Abstract
Both Campylobacter- and Shigella-induced invasive enteritis are common in under-5 Bangladeshi children. Our study aimed to determine the factors associated with Campylobacter and Shigella enteritis among under-5 children, the post-infection worsening growth, and the household cost of invasive enteritis. Data of children having Shigella (591/803) and Campylobacter (246/1148) isolated from the fecal specimen in Bangladesh were extracted from the Global Enteric Multicenter Study (GEMS) for the period December 2007 to March 2011. In multiple logistic regression analysis, fever was observed more frequently among shigellosis cases [adjusted OR 2.21; (95% CI 1.58, 3.09)]. Breastfeeding [aOR 0.55; (95% CI 0.37, 0.81)] was found to be protective against Shigella. The generalized estimating equations multivariable model identified a negative association between Shigella and weight-for-height z score [aOR − 0.11; (95% CI − 0.21, − 0.001)]; a positive association between symptomatic Campylobacter and weight-for-age z score [aOR 0.22; (95% CI 0.06, 0.37)] and weight-for-height z score [aOR 0.22; (95% CI 0.08, 0.37)]. Total costs incurred by households were more in shigellosis children than Campylobacter-induced enteritis ($4.27 vs. $3.49). Households with low-level maternal education tended to incur less cost in case of their shigellosis children. Our findings underscore the need for preventive strategies targeting Shigella infection, which could potentially reduce the disease burden, associated household costs, and child growth faltering.
Similar content being viewed by others
Introduction
Campylobacter, Shigella, Salmonella, and diarrheagenic Escherichia coli constitute the major bacterial pathogens that often cause acute invasive gastrointestinal infections1,2,3. A fundamental distinction among bacterial pathogens involves the capacity to invade intestinal epithelial cells and multiply within the gut mucosa, consequently exerting biological constraints on attempts to control their spread among susceptible populations4. Shigella continues to play a significant role in the etiology of dysentery and inflammatory diarrhea while Campylobacter is one of the most commonly isolated bacteria in infants with diarrhea. Watery or bloody diarrhea, fever, and abdominal pain are the characteristics of infection by Shigella and Campylobacter5. Campylobacter-induced acute gastroenteritis is difficult to differentiate from Shigella-associated gastroenteritis on the sole basis of clinical symptoms or routine stool examination and thus stool culture is required for a conclusive diagnosis of the causative organism.
Enteric infections can trigger inflammatory immune responses that can lead to chronic inflammation of the intestine and subsequent morphological changes. This interrupts nutrient absorption ability and leads to sequelae6. Studies investigating the effects of Campylobacter and Shigella on growth in children have been diverse and limited. The relationship between Shigella and enterotoxigenic Escherichia coli-associated with weight gain and linear growth has been investigated among children in Bangladesh aged between 0 and 5 years7. Household costs of diarrhea mediated by Campylobacter and Shigella have been reported to exert adverse consequences on the household economy8.
However, there is inadequate data on growth among children who are asymptomatic carriers or are suffering from invasive entireties by Campylobacter and Shigella. Moreover, there is an evident knowledge gap regarding the economic implications of infections caused by these two aforementioned infectious organisms in low- and- middle-income countries like Bangladesh.
Global Enteric Multicenter Study (GEMS) was a prospective case–control study conducted across 7 sites in sub-Saharan Africa and South Asia. In this present study, we aimed to compare the demographics, housing, animal exposure, clinical presentation, and associated factors among under-5 children with invasive enteritis associated with Campylobacter and Shigella infection in Bangladesh; evaluate the association between invasive enteritis and growth among under 5 children; and estimate household costs associated with invasive enteritis.
Results
Co-pathogens isolated from Shigella and Campylobacter positive children
In the Bangladesh site, Shigella and Campylobacter positive children having one or more bacterial, viral, and protozoal co-pathogens have been identified (Table S1). Shigella flexneri (28.77%) and Shigella sonnei (10.62%) were more frequently isolated among the cases with moderate-to-severe diarrhea (MSD) in comparison to the asymptomatic children. Campylobacter jejuni was found in almost 13% of both the symptomatic cases and asymptomatic healthy controls (Table S2).
Characteristics of Shigella-positive children having moderate-to-severe diarrhea (MSD) in Bangladesh
About 44.3% of the Shigella-positive children with MSD were aged 24–59 months and half of them were female. Giardia was found to be the most common co-pathogen (Table 1) in the Shigella positive children compared to the Shigella negative children. A significantly higher proportion of under-five children with MSD and associated Shigella infection were stunted, wasted and underweight. The duration of diarrhea before coming to the facility was less. The children had more frequent visible blood in the stool, less vomiting, and more often presented with a history of fever during admission. Regarding the inclusion criteria for MSD, the children more commonly had dysentery and required hospital admission. Caregivers of the Shigella-positive children practiced handwashing less frequently before nursing the child/preparing baby food and after cleaning the child; one-fourth of them belonged to middle and upper middle-class families. Shigella-positive children were less often breastfed. Their stool examinations reported the habitual presence of fecal red blood cell (RBC) and mucus more frequently in comparison to the stool specimens of Shigella-negative children.
Characteristics of Campylobacter-positive children having MSD
About 65% of the Campylobacter-positive children with MSD were aged 0–11 months and 42% of them were female; less often had malnutrition; mostly presented with visible blood in stool and fever during admission, less frequently presented with vomiting. Among the inclusion criteria of MSD, more children had dysentery and required less hospital admission. They had a large family size, and more under-5 children in the house; more frequently had toilet facility at the house; practiced handwashing more commonly before nursing the child/preparing baby food; less often washed hand with soap. Eighteen percent of children were from wealthy families and were more commonly breastfed. Their stool examinations reported the frequent presence of fecal RBC and mucus compared to the stool specimens of Campylobacter-negative MSD children. EAEC was found more often as co-pathogens (Table 1) in the Campylobacter-positive children.
Multiple logistic regression reveals that MSD children with Shigella were significantly associated with the presence of blood in stool [OR 2.41 (95% CI 1.56, 3.70)], usually presented with fever [OR 2.21 (95% CI 1.58, 3.09)], less often had features of dehydration like sunken eyes [OR 0.31 (95% CI 0.18, 0.52)]; and were less frequently breastfed [OR 0.55 (95% CI 0.37, 0.81)]. However, fecal RBC [OR 1.61 (95% CI 1.20, 2.17)] and mucus [OR 2.46 (95% CI 1.38, 4.37)] were more often reported during stool examinations among MSD children with Shigella (Table 2) compared to the children without fecal Shigella. Conversely, Campylobacter positive MSD children were associated with blood in stool [OR 2.57 (95% CI 1.72, 3.81)]; more often sought healthcare service; and had a significantly fewer history of fever [OR 0.70 (95% CI 0.51, 0.96)] upon attendance to the health facility compared to the children without fecal Campylobacter (Table 2).
Child growth with fecal Shigella and Campylobacter
In the unadjusted model, the mean height-for-age z score (HAZ) was found to be elevated at the endline but the mean weight-for-age z score (WAZ) and weight-for-height z score (WHZ) were reduced in the endline (Fig. 1) among the fecal Shigella and Campylobacter-positive under-5 children.
In Table 3, the findings of generalized estimating equations (GEE) modeling have been presented. A significant association between Shigella infection and WHZ was shown in the unadjusted GEE model. After adjusting for other covariates, namely: age, gender, MSD, breastfeeding status, mother’s education, number of people regularly sleeping in the house, number of under-5 children at house, handwashing material, hand washing before nursing a child and after cleaning the child, access to toilet facility, the main source of drinking water, wealth index, co-pathogens Cryptosporidium and Giardia, comorbidity (pneumonia, diarrhea, dysentery, malaria, typhoid), the multivariable model revealed a significant negative association between WHZ [Coefficient: − 0.11 (95% CI − 0.21, − 0.001) and Shigella, indicating that children having Shigella infection irrespective of MSD had poor growth. On the other hand, a significant positive association of WAZ [Coef.: 0.22 (95% CI 0.06, 0.37)] and WHZ [Coef.: 0.22 (95% CI 0.08, 0.37)] with Campylobacter infections indicated symptomatic children having Campylobacter infection had improved growth (Table 3). The analysis was replicated without adjusting the significant co-pathogens (Cryptosporidium and Giardia) and no difference in the adjusted mean growth was found (Table S3).
Expenditures by type of cost
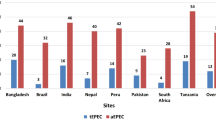
Among cases with nonzero costs (Shigella, n = 591, and Campylobacter, n = 246), the total cost was higher in the Shigella-positive cases ($4.17) compared to the Campylobacter-positive cases ($3.49). Mean direct medical costs to households were similar in both Shigella and Campylobacter ($2.95 vs $2.32). The indirect cost was 3.22 USD among the Shigella-mediated enteritis cases and 4.74 USD for Campylobacter-mediated enteritis cases, but the total direct cost was similar in both the Shigella-mediated and Campylobacter-mediated enteritis cases (Table S4).
Determinants of costs
The relationship between the wealth index, gender, education, age, severity and duration, household direct medical costs and overall costs were investigated (Table 4). There was no difference in total costs for both the Shigella-mediated and Campylobacter-induced enteritis. Both enteritis indicated a higher cost with higher levels of maternal education, especially for household total costs in the case of Shigella-mediated enteritis (p = 0.046). Higher household total costs with a greater duration of hospital stay were observed in both cases (p < 0.05) (Table 4).
Discussion
In our study, children with Shigellosis and Campylobacter infections presenting with dysentery were compared to fecal Shigella and Campylobacter negative children. Our findings were comparable to other studies9,10,11, despite being unable to exclude the co-pathogens responsible for dysentery in shigellosis and Campylobacter infections.
Campylobacter-positive children reported a significantly lower incidence of fever at admission in comparison to Campylobacter-negative children. However, fever was more common in the case of Shigella-positive children compared to Shigella-negative children. It was probably due to mild, often self-limiting Campylobacter infections that needed only supportive treatment12. This observation was similar to a study conducted in the north of Israel12. Henceforth, fever on admission associated with dysentery will be helpful for the clinicians to differentiate between shigellosis and Campylobacter infections among under-5 children.
In our study, Shigella-positive children less often presented with sunken eyes. Findings from a study conducted in a large urban diarrhea treatment facility in Bangladesh reported frequent presentation of shigellosis with some or severe dehydration in children13,14,15. In the case of Campylobacter infection, we observed no association with inclusion criteria of MSD and our findings were consistent with a study among Canadian children, where dehydration was not reported to be a common presenting feature of Campylobacter-mediated enteritis16 and a similar finding was observed in a large waterborne outbreak of Campylobacter jejuni in Norway17.
In our analysis, breastfeeding was found to be a protective factor for shigellosis. Very little is known about the impact of breastfeeding on Shigella-related diarrheal diseases. Another research from Bangladesh studied the children up to the first three years of age and found that breastfed children up to 35 months of age had a higher level of immunity against severe shigellosis18. In rural Mozambique, breastfeeding was also found protective for diarrhea caused by Shigella19.
We observed a significant negative association of Shigella-mediated enteritis with weight-for-height z score. In other studies, researchers have indicated a similar type of results7,20. Malnourished children have been found to present with longer duration of illness and deep ulcerations in the colon. Both acute and prolonged episodes of shigellosis may result in extensive loss of blood from the colonic ulcerations21. Thus, in addition to the other effects of diarrhea, shigellosis results in loss of serum protein that, for children on a marginal diet, must be compensated by increased protein intake for optimal growth to occur22,23. This fecal protein loss may have been partially responsible for growth faltering in children with shigellosis. However, in our study, we found no effect of shigellosis in limiting linear growth.
In several studies, researchers observed an association between Campylobacter infection and reduced weight gain as well as reduced linear growth20,24. However, in our study, we did not find any association of asymptomatic Campylobacter with child anthropometry indices. There was a positive association between symptomatic Campylobacter infection with child growth. This may be because of the treatment of symptomatic episodes with antibiotics. Thus, antibiotic treatment may be a confounding factor in estimating the true effect of Campylobacteriosis on child growth. Similar findings were observed in a systematic review and meta-analysis of Campylobacter infection25. In our study, stunting, wasting, or underweight, in any form, were not associated with invasive enteritis caused by Campylobacter or Shigella. Since the risk of diarrheal disease among severely malnourished children may be higher compared to that in the well-nourished children, our population may be less than ideally suited to disentangling this impact, limiting our ability to assess whether the association between Campylobacter and growth during enrollment was mediated by the nutritional deficit.
In our study population, Cryptosporidium and Giardia were prevalent and they are known to influence the growth of children26,27,28. However, their impact can also be eliminated in this situation, as we controlled their effect during GEE modeling for both Campylobacter and Shigella infections. The analysis was repeated to those children not infected with Cryptosporidium and Giardia. The difference in adjusted mean growth was measured in terms of the HAZ, WAZ, and WHZ score, which was observed to be almost the same. Other studies which used a single assessment of nutritional status to establish a possible link between Shigella or Campylobacter with malnutrition have not been able to distinguish between the growth effect of Shigella or Campylobacter-mediated enteritis or the increased vulnerability of malnourished children to infection.
Medical costs differed by sex, with direct costs being higher for girls suffering from Shigella enteritis, and higher for boys suffering from Campylobacter enteritis, with no difference in overall costs between Shigella and Campylobacter-mediated enteritis. Given the evidence revealed in the literature that household spending on health care, food, and education sometimes favors boys over girls, these findings warrant further exploration29,30. We also observed evidence of lower total costs for children with lower maternal education levels in the case of Shigella-mediated enteritis. Literate mothers incurred higher costs for the treatment of Shigella infections in comparison to illiterate mothers. This was more likely because of illiteracy to curtail or prolong care-seeking. This brings with it the danger that delayed care leading to more adverse outcomes among illiterate mothers’ children. We do not have any ready explanation for this observation but further studies may address these issues.
Total medical costs were amplified by the increased duration of hospital stay in cases of both Campylobacter and Shigella-mediated enteritis. Another study from Northern Ghana also reported higher hospital costs in inpatients than those who received outpatient treatment31.
Unbiased sampling following a standard protocol32, a large sample size33, and high-quality laboratory performance32 were the strengths of our analysis. In this study, we aimed to determine the factors associated with both symptomatic and asymptomatic Shigella and Campylobacter infections among under-5 children. A single home follow-up visit approximately 60 days after enrollment was a valuable addition to the results of this research, which allowed us to understand the growth outcomes of children during the vulnerable times of their lives.
Nevertheless, our study has several limitations warranting a careful interpretation of the results when explaining these findings. It includes the inability to determine the relationship between maternal age and BMI, gestational age, and birth weight data for child growth failure. Due to a limited number of samples, we could not conclude the differential effects of Campylobacter and Shigella by species. This study did not evaluate the antimicrobial susceptibility patterns. Additionally, the cost of adverse outcomes and mental effects (such as distress and tiredness) of diarrheal disease caregivers have not been clarified in the current study. Moreover, the study was conducted in a sub-district of Bangladesh, so the results may not be generalizable for the whole country.
In conclusion, the use of clinical predictors may make it possible to target appropriate empiric antimicrobial therapy for children most likely to have invasive enteritis in resource-constrained settings. Our findings underscore the need for preventive strategies targeting Shigella, which could potentially reduce the disease burden and its sequelae such as child growth faltering during the first 5 years of life. Results also indicate the economic burden of households. Appropriate coping mechanisms may be undertaken to alleviate this burden. This may have public health implications particularly in the case of households with illiterate mothers or childhood invasive enteritis, mainly in the case of girls.
Method
Study site
Related data were extracted from the Global Enteric Multicenter Study (GEMS), Bangladesh site database34. The location of the GEMS Bangladesh site was in a rural community, situated in the Mirzapur sub-district of Tangail, Bangladesh. Details about the study site have been reported elsewhere34,35,36.
Study design and study participants
The design and methodology of the GEMS were mentioned earlier36. Briefly, data were extracted from cases and controls enrolled at the GEMS Bangladesh site, a three-year research during December 2007 and March 2011. GEMS was a prospective matched case–control study conducted for 36 months at 7 sites where demographic surveillance systems (DSS) regularly updated censused populations. The sampling frame comprised children aged < 60 months residing within each site’s DSS area. Children brought to sentinel health centres serving each DSS-respondent-children were assessed to match with the inclusion criteria for MSD irrespective of their socioeconomic status. Every fortnight, 8–9 cases per age stratum (0–11, 12–23, and 24–59 months) per site were targeted for enrolment. Within 14 days of each case enrolled, they undertook to enroll 1–3 randomly selected age- and sex-matched controls from the same or nearby communities36,37,38. The research had a well-defined standardized protocol for recruitment36. The published37, working hypothesis35, epidemiology36, clinical39, laboratory36, and statistical methods40 of GEMS have been described elsewhere41. In this analysis, we enrolled 1394 (36.12%) under-5 children from a total of 3859 children enrolled in the GEMS Bangladesh site. There were 648 (16.79%) Shigella-positive and 673 (17.44%) Campylobacter-positive under-5 children enrolled in Bangladesh. Among MSD cases, there were 591 (42.40%) Shigella-positive cases and 803 (57.60%) Shigella-negative controls, and 246 (17.65%) Campylobacter-positive cases, and 1148 (82.35) Campylobacter-negative controls (Fig. 2). Only 46 (< 5%) children had both Campylobacter and Shigella present in the stool.
Collection of stool samples and stool microbiology
Stool specimens for the GEMS were examined for every child at the time of enrolment using the GEMS laboratory procedure protocol42,43,44.
Shigella and Campylobacter spp. isolation
Tests for isolation of both Campylobacter and Shigella spp. used in GEMS have been described elsewhere43.
Variable of interest
Anthropometry
During enrollment and the 60-day follow-up visit length/ height, weight, and MUAC for each child were measured; descriptions of measurement methods are mentioned elsewhere36,45,46. The height/length-for-age, weight-for-age, and weight-for-height z-scores (HAZ, WAZ, and WHZ) have been calculated by WHO SAS macro using the WHO Child Growth Standards for the reference population47, 48.
Diarrhea
Passage of ≥ 3 abnormally loose or watery stools per 24 h36, 49.
Fever, vomiting, and dysentery
Many of the factors, such as vomiting (approximately 3 times/day) and fever on admission (measured at least 38 °C) and dysentery as the presence of visible blood in stools, can only be evaluated retrospectively36.
Inclusion criteria for MSD
Every child was evaluated for diarrhea and study enrollment eligibility. The episode had to be current (initiated after around 7 days without diarrhea), acute (initiated within the previous 7 days), and at least one of the following characteristics for moderate-to-severe diarrhea (MSD) had to be met: sunken eyes (confirmed by parent or caretaker as more than usual; loss of skin turgor (abdominal skin pinch with slow [≤ 2 s] or very slow [> 2 s] recoil); intravenous rehydration administered or prescribed; dysentery (visible blood in loose stools); or hospitalized with diarrhea or dysentery50.
Breastfeeding status
Breastfed referred to both exclusive and partially breastfeed children.
Socio-demographic information
This involved data from the participant’s household (defined as a group of people sharing a cooking fire) which included mother as a primary caretaker, education of mother (illiterate or literate), and size of household (including the number of children < 5 years of age, number of people regularly sleeping in the house). The explanatory variables were known to be building materials (cement or non-cement), the practice of handwashing (before nursing or preparing baby food; after handling animals, and cleaning a child), access and the main source of drinking water (tube well and non-tube well water), water treatment (water treatment method of drinking water available or not), improved sanitation facilities (an available toilet facility for disposal of human fecal waste or not), pets on the premises (sheep, goat, rodent/fowl, cow, dog, and cat), and methods for hand washing (water with soap or without soap).
Wealth index
Based on the wealth index quintiles (poor, lower middle, middle, upper middle, and rich), households were categorized into socio-economic status (SES) to determine potential associated factors for disease as well as indicators for constructing a wealth index for each site36,51.
Duration of hospital stay
The outcome was described by using a total duration of hospital stay (less than 4 days and ≥ 4 days).
Household follow-up visit
GEMS field staff members visited each enrolled child's household roughly 60 days after enrollment (acceptable range, 50–90 days). During these follow-up household visits, detailed comorbidity data (typhoid, pneumonia, diarrhea, and dysentery) were obtained36.
Child growth
Only case–control (Campylobacter and Shigella positive and negative) sets of data on both enrollment and follow-up HAZ, WAZ, and WHZ for participants enrolled in GEMS were included in our study36. We used weighted means of baseline and endline HAZ, WAZ, and WAZ (n = 648 vs. 640) for Shigella-positive children; and HAZ, WAZ, and WAZ (n = 673 vs. 660) for Campylobacter-positive children irrespective of MSD from enrolment to follow-up respectively from GEMS Bangladesh site.
Household cost
Direct medical costs, direct non-medical costs, indirect costs, and overall costs per study child (fecal Campylobacter and Shigella positive MSD) were analyzed for care-seeking from the medical facilities for the treatment of a given episode of MSD, all of which were converted to the current US dollar rate. Direct medical expenses were classified as both informal and formal expenses, with the former representing treatment given by a local healer or pharmacist and the latter combining all health centers, hospitals, and licensed practitioners. Direct non-medical costs were split down by transport and other costs, while indirect costs were either time costs or other costs.
Statistical methods
Considering mean and standard deviation (SD) for continuous variables and frequency as a percentage to summarize the data for categorical variables, we reported the child, maternal, and household-level characteristics. Student's t test for continuous variables was performed to compare the mean differences, and changes in proportions were compared by the Chi-squared (χ2) test. Since Shigella and Campylobacter infection were binary indicators, we performed multiple logistic regression analyses to identify the significantly associated factors of Shigella and Campylobacter infections in children aged < 5 years for Shigella and Campylobacter positive children having MSD. The covariates were adjusted for multiple logistic regression models using a stepwise forward selection method if associated with p value < 0.25 in the simple model52, whereas other relevant variables such as age and sex were adjusted for a p value < 0.25 due to biological as well as public health importance as more traditional levels such as 0.05 can fail in identifying variables known to be of importance. All covariates were included in the subsequent models to obtain an adjusted final model. Adjusted odds ratios (aOR) with a 95% confidence interval (CI) as the strength of the associations were determined from multiple logistic regression. The relationship of explanatory variables (presence of fecal Campylobacter and Shigella) with the continuous outcome variables (HAZ, WAZ, and WHZ) were examined longitudinally using generalized estimating equations (GEE) with exchangeable correlation and identity link function53,54. The variance inflation factor (VIF) was calculated to assess multicollinearity and no variable with a VIF > 5 was identified. Some cases incurred no expenditures for both medical and total costs, and the remainder created a right-skewed distribution. For MSD cases, descriptive statistics (means and SD) for costs were estimated. The analysis of variance (ANOVA) was used to independently assess the results of the economic status of households, maternal education, sex of a child, age, and duration of illness. We determined the strength of the association by estimating the coefficients and their 95% CIs. A probability of less than 0.05 was assumed statistically significant. All data were analyzed using STATA version 15.0 IC (College Station, TX: Stata Corp LLC).
Ethical consideration
The ethical committees and the respective research review boards at the University of Maryland School of Medicine and the committees overseeing each site and their collaborating partners from other institutions approved the clinical protocol, consent forms, case report forms, field methods, and other supportive materials prior to the start of the study. All methods were performed in accordance with the relevant guidelines and regulations. The signed informed consent for the inclusion of children in the study was obtained from the parents/guardians of the children (both sick MSD cases and healthy controls).
Data availability
This study analyzed a publicly accessible GEMS dataset. This data can be found here: ClinEpiDB [https://clinepidb.org/ce/app/record/dataset/DS_841a9f5259].
References
Gascón, J. et al. Diarrhea in children under 5 years of age from Ifakara, Tanzania: A case–control study. J Clin Microbiol. 38(12), 4459–4462 (2000).
Mulatu, G., Beyene, G. & Zeynudin, A. Prevalence of Shigella, Salmonella and Campylobacter species and their susceptibility patters among under five children with diarrhea in Hawassa town, south Ethiopia. Ethiop. J. Health Sci. 24(2), 101–108 (2014).
Amieva, M. R. Important bacterial gastrointestinal pathogens in children: a pathogenesis perspective. Pediatr. Clin. N Am. 52(3), 749–777, vi (2005).
Keusch, G. T. The epidemiology and pathophysiology of invasive bacterial diarrheas. in Diarrhea and malnutrition 45–72 (Springer, Berlin, 1983).
Same, R. G. & Tamma, P. D. Campylobacter infections in children. Pediatr. Rev. 39(11), 533–541 (2018).
Crane, R. J., Jones, K. D. J. & Berkley, J. A. Environmental enteric dysfunction: an overview. Food Nutr. Bull. 36(1 Suppl), S76–S87 (2015).
Black, R. E., Brown, K. H. & Becker, S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 73(6), 799–805 (1984).
Rheingans, R. et al. Determinants of household costs associated with childhood diarrhea in 3 South Asian settings. Clin. Infect. Dis. 55(Suppl 4), S327-335 (2012).
Soofi, S. B. et al. A comparison of disease caused by Shigella and Campylobacter species: 24 months community based surveillance in 4 slums of Karachi, Pakistan. J. Infect. Public Health 4(1), 12–21 (2011).
Gillespie, I. A. et al. Investigating vomiting and/or bloody diarrhoea in Campylobacter jejuni infection. J. Med. Microbiol. 55(6), 741–746 (2006).
Kahsay, A. G. & Teklemariam, Z. Prevalence of Shigella among diarrheic children under-5 years of age attending at Mekelle health center, north Ethiopia. BMC. Res. Notes 8, 788–788 (2015).
Sakran, W. et al. Campylobacter gastroenteritis in children in north-eastern Israel comparison with other common pathogens. Sci Rep. 10(1), 5823 (2020).
Huskins, W. C., Griffiths, J. K., Faruque, A. S. G. & Bennish, M. L. Shigellosis in neonates and young infants. J. Pediatr. 125(1), 14–22 (1994).
Chisti, M. J. et al. Characteristics of children with shigella encephalopathy: Experience from a large urban diarrhea treatment center in Bangladesh. Pediatr. Infect. Dis. J. 29(5), 444–447 (2010).
Soofi, S. B. et al. A comparison of disease caused by Shigella and Campylobacter species: 24 months community based surveillance in 4 slums of Karachi, Pakistan. J. Infect. Public Health 4(1), 12–21 (2011).
Karmali, M. A. & Fleming, P. C. Campylobacter enteritis in children. J. Pediatr. 94(4), 527–533 (1979).
Mortensen, N. et al. Characteristics of hospitalized patients during a large waterborne outbreak of Campylobacter jejuni in Norway. PLoS One. 16(3), e0248464 (2021).
Clemens, J. D. et al. Breast feeding as a determinant of severity in Shigellosis: Evidence for protection throughout the first three years of life in Bangladeshi children. Am. J. Epidemiol. 123(4), 710–720 (1986).
Vubil, D. et al. Clinical features, risk factors, and impact of antibiotic treatment of diarrhea caused by Shigella in children less than 5 years in Manhiça District, rural Mozambique. Infect. Drug Resist. 11, 2095–2106 (2018).
Lee, G. et al. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr. Infect. Dis. J. 33(10), 1004–1009 (2014).
Keusch, G. T. Shigellosis. In Bacterial infections of humans 699–724 (Springer, Berlin, 2009).
Whitehead, R. G. Protein and energy requirements of young children living in the developing countries to allow for catch-up growth after infections. Am. J. Clin. Nutr. 30(9), 1545–1547 (1977).
Pencharz, P. B. Protein and energy requirements for “optimal” catch-up growth. Eur. J. Clin. Nutr. 64(Suppl 1), S5-7 (2010).
Lee, G. et al. Symptomatic and asymptomatic campylobacter infections associated with reduced growth in Peruvian Children. PLoS Neglect. Trop. Dis. 7(1), e2036 (2013).
Ternhag, A., Asikainen, T., Giesecke, J. & Ekdahl, K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin. Infect. Dis. 44(5), 696–700 (2007).
Prado, Md. S. et al. Asymptomatic giardiasis and growth in young children: A longitudinal study in Salvador, Brazil. Parasitology. 131(Pt 1), 51–56 (2005).
Simsek, Z., Zeyrek, F. Y. & Kurcer, M. A. Effect of Giardia infection on growth and psychomotor development of children aged 0–5 years. J. Trop. Pediatr. 50(2), 90–93 (2004).
Checkley, W. et al. Effects of Cryptosporidium parvum infection in Peruvian children: Growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 148(5), 497–506 (1998).
Nguyen, H. & Knowles, J. Demand for voluntary health insurance in developing countries: the case of Vietnam’s school-age children and adolescent student health insurance program. Soc. Sci. Med. 71(12), 2074–2082 (2010).
Pandey, A. et al. Gender differences in healthcare-seeking during common illnesses in a rural community of West Bengal, India. J. Health Popul. Nutr. 20(4), 306–311 (2002).
Aikins, M., Armah, G., Akazili, J. & Hodgson, A. Hospital health care cost of diarrheal disease in Northern Ghana. J. Infect. Dis. 202(Supplement_1), S126–S130 (2010).
Kotloff, K. L. et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 55(Suppl_4), S232–S245 (2012).
Blackwelder, W. C. et al. Statistical methods in the global enteric multicenter study (GEMS). Clin. Infect. Dis. 55(Suppl_4), S246–S253 (2012).
Khatun, F. et al. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008). Epidemiol. Infect. 139(3), 446–452 (2011).
Farag, T. H. et al. Some epidemiologic, clinical, microbiologic, and organizational assumptions that influenced the design and performance of the Global Enteric Multicenter Study (GEMS). Clin. Infect. Dis. 55(Suppl 4), S225–S231 (2012).
Kotloff, K. L. et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 55(Suppl 4), S232-245 (2012).
Levine, M. M., Kotloff, K. L., Nataro, J. P. & Muhsen, K. The Global Enteric Multicenter Study (GEMS): Impetus, rationale, and genesis. Clin. Infect. Dis. 55(Suppl 4), S215–S224 (2012).
Kotloff, K. L. et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case–control study. Lancet (London, England). 382(9888), 209–222 (2013).
Livio, S. et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 59(7), 933–941 (2014).
Blackwelder, W. C. et al. Statistical methods in the Global Enteric Multicenter Study (GEMS). Clin. Infect. Dis. 55(Suppl 4), S246-253 (2012).
Sow, S. O. et al. The burden of cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 10(5), e0004729–e0004729 (2016).
Lindsay, B. et al. Microbiota that affect risk for shigellosis in children in low-income countries. Emerg. Infect. Dis. 21(2), 242–250 (2015).
Panchalingam, S. et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin. Infect. Dis. 55(Suppl 4), S294-302 (2012).
Howard, G., Ahmed, M. F., Shamsuddin, A. J., Mahmud, S. G. & Deere, D. Risk assessment of arsenic mitigation options in Bangladesh. J. Health Popul. Nutr. 24(3), 346 (2006).
Cogill, B. Anthropometric indicators measurement guide. (2001).
El Mouzan, M. I. et al. Prevalence of malnutrition in Saudi children: A community-based study. Ann. Saudi Med. 30(5), 381–385 (2010).
World Health O. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight -for-height and body mass index-for-age: Methods and development (World Health Organization, 2006).
World Health Organization. WHO child growth standards, SAS macro (version 3.2. 2). in World Health Organization Geneva (Switzerland, 2012).
Baqui, A. H. et al. Methodological issues in diarrhoeal diseases epidemiology: Definition of diarrhoeal episodes. Int. J. Epidemiol. 20(4), 1057–1063 (1991).
Kotloff, K. L. et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: Epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 55(Suppl 4), S232-245 (2012).
Filmer, D. & Pritchett, L. H. Estimating wealth effects without expenditure data—or tears: An application to educational enrollments in states of India. Demography 38(1), 115–132 (2001).
Bursac, Z., Gauss, C. H., Williams, D. K. & Hosmer, D. W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 3(1), 17 (2008).
Diggle, P., Liang, K.-Y. & Zeger, S. L. Longitudinal data analysis Vol. 5, 13 (Oxford University Press, New York, 1994).
Das, S., Alam, M. A., Mahfuz, M., Arifeen, S. E. & Ahmed, T. Relative contributions of the correlates of stunting in explaining the mean length-for-age z-score difference between 24-month-old stunted and non-stunted children living in a slum of Dhaka, Bangladesh: Results from a decomposition analysis. BMJ Open. 9(7), e025439 (2019).
Acknowledgements
We acknowledge with gratitude the commitment of the Global Enteric Multicenter Study (GEMS) in collaboration with the Center for Vaccine Development, University of Maryland School of Medicine, USA. GEMS received a grant from the Bill & Melinda Gates Foundation. The authors are grateful to GEMS staff, parents, and children for their contributions. This research protocol was funded by core donors who provide unrestricted support to icddr,b for its operations and research. Current donors providing unrestricted support include the Governments of Bangladesh, Canada, Sweden, and the UK. We gratefully acknowledge our core donors for their support and commitment to icddr,b's research efforts.
Author information
Authors and Affiliations
Contributions
A.S.G.F., T.A., and R.D. made major contributions to the design and implementation; M.A.H. managed the data set and gave technical support; R.D. analyzed the data, constructed the tables, figures, and wrote the manuscript's initial draft. M.A.H., M.J.C., T.A., and A.S.G.F. have critically revised for essential intellectual content; TA gave final approval to the version to be published. Every author was sufficiently involved in the research to take on public responsibility for content related sections. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, R., Haque, M.A., Chisti, M.J. et al. Associated factors, post infection child growth, and household cost of invasive enteritis among under 5 children in Bangladesh. Sci Rep 11, 12738 (2021). https://doi.org/10.1038/s41598-021-92132-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92132-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.