, Nümbrecht, Germany) with 5 × 104 cells in each well. For proliferation, cells were cultivated with 0 mM Gln, 10 mM Gln or 10 mM Ala supplemented growth medium. Cells were collected with Qiazol lysis reagent (Qiagen, Hilden, Germany) at day 2 and 3 after seeding. For differentiation, cells were cultivated in the same way during the proliferation period until day 3. At day 3 (80–90% confluence of cells), differentiation medium (96% Gln-free Alpha MEM medium, 2% FBS, 1% penicillin–streptomycin, 1% amphotericin B and 0.28 mM Gln) supplemented with 0 mM Gln, 10 mM Gln or 10 mM Ala was applied to the cells after washing once with PBS. The differentiation medium was replaced every 3 days. The myogenic cells were collected with Qiazol lysis reagent at days 0, 3 and 6 after induction of differentiation. For each plate, duplicate wells of supplementation were performed in three independent cultivation experiments.
RNA isolation and cDNA synthesis
Muscle RNA was extracted with an RNeasy Fibrous Tissue Mini Kit (Qiagen) from 70 to 90 mg of MLD, while RNA of cultured myogenic cells was isolated with Qiazol lysis reagent following the standard protocol. All RNA was stored in − 80 °C until subsequent analysis. A NanoDrop ND-1000 spectrophotometer (Peqlab, Erlangen, Germany) was used to determine RNA concentration; and RNA integrity was determined using the Experion Automated Electrophoresis System and the RNA StdSens analysis chip (Bio-Rad, Munich, Germany) following the manufacturer’s instructions. Then, cDNA was synthesized with an iScript cDNA synthesis kit (Bio-Rad) in a 20 μL reaction volume from 1000 ng (muscle tissue) or 600 ng RNA (myogenic cells) and stored in − 20 °C. Primers of reference and target genes were adopted from literature58,59,60,61,62,63, shown in Table 2. All primers were synthesized by a commercial company (Sigma-Aldrich). The annealing temperature of all primers was 60 °C. Qualitative polymerase chain reaction (PCR) was performed and the products were subjected to agarose gel electrophoresis to test the primers and products.
Quantitative PCR (qPCR)
The qPCR was performed with FastStart Essential DNA Green Master using a LightCycler 96 real-time qPCR system (Roche, Basel, Switzerland) as described elsewhere64. Quantitation cycle (Cq) value was calculated by the LightCycler 96 system software. All samples were measured in duplicates. Efficiencies of amplifications were analyzed with standard curves from qPCR with serial cDNA dilutions (1, 1/10, 1/50, 1/100, 1/200) and were within 1.8–2.1. The mRNA abundance of target genes was normalized to two reference genes, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ)58 and peptidylprolyl isomerase A (PPIA)59, and was calculated as normalized relative quantities (NRQ)65.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) with the MIXED procedure of SAS statistical software (Version 9.4, SAS Inst., Cary, USA). For animal data analyses, BiW (LBW, NBW), supplementation (Gln, Ala), age (5, 12, 26 dpn) and their respective interactions were included as fixed factors and sow as random factor. The SLICE statement was used to enable the partitioned analysis of the least-squares means (LSmeans) for the interaction between BiW and supplementation within the same age. For analysis of xCELLigence assays, BiW (LBW, NBW), supplementation (Gln, Ala), concentration of supplementation (0, 0.5, 5, 10 mM), and their respective interactions were included as fixed factors. Cell indexes in time intervals before (8–48 h) and after (56–78 h) medium change as well as the respective stimulation effects were analyzed using the same model, but as repeated measurements with “compound symmetry” as covariance structure and group effect BiW × supplementation × concentration. For analysis of myogenic cell proliferation and differentiation assays, BiW (LBW, NBW), supplementation (no supplementation, Gln, Ala), day of proliferation (2, 3) or differentiation (0, 3, 6) and their respective interactions were considered as fixed factors. The SLICE statement of the MIXED procedure was used to enable the partitioned analysis of the LSmeans for the interaction between supplementation within the same cultivation day. Tukey–Kramer test was applied to analyze pairwise differences. Values are presented as LSmeans and standard errors (SE). Differences were considered significant if p ≤ 0.05, or a trend if 0.05 < p ≤ 0.1.
References
Wu, G., Bazer, F. W., Wallace, J. M. & Spencer, T. E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 84, 2316–2337. https://doi.org/10.2527/jas.2006-156 (2006).
Rehfeldt, C. & Kuhn, G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J. Anim. Sci. 84(Suppl), E113-123. https://doi.org/10.2527/2006.8413_supple113x (2006).
Davis, T. A. & Fiorotto, M. L. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care 12, 78–85. https://doi.org/10.1097/MCO.0b013e32831cef9f (2009).
Wilson, F. A. et al. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J. Nutr. 139, 1873–1880. https://doi.org/10.3945/jn.109.106781 (2009).
Jamin, A., Seve, B., Thibault, J. N. & Floc’h, N. Accelerated growth rate induced by neonatal high-protein milk formula is not supported by increased tissue protein synthesis in low-birth-weight piglets. J. Nutr. Metab. 2012, 545341. https://doi.org/10.1155/2012/545341 (2012).
Wan, H. et al. Dietary supplementation with beta-hydroxy-beta-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets. Br. J. Nutr. 115, 1360–1369. https://doi.org/10.1017/S0007114516000465 (2016).
Madsen, J. G., Seoni, E., Kreuzer, M., Silacci, P. & Bee, G. Influence of l-carnitine and l-arginine on protein synthesis and maturation of the semitendinosus muscle of lightweight piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 102, 440–451. https://doi.org/10.1111/jpn.12765 (2018).
Davis, T. A., Suryawan, A., Orellana, R. A., Nguyen, H. V. & Fiorotto, M. L. Postnatal ontogeny of skeletal muscle protein synthesis in pigs. J. Anim. Sci. 86, E13-18. https://doi.org/10.2527/jas.2007-0419 (2008).
Wu, G. & Knabe, D. A. Free and protein-bound amino acids in sow’s colostrum and milk. J. Nutr. 124, 415–424. https://doi.org/10.1093/jn/124.3.415 (1994).
Curi, R. et al. Molecular mechanisms of glutamine action. J. Cell Physiol. 204, 392–401. https://doi.org/10.1002/jcp.20339 (2005).
Li, P. et al. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids 37, 709–716. https://doi.org/10.1007/s00726-008-0196-5 (2009).
Burrin, D. G. & Reeds, P. J. Alternative fuels in the gastrointestinal tract. Curr. Opin. Gastroenterol. 13, 165–170 (1997).
Cory, J. G. & Cory, A. H. Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: Asparaginase treatment in childhood acute lymphoblastic leukemia. In Vivo 20, 587–589 (2006).
Flynn, N. E. & Wu, G. An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am. J. Physiol. 271, R1149-1155. https://doi.org/10.1152/ajpregu.1996.271.5.R1149 (1996).
Wu, G. et al. Triennial Growth Symposium: Important roles for l-glutamine in swine nutrition and production. J. Anim. Sci. 89, 2017–2030. https://doi.org/10.2527/jas.2010-3614 (2011).
Zhao, Y. et al. Effects of oral glutamine supplementation on early postnatal muscle morphology in low and normal birth weight piglets. Animals (Basel) 10, 2020. https://doi.org/10.3390/ani10111976 (1976).
Rudar, M., Fiorotto, M. L. & Davis, T. A. Regulation of muscle growth in early postnatal life in a swine model. Annu. Rev. Anim. Biosci. 7, 309–335. https://doi.org/10.1146/annurev-animal-020518-115130 (2019).
Moss, F. P. & Leblond, C. P. Nature of dividing nuclei in skeletal muscle of growing rats. J. Cell Biol. 44, 459–462. https://doi.org/10.1083/jcb.44.2.459 (1970).
Shamim, B., Hawley, J. A. & Camera, D. M. Protein availability and satellite cell dynamics in skeletal muscle. Sports Med. 48, 1329–1343. https://doi.org/10.1007/s40279-018-0883-7 (2018).
Moss, F. P. & Leblond, C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 170, 421–435. https://doi.org/10.1002/ar.1091700405 (1971).
Wang, X. Q. et al. The differential proliferative ability of satellite cells in Lantang and Landrace pigs. PLoS ONE 7, e32537. https://doi.org/10.1371/journal.pone.0032537 (2012).
Sambasivan, R. & Tajbakhsh, S. Skeletal muscle stem cell birth and properties. Semin. Cell Dev. Biol. 18, 870–882. https://doi.org/10.1016/j.semcdb.2007.09.013 (2007).
Schmidt, M., Schuler, S. C., Huttner, S. S., von Eyss, B. & von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell Mol. Life Sci. 76, 2559–2570. https://doi.org/10.1007/s00018-019-03093-6 (2019).
Mesires, N. T. & Doumit, M. E. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am. J. Physiol. Cell Physiol. 282, C899–C906. https://doi.org/10.1152/ajpcell.00341.2001 (2002).
Zammit, P. S. et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119, 1824–1832. https://doi.org/10.1242/jcs.02908 (2006).
Patruno, M., Caliaro, F., Martinello, T. & Mascarello, F. Expression of the paired box domain Pax7 protein in myogenic cells isolated from the porcine semitendinosus muscle after birth. Tissue Cell 40, 1–6. https://doi.org/10.1016/j.tice.2007.08.006 (2008).
Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786. https://doi.org/10.1016/s0092-8674(00)00066-0 (2000).
Ropka-Molik, K., Eckert, R. & Piórkowska, K. The expression pattern of myogenic regulatory factors MyoD, Myf6 and Pax7 in postnatal porcine skeletal muscles. Gene Expr. Patterns 11, 79–83. https://doi.org/10.1016/j.gep.2010.09.005 (2011).
Zammit, P. S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 72, 19–32. https://doi.org/10.1016/j.semcdb.2017.11.011 (2017).
Almeida, C. F., Fernandes, S. A., Ribeiro Junior, A. F., Keith Okamoto, O. & Vainzof, M. Muscle satellite cells: Exploring the basic biology to rule them. Stem Cells Int. 2016, 1078686. https://doi.org/10.1155/2016/1078686 (2016).
Liu, X. et al. Maternal dietary protein affects transcriptional regulation of myostatin gene distinctively at weaning and finishing stages in skeletal muscle of Meishan pigs. Epigenetics 6, 899–907. https://doi.org/10.4161/epi.6.7.16005 (2011).
Doumit, M. E. & Merkel, R. A. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell 24, 253–262. https://doi.org/10.1016/0040-8166(92)90098-r (1992).
Qin, L. L. et al. Mechano growth factor (MGF) promotes proliferation and inhibits differentiation of porcine satellite cells (PSCs) by down-regulation of key myogenic transcriptional factors. Mol. Cell Biochem. 370, 221–230. https://doi.org/10.1007/s11010-012-1413-9 (2012).
Collins, C. A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301. https://doi.org/10.1016/j.cell.2005.05.010 (2005).
Miersch, C. et al. Molecular and functional heterogeneity of early postnatal porcine satellite cell populations is associated with bioenergetic profile. Sci. Rep.-UK 7, 45052. https://doi.org/10.1038/srep45052 (2017).
Miersch, C., Stange, K. & Röntgen, M. Separation of functionally divergent muscle precursor cell populations from porcine juvenile muscles by discontinuous Percoll density gradient centrifugation. BMC Cell Biol. 19, 2. https://doi.org/10.1186/s12860-018-0156-1 (2018).
Cardasis, C. A. & Cooper, G. W. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: A satellite cell-muscle fiber growth unit. J. Exp. Zool. 191, 347–358. https://doi.org/10.1002/jez.1401910305 (1975).
Baksh, D., Song, L. & Tuan, R. S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J. Cell Mol. Med. 8, 301–316. https://doi.org/10.1111/j.1582-4934.2004.tb00320.x (2004).
Schultz, E. & McCormick, K. M. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123, 213–257. https://doi.org/10.1007/BFb0030904 (1994).
Zammit, P. S., Partridge, T. A. & Yablonka-Reuveni, Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J. Histochem. Cytochem. 54, 1177–1191. https://doi.org/10.1369/jhc.6R6995.2006 (2006).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67. https://doi.org/10.1152/physrev.00043.2011 (2013).
Aiello, D., Patel, K. & Lasagna, E. The myostatin gene: An overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 49, 505–519. https://doi.org/10.1111/age.12696 (2018).
Liu, C. & Lin, J. D. PGC-1 coactivators in the control of energy metabolism. Acta Biochim. Biophys. Sin. (Shanghai) 43, 248–257. https://doi.org/10.1093/abbs/gmr007 (2011).
Yamamoto, M. et al. Loss of MyoD and Myf5 in skeletal muscle stem cells results in altered myogenic programming and failed regeneration. Stem Cell Rep. 10, 956–969. https://doi.org/10.1016/j.stemcr.2018.01.027 (2018).
Lin, J. et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801. https://doi.org/10.1038/nature00904 (2002).
Rowe, G. C. et al. Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle. Cell Rep. 3, 1449–1456. https://doi.org/10.1016/j.celrep.2013.04.023 (2013).
Felicioni, F. et al. Postnatal development of skeletal muscle in pigs with intrauterine growth restriction: Morphofunctional phenotype and molecular mechanisms. J. Anat. 236, 840–853. https://doi.org/10.1111/joa.13152 (2020).
Metzger, K., Tuchscherer, A., Palin, M. F., Ponsuksili, S. & Kalbe, C. Establishment and validation of cell pools using primary muscle cells derived from satellite cells of pig skeletal muscle. In Vitro Cell Dev. Biol. Anim. 56, 193–199. https://doi.org/10.1007/s11626-019-00428-2 (2020).
Kho, D. et al. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors (Basel) 5, 199–222. https://doi.org/10.3390/bios5020199 (2015).
Nissen, P. M. & Oksbjerg, N. In vitro primary satellite cell growth and differentiation within litters of pigs. Animal 3, 703–709. https://doi.org/10.1017/s1751731109003929 (2009).
Stange, K., Miersch, C., Sponder, G. & Rontgen, M. Low birth weight influences the postnatal abundance and characteristics of satellite cell subpopulations in pigs. Sci. Rep. https://doi.org/10.1038/s41598-020-62779-1 (2020).
Wu, G. & Thompson, J. R. The effect of glutamine on protein turnover in chick skeletal muscle in vitro. Biochem. J. 265, 593–598. https://doi.org/10.1042/bj2650593 (1990).
Haynes, T. E. et al. l-Glutamine or l-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37, 131–142. https://doi.org/10.1007/s00726-009-0243-x (2009).
Wu, G. et al. Functional amino acids in swine nutrition and production. In Dynamics in Animal Nutrition (ed. J. Doppenberg) 69–98 (Wageningen Academic Publishers, The Netherlands, 2010).
Pallafacchina, G. et al. An adult tissue-specific stem cell in its niche: A gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 4, 77–91. https://doi.org/10.1016/j.scr.2009.10.003 (2010).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412. https://doi.org/10.1371/journal.pbio.1000412 (2010).
Mau, M., Oksbjerg, N. & Rehfeldt, C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell Dev. Biol. Anim. 44, 1–5. https://doi.org/10.1007/s11626-007-9069-6 (2008).
Erkens, T. et al. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol. 6, 41. https://doi.org/10.1186/1472-6750-6-41 (2006).
Feng, X. et al. Selection of reference genes for gene expression studies in porcine skeletal muscle using SYBR green qPCR. J. Biotechnol. 150, 288–293. https://doi.org/10.1016/j.jbiotec.2010.09.949 (2010).
Patruno, M. et al. Myostatin shows a specific expression pattern in pig skeletal and extraocular muscles during pre- and post-natal growth. Differentiation 76, 168–181. https://doi.org/10.1111/j.1432-0436.2007.00189.x (2008).
Rehfeldt, C. et al. Limited and excess protein intake of pregnant gilts differently affects body composition and cellularity of skeletal muscle and subcutaneous adipose tissue of newborn and weanling piglets. Eur. J. Nutr. 51, 151–165. https://doi.org/10.1007/s00394-011-0201-8 (2012).
Maak, S., Wicke, M. & Swalve, H. Analysis of gene expression in specific muscles of swine and turkey. Arch. Anim. Breed 48, 135–140 (2005).
Liu, J. et al. Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS ONE 7, e34835. https://doi.org/10.1371/journal.pone.0034835 (2012).
Schering, L., Albrecht, E., Komolka, K., Kuhn, C. & Maak, S. Increased expression of thyroid hormone responsive protein (THRSP) is the result but not the cause of higher intramuscular fat content in cattle. Int. J. Biol. Sci. 13, 532–544. https://doi.org/10.7150/ijbs.18775 (2017).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19. https://doi.org/10.1186/gb-2007-8-2-r19 (2007).
Acknowledgements
We thank E. Schwitulla, S. Foß, F. Feldt, K. Gürtler, A. Berndt and M. Jugert-Lund for excellent technical support, Dr. S. Wimmers for lending the xCELLigence device, Dr. A. Tuchscherer for statistical advice, Dr. R. Pfuhl and the staff of the experimental slaughterhouse, as well as the EAS team.
Funding
Open Access funding enabled and organized by Projekt DEAL. Yaolu Zhao was funded by a China Scholarship Council (CSC) grant. The project was partly funded by Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (Grant number ME 1420/10-1).
Author information
Authors and Affiliations
Contributions
E.A. and C.C.M. designed the study; Y.Z., E.A. and K.S. performed the experiments; E.A. and S.M. supervised the investigation; Z.L., J.S. and Q.L.S. performed the animal experiments; Y.Z. and E.A. performed final analysis and drafted the manuscript; S.M., E.A., K.S., C.C.M., Z.L., J.S. and Q.L.S. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Albrecht, E., Stange, K. et al. Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets. Sci Rep 11, 13432 (2021). https://doi.org/10.1038/s41598-021-92959-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92959-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.