Abstract
Increasing evidence suggests that obstructive sleep apnea (OSA) is a metabolic syndrome-related disease; however, the association between nonalcoholic fatty liver disease (NAFLD) and OSA is not firmly established. In this study, we investigated the relationship between NAFLD and OSA in a general population drawn from a nationwide population-based cohort. Data from the Korean National Health Insurance System between January 2009 and December 2009 were analyzed using Cox proportional hazards model. NAFLD was defined as a fatty liver index (FLI) ≥ 60 in patients without excessive alcohol consumption (who were excluded from the study). Newly diagnosed OSA during follow-up was identified using claims data. Among the 8,116,524 participants, 22.6% had an FLI score of 30–60 and 11.5% had an FLI ≥ 60. During median follow-up of 6.3 years, 45,143 cases of incident OSA occurred. In multivariable analysis, the risk of OSA was significantly higher in the higher FLI groups (adjusted hazard ratio [aHR] 1.15, 95% confidence interval [CI] 1.12–1.18 for FLI 30–60 and aHR 1.21, 95% CI 1.17–1.26 for FLI ≥ 60). These findings were consistent regardless of body mass index and presence of abdominal obesity. In conclusion, a high FLI score may help identify individuals with a high risk of OSA. Understanding the association between NAFLD and OSA may have clinical implications for risk-stratification of individuals with NAFLD.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent collapse of the upper respiratory tract during sleep, leading to intermittent hypoxia, snoring, and sleep fragmentation. It is a prevalent disease, affecting 7–20% of the general population1 and up to 48–70% of obese populations2,3. Cumulative studies have shown that OSA is associated with various metabolic diseases, including hypertension4, glucose intolerance5, and dyslipidemia6, as well as increased all-cause mortality7.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. Its prevalence is increasing and is now as high as 20–30% in Asia8. NAFLD may progress in some instances to steatohepatitis, hepatic fibrosis, and cirrhosis9 and hepatic steatosis is frequently associated with other disorders, including cardiovascular disease10, diabetes11, chronic kidney disease12, and colorectal cancer13. Like OSA, NAFLD is closely related to obesity14. In particular, the hallmark of OSA—chronic intermittent hypoxia during sleep—has been associated with NAFLD15,16,17.
Previous studies regarding the association between NAFLD and OSA were conducted to investigate the risk of hepatic steatosis in patients with OSA. The risk of OSA in patients with NAFLD, however, has received little attention. Since the prevalence of diagnosed OSA in patients with NAFLD is relatively low in Asian countries18, it is difficult to evaluate the risk of OSA in patients diagnosed with NAFLD. Thus, we used a nationwide population-based cohort of participants in a health screening program to investigate the risk of incident OSA during follow-up in people with hepatic steatosis.
Methods
Data source
In this study, we obtained information from the database of the Korean National Health Insurance System (NHIS), which is the national insurer managed by the Korean government and to which approximately 97% of the Korean population subscribes19. The NHIS database contains health records, including sociodemographic data (age, sex, and income level), medical diagnosis (based on International Classification of Diseases, 10th revision [ICD-10]), treatment data, and health examination results (lifestyle and laboratory test results), for the Korean population. NHIS recommends that subscribers undergo a standardized medical examination at least biennially. Eligible members can get the examination at medical institutions including private clinics and hospitals, and public health centers engaged voluntarily for the national screening program.
Study sample
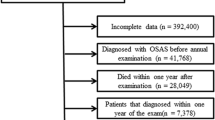
A total of 10,505,818 individuals underwent an annual or biennial evaluation provided by the NHIS in 2009. Patients who met the following criteria were excluded from the study: < 20 years of age (n = 15,317); excessive alcohol consumption (≥ 30 g of alcohol/day) (n = 679,743); diagnosis of liver cirrhosis (K703, K746) or any hepatitis (B15-B19) (n = 989,161), cancer (n = 118,243), or OSA (G473) (n = 25,771); or incomplete information (n = 561,059); these data included smoking history (n = 63,282), exercise (n = 158,986), alcohol history (n = 160,159), body mass index (n = 12,966), waist circumference (n = 7,576), blood pressure (n = 12,752) and laboratory findings (n = 145,338).
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (E-2002-015-1098) and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. The requirement for informed consent from individuals was waived because de-identified secondary data were used.
Clinical parameters and biochemical analysis
As described previously20, standardized self-reported questionnaires and, clinical parameters including laboratory tests were used to collect data at the time of enrollment. Briefly, age, sex, smoking status (non-smoker, ex-smoker, and current smoker), and alcohol consumption (frequency [0–7 days/week] and amount consumed on one occasion) were used. Regular physical exercise was defined as engaging in exercise on a routine basis with moderate to high-intensity activity ≥ 3 times/week. Income level was dichotomized at the lowest 20%. Comorbidities were defined using ICD 10 diagnosis codes, prescription information in the year prior to health screening, and health screening results. The criteria for hypertension were I10–13 or I15 claim codes plus ≥ 1 prescription of an antihypertensive agent, or systolic/diastolic blood pressure ≥ 140/90 mmHg); the criteria for diabetes were E11–14 claim codes plus ≥ 1 prescription of an antidiabetic medication per year, or a fasting glucose level ≥ 126 mg/dL; and the criteria for dyslipidemia were E78 claim code plus ≥ 1 prescription of a lipid-lowering agent, or total cholesterol ≥ 240 mg/dL. Chronic kidney disease was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 (estimated using the Modification of Diet in Renal Disease equation).
Body mass index (BMI) was calculated as weight (kg) divided by the square of the person’s height (m). Waist circumference (WC) was measured by a well‐trained person at the midpoint between the lower costal margin and the iliac crest. After an overnight fast of ≥ 8 h, blood specimens were obtained from each participant.
Surrogate measure of fatty liver
Although ultrasonography is a first-line screening technique in clinical practice21, ultrasonography is not included in the NHIS mass screening program. Therefore, biochemical data were used to identify NAFLD. These data were used to calculate the fatty liver index (FLI) score according to the below formula (in which GGT refers to gamma-glutamyl transferase)22. This score ranges from 0 to 100, with < 30 representing low risk of a fatty liver and ≥ 60 representing high risk of a fatty liver.
Study outcome
The study population was followed from baseline to the date of OSA diagnosis or until December 31, 2015, whichever occurred first. The primary endpoint of this study was incident OSA, which was defined as ≥ 1 one claim using the ICD‐10 code G473.
Statistical analyses
Statistical analysis is similar to a previous study23. Data are presented as mean ± standard deviation for normally distributed continuous variables and as proportions for categorical variables, unless otherwise indicated. Student’s t-test and analysis of variance were used to analyze continuous variables, and differences between nominal variables were compared using the chi-square test. Log transformations were performed for non-normally distributed variables. Incidence rate of the primary outcome was calculated by dividing the number of incident cases by the total follow-up period and presented as per 1000 person-years. We constructed a Cox proportional hazard model (Model 1) adjusted for age and sex, and the multivariate analysis included more potential confounders: smoking, alcohol consumption, exercise24, income25, hypertension, dyslipidemia, diabetes and BMI (Model 2), WC instead of BMI (Model 3), and lipid accumulation product (LAP)26 instead of BMI or WC (Model 4). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.Rproject.org). A two-sided P value < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
The prevalence of NAFLD, based on an FLI ≥ 60, was 11.5%. The total population was divided into three groups according to FLI score: < 30, 30–60, and ≥ 60. The baseline characteristics of each group are shown in Table 1. Compared to the lowest FLI group, the higher FLI groups had higher rates of males, current smokers, alcohol consumers, and people with a higher income level. Hypertension, dyslipidemia, and diabetes mellitus were also associated with a higher FLI. Most anthropometric and laboratory variables (including BMI, WC, total cholesterol, triglycerides, high-density lipoprotein cholesterol, and fasting glucose) were less metabolically favorable in people in the higher FLI groups than in those in the lowest FLI group (P < 0.001).
Risk of incident OSA in people with NAFLD
During the mean 6.3 years of follow-up period, the OSA incidence rate was higher in subjects with a high (≥ 60) FLI than in those with a low (< 30) FLI (1.831 vs 0.567 outcomes per 1000 person-years, Table 2). After adjusting for age and sex, the risk of OSA was significantly higher in the higher FLI groups, compared with the lowest FLI group (hazard ratio [HR] 1.68, 95% confidence interval [CI] 1.64–1.72 for FLI 30–60 and HR 2.42, 95% CI 2.36–2.48 for FLI ≥ 60). After adjusting for age, sex, smoking, alcohol consumption, physical exercise, income, BMI, diabetes, hypertension, and dyslipidemia, these associations remained significant (adjusted hazard ratio [aHR] 1.15, 95% CI 1.12–1.18 for FLI 30–60 and aHR = 1.21, 95% CI 1.17–1.26 for FLI ≥ 60). When we adjusted waist circumference (Model 3), and LAP (Model 4) instead of BMI, an increased risk of OSA in the highest FLI group compared with the lowest FLI group, still observed (Table 2).
OSA risk according to obesity, abdominal obesity, and other factors
When we stratified the population according to the BMI, the risk of OSA was significantly increased in higher FLI groups (FLI 30–60 and ≥ 60) in both the non-obese (BMI < 25), and the obese subjects (BMI ≥ 25) (Table 3). The risk of OSA was highest in patients with the highest FLI and obesity (aHR 2.58, 95% CI 2.51–2.66). When stratified according to WC, the risk of OSA was significantly increased in higher FLI groups (30–60 and ≥ 60) in patients with abdominal obesity (WC ≥ 90 cm for males and ≥ 80 cm for females), as well as in those without abdominal obesity. The risk of OSA was highest in patients with the highest FLI and abdominal obesity (aHR 2.60, 95% CI 2.53–2.68).
Next, we investigated various factors affecting the risk of OSA in patients with NAFLD (FLI ≥ 60). When stratified by age, sex, BMI, and abdominal obesity, the risk of OSA was significantly higher in NAFLD patients with younger age, male sex, obesity and abdominal obesity than in those with an older age, female sex, without obesity and abdominal obesity (Fig. 1).
Forest plot shows the HRs of the association between FLI ≥ 60 and OSA in different subgroups of participants; age < 40, 40 ≤ age < 65 vs age ≥ 65 years, male vs female, nonobese (BMI < 25) vs obese (BMI ≥ 25), no-abdominal obesity vs abdominal obesity. HRs are represented by black square; 95% CI are denoted by horizontal whiskers. FLI fatty liver index, OSA obstructive sleep apnea, BMI body mass index, HR hazard ratio, CI confidence interval.
Discussion
In this nationwide population-based study involving most Korean adults, we found that NAFLD (defined by FLI) was significantly associated with increased risk of OSA, even after adjusting for multiple metabolic variables. Moreover, the risk of OSA was higher in NAFLD patients who were younger, male, or obese than in those who were older, female, or non-obese.
Many studies have shown that patients with OSA have a higher incidence of NAFLD than the normal population, suggesting a link between hepatic injury and hypoxia in OSA15,17. A French study of 1285 patients with OSA found a linear relationship between OSA severity and hepatic steatosis index27. The relationship was independent of confounding factors, thereby suggesting that liver damage may occur during OSA. Similarly, an in vitro study showed that chronic intermittent hypoxia in obese mice caused hyperlipidemia by inhibiting clearance of triglyceride-rich lipoproteins28.
To date, most studies have evaluated the increased risk of hepatic steatosis in patients with OSA. Few studies have examined the converse association: the risk of OSA in patients with hepatic steatosis. One study reported that the overall prevalence of OSA was significantly higher in patients with fibrosis, compared to those without fibrosis, in NAFLD patients with chronically elevated liver enzymes29. Similarly, the apnea–hypopnea index was higher in patients with hepatic fibrosis than in those without fibrosis in NAFLD patients with severe obesity30. Another study reported that polysomnographic parameters, including apnea–hypopnea index and oxygen desaturation index, were significantly higher in patients with moderate or severe NAFLD, compared with individuals without NAFLD31. A population-based study based on the United States National Health and Nutrition Examination Survey showed that NAFLD, defined by elevated liver enzymes, was associated with sleep disorders diagnosed using sleep disorder questionnaires32. In agreement with these previous results, we found that the risk of OSA increased in a dose-dependent manner as FLI increased, supporting the presence of a close link between OSA and NAFLD.
The major confounding variable when analyzing the link between NAFLD and OSA is obesity. Measures of obesity include BMI and WC, both of which are associated with an increased risk of OSA33. When we classified subjects according to BMI and WC, the association between FLI and OSA remained in both obese and non-obese subgroups and in both with and without abdominal obesity subgroups. Moreover, patients with the highest FLI and obesity had the highest risk of incident OSA, supporting the important role of obesity in the risk of OSA. Furthermore, the association between NAFLD (FLI ≥ 60) and OSA was significantly higher in patients with a younger age, male sex, or obesity than in patients with an older age, female sex, or no obesity. These results are consistent with those of a study performed at a tertiary center in India, which found that the prevalence of symptomatic OSA was predicted by male sex and obesity18.
Mechanisms underlying the association between NAFLD and OSA are not fully elucidated. Intermittent hypoxia during OSA reverses normal diurnal glucose variation and promotes pancreatic beta-cell replication, which may lead to insulin resistance34. Chronic intermittent hypoxia also upregulates the sympathetic nervous system and increases circulating free fatty acids, hepatic gluconeogenesis, and induces insulin resistance35. Conversely, obesity among patients with NAFLD may lead to upper airway collapse, leading to chronic intermittent hypoxia36. However, the exact mechanism for assigning causal relationship is still known.
Regarding clinical practice, this study provides new insights to suggest the risks NAFLD may have for the the development of sleep impaired breathing. Another strength of this study is that it is a large-scale nationwide cohort study. However, the study has some limitations. First, because of its population-based observational study design, the results should be interpreted cautiously. Second, using FLI as a surrogate marker of NAFLD cannot accurately quantify the presence and amount of steatosis37. Although liver biopsy is considered the gold standard for diagnosing NAFLD, it is not usually performed in healthy individuals. Radiographic techniques, such as ultrasonography or magnetic resonance imaging, are typically used for diagnosing NAFLD in clinical practice. However, FLI (≥ 60) was previously investigated as a surrogate marker for NAFLD and found to accurately diagnose steatosis and correlate with insulin resistance37. Third, because we could not obtain data from polysomnography, which is the gold standard diagnostic test for OSA38, we could not analyze the severity of OSA. Fourth, since the diagnosis of OSA was based on claims data using the ICD-10 code, the number of subjects with OSA may have been underestimated. Although OSA affects approximately 20% of US adults, of whom about 90% are undiagnosed. This may be due to poor awareness of OSA, a lack of routine screening, and the limited number of facilities for sleep study39. Lastly, the diagnostic method of sleep apnea may not be unified. However, the diagnosis of sleep apnea was usually done using polysomnography according to recently published data in Korea40,41. Since the aim of this study was not about the exact diagnosis of OSA, but to examine the relationship between clinically significant OSA and NAFLD, we used the ICD-code to define OSA as previous study42. Further investigations using more accurate measures to diagnose OSA are necessary to confirm our results.
In conclusion, a high FLI score may help identify individuals with a high risk of OSA. Further study should be done to identify the risk profile for OSA in NAFLD patients. Understanding the association between NAFLD and OSA may have clinical important implications for reducing the incidence of these comorbidities.
Abbreviations
- OSA:
-
Obstructive sleep apnea
- NAFLD:
-
Nonalcoholic fatty liver disease
- FLI:
-
Fatty liver index
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NHIS:
-
National Health Insurance System
- ICD:
-
International Classification of Diseases
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- GGT:
-
Gamma-glutamyl transferase
- LAP:
-
Lipid accumulation product
References
Theorell-Haglow, J. et al. Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults—What do we know? A clinical update. Sleep Med. Rev. 38, 28–38 (2018).
Al Lawati, N. M., Patel, S. R. & Ayas, N. T. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog. Cardiovasc. Dis. 51, 285–293 (2009).
Ceccato, F., Bernkopf, E. & Scaroni, C. Sleep apnea syndrome in endocrine clinics. J. Endocrinol. Investig. 38, 827–834 (2015).
Nieto, F. J. et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283, 1829–1836 (2000).
Punjabi, N. M. et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am. J. Epidemiol. 160, 521–530 (2004).
Siarnik, P. et al. Obstructive sleep apnea and dyslipidemia. Vnitr Lek. 64, 934–938 (2018).
Young, T. et al. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108, 246–249 (2009).
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40, 1387–1395 (2004).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: Old questions and new insights. Science 332, 1519–1523 (2011).
Wong, V. W. et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 60, 1721–1727 (2011).
Adams, L. A. et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: A community-based cohort study. Am. J. Gastroenterol. 105, 1567–1573 (2010).
Targher, G. et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51, 444–450 (2008).
Wong, V. W. et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 60, 829–836 (2011).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Minville, C. et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest 145, 525–533 (2014).
Aron-Wisnewsky, J., Clement, K. & Pepin, J. L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism 65, 1124–1135 (2016).
Tanne, F. et al. Chronic liver injury during obstructive sleep apnea. Hepatology 41, 1290–1296 (2005).
Agrawal, S. et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol. Int. 9, 283–291 (2015).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15 (2017).
Cho, J. H. et al. Abdominal obesity increases risk for esophageal cancer: A nationwide population-based cohort study of South Korea. J. Gastroenterol. 55, 307–316 (2020).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609 (2012).
Bedogni, G. et al. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Choi, H. S. et al. Obstructive sleep apnea as a risk factor for incident end stage renal disease: A nationwide population-based cohort study from Korea. Clin. Exp. Nephrol. 23, 1391–1397 (2019).
Offenwert, E. V. et al. Physical activity and exercise in obstructive sleep apnea. Acta Clin. Belg. 74, 92–101 (2019).
Dong, L. et al. Prevalence and correlates of obstructive sleep apnea in urban-dwelling, low-income, predominantly African-American women. Sleep Med. 73, 187–195 (2020).
Kahn, H. S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 5, 26 (2005).
Trzepizur, W. et al. Association between severity of obstructive sleep apnea and blood markers of liver injury. Clin. Gastroenterol. Hepatol. 14, 1657–1661 (2016).
Drager, L. F. et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur. Heart J. 33, 783–790 (2012).
Petta, S. et al. Obstructive sleep apnea is associated with liver damage and atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One 10, e0142210 (2015).
Mesarwi, O. A. et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep 38, 1583–1591 (2015).
Cakmak, E. et al. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat. Mon. 15, e32655 (2015).
Mir, H. M., Stepanova, M., Afendy, H., Cable, R. & Younossi, Z. M. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): A population-based study. J. Clin. Exp. Hepatol. 3, 181–185 (2013).
Grunstein, R., Wilcox, I., Yang, T. S., Gould, Y. & Hedner, J. Snoring and sleep apnoea in men: Association with central obesity and hypertension. Int. J. Obes. Relat. Metab. Disord. 17, 533–540 (1993).
Yokoe, T. et al. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J. Physiol. 586, 899–911 (2008).
Fletcher, E. C. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 26, 15–19 (2003).
Schelbert, K. B. Comorbidities of obesity. Prim. Care 36, 271–285 (2009).
Fedchuk, L. et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol. Ther. 40, 1209–1222 (2014).
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 13, 479–504 (2017).
Finkel, K. J. et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 10, 753–758 (2009).
Kim, J.-W. et al. Polysomnographic phenotyping of obstructive sleep apnea and its implications in mortality in Korea. Sci. Rep. 10, 13207 (2020).
Lee, J.-E. et al. Mortality of patients with obstructive sleep apnea in Korea. J. Clin. Sleep Med. 9, 997–1002 (2013).
Choi, J. H. et al. Association between obstructive sleep apnoea and breast cancer: The Korean National Health Insurance Service Data 2007–2014. Sci. Rep. 9, 19044 (2019).
Funding
This study was supported by Grant 04-2020-0690 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Contributions
G.E.C. and E.J.C. drafted and revised the manuscript. J.-J.Y., Y.C., and Y.C. collected and reviewed the data, and revised the manuscript. S.-H.P. and D.W.S. performed the statistical analysis and revised the manuscript. K.H. and S.J.Y. conceived the idea, determined the study design, collected the data and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, G.E., Cho, E.J., Yoo, JJ. et al. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci Rep 11, 13473 (2021). https://doi.org/10.1038/s41598-021-92703-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92703-0
This article is cited by
-
The ZJU index is associated with the risk of obstructive sleep apnea syndrome in Chinese middle-aged and older people: a cross-sectional study
Lipids in Health and Disease (2023)
-
Lean or diabetic subtypes predict increased all-cause and disease-specific mortality in metabolic-associated fatty liver disease
BMC Medicine (2023)
-
Non-alcoholic fatty liver disease is associated with decreased bone mineral density in upper Egyptian patients
Scientific Reports (2023)
-
Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome
Signal Transduction and Targeted Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.