Abstract
Deferasirox (DFX) is the newest among three different chelators available to treat iron overload in iron-loading anaemias, firstly released as Dispersible Tablets (DT) and more recently replaced by Film-Coated Tablets (FCT). In this retrospective observational study, pharmacokinetics, pharmacodynamics, and safety features of DFX treatment were analyzed in 74 patients that took both formulations subsequently under clinical practice conditions. Bioavailability of DFX FCT compared to DT resulted higher than expected [Cmax: 99.5 (FCT) and 69.7 (DT) μMol/L; AUC: 1278 (FCT) and 846 (DT), P < 0.0001]. DFX FCT was also superior in scalability among doses. After one year of treatment for each formulation, no differences were observed between the treatments in the overall iron overload levels; however, DFX FCT but not DT showed a significant dose–response correlation [Spearman r (dose-serum ferritin variation): − 0.54, P < 0.0001]. Despite being administered at different dosages, the long-term safety profile was not different between formulations: a significant increase in renal impairment risk was observed for both treatments and it was reversible under strict monitoring (P < 0.002). Altogether, these data constitute a comprehensive comparison of DFX formulations in thalassaemia and other iron-loading anaemias, confirming the effectiveness and safety characteristics of DFX and its applicability for treatment tailoring.
Similar content being viewed by others
Introduction
Iron chelation therapy (ICT) represents a cornerstone in the management of iron overload in patients affected by thalassaemia and other iron-loading conditions, with a significant impact on morbidity and mortality1,2. Among the available drugs, deferasirox (DFX) represents the first-line iron chelator worldwide for the treatment of chronic iron overload due to blood transfusions in patients from the age of 2 years and older and for non-transfusion-dependent thalassaemia syndromes in patients aged 10 years and older3,4.
DFX is a tridentate ligand, with high affinity and specificity for iron. The active form is highly lipophilic and bound almost exclusively to serum albumin5,6. After absorption, the time to reach maximum plasma concentration (Tmax) is 1–4 h postdose; maximum plasma concentration (Cmax) and Area Under the concentration Curve over 24 h (AUC) linearly increase with dosage after a single administration and under steady-state condition. Its long half-life (t1/2, 11–19 h) allows a once-daily oral regimen. The main pathway of DFX metabolism is via glucuronidation by uridine diphosphate glucuronosyltransferase (UGT); a residual 6% of the prodrug is metabolized by cytochrome P450 (CYP) 1A1 and 2D66. The majority (84%) of DFX and its metabolites is eliminated via bile through multidrug resistance protein 2 (MRP2) and 8% by breast cancer resistance protein (BCRP)6,7; the remaining 8% is excreted in the urine. DFX is known to interact with other drugs (e.g. rifampicin), that could alter significantly its bioavailability8.
Extensive clinical trial programs in adult and paediatric patients with thalassaemia, myelodysplastic syndromes (MDS), sickle-cell disease, and other rare anaemias gathered data about the efficacy and the safety of DFX3,9,10,11, proving it to be comparable to the other chelators3,10,12,13 and demonstrating its undisputed advantage for patient compliance and adherence to long-term treatment10,12,13,14. However, increasing evidence suggests that some patients under DFX treatment do not achieve adequate chelation, even if receiving a maximal dose of the drug (poor responders). A small-scale study observed that poor responders had significantly lower systemic drug exposure compared to controls, identifying the difference in bioavailability to be the main cause of this effect15. Further studies identified polymorphism in UGT1A1, CYP1A1 and CYP1A2 to be predictive factors of inadequate response to DFX treatment16.
DFX long-term administration is known to be associated with various adverse events and mild to severe alterations of hepatic and kidney functions are the most relevant among them. Reports of drug-induced liver injury, including hepatic failure, have been reported during post-approval use of this compound, showing a hepatocellular pattern of injury with marked elevations in serum aminotransferase in the most severe cases17,18. A reversible, mild increase in serum creatinine (sCr) has been reported since the core registration trials19; successively, acute nephrotoxicity related to DFX was reported in cases of proximal tubulopathy and Fanconi syndrome or acute interstitial nephritis. Due to the risk of serious adverse reactions, including acute kidney injury (AKI), hepatic failure, and gastrointestinal (GI) haemorrhage, strict monitoring of renal and hepatic function is recommended when administering DFX4. A comprehensive analysis requested by the FDA Paediatric Advisory Committee established a clear relationship between high-dose DFX [> 30 mg/kg/day (DT) or > 21 mg/kg/day (FCT or sprinkle granules)], low ferritin (< 1000 μg/L) and the risk of AKI. Analyses of pooled data from three clinical studies found that small decreases in estimated glomerular filtration rate (eGFR) among paediatric patients due to small body surface area are associated with significantly increased DFX plasma concentrations, possibly leading to severe nephrotoxicity in case of over-chelation20.
To overcome the GI tolerability and palatability issues of the first licensed preparation in Dispersible Tablets (DFX DT), a Film-Coated Tablets formulation (DFX FCT) was released in 2016 and is now available in most countries. The new formulation allows a single-step administration (without preparation through water dispersion) and less stringent food restrictions compared to DFX DT4. Due to higher bioavailability, the new formulation contains 30% less active principle. The lack of food effect on the bioavailability of DFX FCT formulation is expected to improve adherence to the therapy schedule and lead to more predictable drug exposure. The randomized, open-label, phase II ECLIPSE study compared original DFX DT formulation with the dose-adjusted FCT in patients with thalassaemia and lower-risk MDS and demonstrated similar safety profiles with fewer tolerability issues, together with greater satisfaction and palatability in the FCT group. The imbalance in reported renal events observed between the two treatment arms was likely attributed to a larger proportion of patients receiving a higher than recommended dosage in the FCT group, as well as non-adherence to protocol-recommended dose modifications21. Since the recent introduction of the new formulation, only a few data were reported about long-term effects of DFX FCT outside clinical trial settings22,23,24, and very few independent comparisons of the two formulations are currently available.
Design and methods
Ethics declarations
The study was approved by the local ethical committee (Comitato Etico Interaziendale A.O.U. San Luigi Gonzaga di Orbassano e AA.SS.LL. TO3-TO4-TO5, Registration Number 79/2012) and performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. DFX PK curve assessment has been approved by the hospital licensing committee and included in the clinical routine, fully reimbursed by the public health system. Each patient or legal guardian signed the informed consent.
Study design
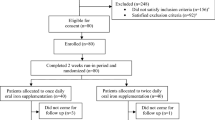
A retrospective observational study was designed based on clinical records of procedures performed for clinical practice and available at Haemoglobinopathies Centre in Orbassano (To), Italy, from 01 January 2011 to 31 March 2019. The aim of the study was to compare pharmacokinetics (PK), pharmacodynamics (PD) and safety of both DT and FCT DFX formulations in a group of patients with thalassaemia and other congenital anaemias. Two separate and subsequent sections were considered and compared to each other: one accounting for data about DFX DT and the other accounting for data about DFX FCT (Suppl. Fig. 1). Each patient took part in both of these sections, so that measures obtained for DT were compared with those obtained for FCT for every participant. All patients were evaluated for PK; in addition, a subgroup defined by inclusion and exclusion criteria was evaluated for PD and safety. The study time was not selected previously and was therefore determined by the available clinical records in the time frame considered.
Inclusion and exclusion criteria
To be included in this study, patients must have had a diagnosis of hereditary iron-loading anaemia or other red blood cell disorders requiring iron chelation therapy. In addition, they must have had a record of at least one PK evaluation for both DFX DT and FCT in the period considered for the study. Patients that performed ≥ 12 months monotherapy with DFX DT and ≥ 12 months monotherapy with DFX FCT and performed ≥ 2 Liver Iron Concentration (LIC) measurements for each treatment period (± 3 months from the time of drug administration, performed ≥ 6 months apart) were included in the analysis of PD, safety and compliance. Patients treated by administration of any other iron chelator than DFX at the time of evaluation were included only in the PK analysis and excluded from the study of PD, safety and compliance. (Fig. 1).
Study Population. Deferasirox (DFX) pharmacokinetic (PK) tests performed for clinical practice between 01 January 2011 and 31 March 2019 were considered. 74 patients who performed one PK tests for DFX dispersible tablets (DT) and one for film-coated tablets (FCT) formulation formed the study population for the PK analysis. Among them, only those who completed at least 1 year of treatment with each formulation alone were further included for the pharmacodynamics and safety analysis of this study.
Patient demographics and clinical characteristics
Data about personal and clinical condition (gender, age, Body Mass Index (BMI), state of pubertal activation according to Tanner’s scale), were collected for all patients from the clinical records available in the study period. Diagnoses were collected from clinical records. Diagnoses of β-thalassaemia were classified as thalassaemia major or intermedia, according to their clinical presentation (β-thalassaemia major entails lifelong regular transfusion requirement while thalassaemia intermedia is referred to patients who do not require transfusions or may require occasional or even frequent transfusions for defined periods of time). DFX DT (Exjade, Novartis Pharma) and DFX FCT (Exjade, Novartis Pharma) were administered as standard of care in successive and separate times. Data about administered doses were collected from clinical records.
Pharmacokinetics
PK study was performed in a controlled setting, where patients underwent ≥ 48 h washout from last dose of ICT, if not naive. Possible confounding or PK-altering factors, such as occasional administration of non-steroidal anti-inflammatory drugs or ethanol consumption were prohibited for ≥ 48 h before performing PK. A trained nurse supplied patients with the indicated dose of DT formulation, carefully ensuring a proper dispersion and administration, while FCT was supplied directly in the required amount of tablets. Patients were fasting before DT administration, while a light meal was allowed according to each patient’s preference and usual custom for FCT. Serum DFX concentrations were measured by HPLC–UV method, as previously described25. Briefly, a simple protein precipitation extraction procedure was applied on 500 µL of plasma aliquots: 100 µL of internal standard (IS) working solution, made at the final concentration of 100 µg/mL in methanol and used immediately, was added to plasma sample. Then 750 µL of protein precipitation solution (methanol:acetonitrile 50:50, v/v) was added to each sample. After brief mixing (30 s), samples were centrifuged at 12,000 rpm for 15 min and 800 µL of the obtained supernatant were transferred to vials, for injection in column (20 µL). Chromatographic separation was achieved on a C18 reverse phase column and eluate was monitored at 295 nm, with 8 min of analytical run. Quantification of DFX was performed by IS use: calibration curves, over the concentration range chosen, were built with the spike height ratios of each standard calibration and IS, and fitted using linear regression. This method has been validated following Food and Drug Administration procedures: mean intra and inter day variability was 4.64 and 10.55%; mean accuracy was 6.27%; mean extraction recovery 91.66%. Calibration curves ranged from 0.078125 to 40 µg/mL. Limit of quantification was set at 0.15625 while limit of detection at 0.078125 µg/mL. For each patient, a 5-timepoints PK curve was obtained, measuring DFX plasma concentration at 0 h (before administration) and 2 h, 4 h, 6 h and 24 h after administration. Maximum plasma concentration (Cmax) and Area Under Curve (AUC) were calculated accordingly.
Pharmacodynamics and safety
Serum ferritin (FTN) determined by chemiluminescence and Liver Iron Concentration (LIC) measured by superconducting quantum interference device (SQUID) were collected from the clinical records available in the study period. The single reported value of FTN is calculated as the mean of all FTN values available in the time ± 1.5 months from the date of LIC measure. In order to account for different times of observation and different conditions at baseline for each patient, the rate of variation of FTN and LIC (ΔFTN and ΔLIC, respectively) was considered instead of the absolute difference. Briefly, the difference between baseline and end-of-study values of FTN and LIC was normalized for the actual days of observation). For patients transfused during the study period, mean iron intake of the previous year was calculated from their clinical records, based on the number of RBC units received, using the formula: transfusion volume x haematocrit x 1.08, as reported previously26,27. Compliance was assessed from patients’ self-report and validated by drug accountability and physicians’ estimation. Concomitant medications administered in the study period were collected from clinical records. Data regarding safety (alanine aminotransferase (ALT), aspartate aminotransferase (AST), sCr, protein/creatinine ratio) were collected from all clinical records available in the study period. eGFR was calculated using Cockcroft-Gault and Bedside Schwartz formula for patients aged ≥ 14 and < 14 years, respectively. Acute Kidney Injury (AKI) was defined according to guidelines as previously described20, specifically, an episode of AKI was reported if reaching any of the following thresholds: eGFR < 90 mL/min/1.73 m2 if baseline ≥ 100 mL/min/1.73 m2 or eGFR < 75% of baseline if otherwise and any case of eGFR < 60 mL/min/1.73 m2). Chronic Kidney Disease (CKD) risk was defined according to guidelines28. For PD and safety analysis, study time was defined as the period between two subsequent LIC measures, considered as the treatment period. The end of study was defined as the last measure obtained within the treatment period.
Statistical analysis
Continuous variables were described by mean and standard deviation and categorical variables by frequency and percentage. Wilcoxon matched-pairs test and Spearman's r index were used to analyze differences and correlations between DFX DT and DFX FCT treatments. Exact Clopper–Pearson test was used to analyze differences between probabilities of events. A significance threshold of 0.05 was considered for all tests. A quantile regression was used to analyze the correlation between PK or PD parameters and dose adjusted by age, BMI, sex and pubertal activation. Median regression was used in this analysis. The quantile regression was chosen to avoid data transformation due to the non-normal distribution of the errors and 95% confidence intervals were estimated by bootstrapping (500 samples). Statistics were calculated using Statsoft Statistica 10 or R software version 4.0.229.
Results
Patients' characteristics
In the period considered, 123 and 210 patients underwent at least one PK evaluation for DFX DT and FCT administration, respectively. Seventy-four patients diagnosed with β-thalassaemia major (61), β-thalassaemia intermedia (11), Diamond-Blackfan anaemia (1) and Stomatocytosis (1) met inclusion criteria for PK analysis; among them, 36 (49%) were female. Mean age was 32 ± 11 years at DT and 37 ± 11 years at FCT study time (Table 1). Nine were paediatrics. Forty-nine out of 74 patients were subsequently included in PD and safety analysis. Twenty-five were excluded according to exclusion criteria (Fig. 1). Mean observational study time was 11.6 ± 3.5 and 11.3 ± 3.3 months per patient for DT and FCT, respectively, corresponding to a total time of 47.5 (DT) and 46.1 years (FCT). Actual administered dose was adjusted during the study time when clinically indicated; however, no statistically significant differences were observed between doses tested at PK and mean administered dose. No patients had a prescription for any drug known to potentially modify DFX plasma concentration.
Pharmacokinetics analysis
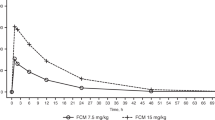
When performing PK measurement, DFX testing dose was established for each patient according to their clinical condition. Overall, it was tested at a mean dose of 26.1 ± 6.9 and 15.5 ± 5.2 mg/kg for DT and FCT, respectively. FCT dose tested at PK was therefore 41% lower than DT. Despite the lower dose tested, both Cmax and AUC were significantly superior in the FCT formulation, and specifically mean Cmax measured 71.0 ± 35.2 (DT) and 101.5 ± 40.3 (FCT) μMol/L (P < 0.001) and AUC measured 855.0 ± 499 (DT) and 1301.3 ± 658 (FCT) (P < 0.001). (Fig. 2A–D). Variation coefficients of the mean measured 50.6% (DT) and 40.5% (FCT) for Cmax and 58.9% (DT) and 51.5% (FCT) for AUC. Similar results were obtained when considering patients with different diagnoses in separate groups, i.e. thalassaemia major, thalassaemia intermedia and others. (Suppl. Fig. 2 + Suppl. Tab. 1). Successively, the correlation between the dose administered at PK and bioavailability indexes was analyzed. Spearman’s r (dose-Cmax) measured 0.32 (P = 0.006) and 0.53 (P < 0.0001) and Spearman’s r (dose-AUC) measured 0.27 (P = 0.02) and 0.45 (P < 0.0001) for DT and FCT, respectively. (Fig. 2E,F). When considering each diagnosis separately, the highest correlation was observed for patients affected by thalassaemia intermedia, resulting in a Spearman’s r (dose-Cmax) of 0.93 (P = 0.0001). (Suppl. Tab. 2). To better evaluate factors that could act as modifiers of drug exposure, correlation with gender, age, state of pubertal activation, and BMI were also investigated. None of these factors contributed to a significant difference in DFX bioavailability (Suppl. Tab. 3).
Pharmacokinetics: (a) Mean PK curve at all doses, for DFX DT and FCT. Blood samples were obtained pre-dose and at 2, 4, 6 and 24 h post dose administration. Mean and standard deviation are shown. (b–d) DFX was tested at a mean dose of 26.1 ± 6.9 (DT) and 15.5 ± 5.2 (FCT) mg/kg (− 41%), resulting in a maximum plasma concentration (Cmax) of 71.0 ± 35.2 (DT) and 101.5 ± 40.3 (FCT) µMol/L (+ 43%) and an Area Under the Curve (AUC) of 855.0 ± 499 (DT) and 1301.3 ± 658 (+ 52%). Error bars: standard deviation. (e) Cmax correlated significantly with dose for both formulations, but the observed correlation was stronger for FCT: Spearman's r (dose-Cmax) = 0.32 (P = 0.006) and 0.53 (P < 0.0001) for DT and FCT, respectively. (f) AUC correlated significantly with dose for both formulations, but the observed correlation was stronger for FCT: Spearman's r (dose-AUC) = 0.27 (P = 0.02) and 0.45 (P < 0.0001) for DT and FCT, respectively.
Pharmacodynamics analysis
To evaluate the effect of DFX administration over the treatment period, ΔFTN and ΔLIC levels registered for DT and FCT treatment were measured in a subset of patients. Baseline levels of iron overload were similar among treatment periods with each formulation. Specifically, FTN levels were not different, while LIC levels were slightly more elevated at the beginning of FCT study time (Table 1). No significant variations were observed for the whole group, nor for any of the diagnoses considered separately. (Fig. 3A,B, Suppl. Fig. 3 + Suppl. Tab. 4). The iron input calculated for those patients receiving blood transfusions did not differ significantly. (Suppl. Fig. 4).
Pharmacodynamics. Iron overload changing during treatment measured by yearly variation of serum ferritin (ΔFTN) (a) and LIC (ΔLIC) (b) . Mean ΔFTN measured + 277 ± 1473 (DT) and − 24 ± 992 (FCT) ng/mL/year (ns); ΔLIC measured − 0.4 ± 3.2 (DT) and − 0.1 ± 2.7 (FCT) mg/g Fe d.w./year (ns). Correlation between administered dose of DFX FCT and iron overload variation measured both as (c) ΔFTN and (d) ΔLIC. Spearman's r (dose-ΔFTN) = − 0.54 (P < 0.0001) and Spearman's r (dose-ΔLIC) = − 0.32 (P = 0.02). (e) DFX FCT dose plotted as variation from the mean and correspondent ΔFTN variation for each patient. (f) ΔFTN in patients who received a DFX FCT dose lower (range 6.4–15.3 mg/kg/day; N = 28) or higher (range 15.6–28.2 mg/kg/day; N = 21) than the mean of all FCT doses considered in the study. (P = 0.001). Whisker plots show median (line), 25 to 75 percentile (box), 5–95 percentile (error bar) and individual results out of previous ranges (dots). ns: not significant.
The correlation among dose and ΔFTN or ΔLIC levels variation was assessed for both formulations, resulting in a significant dependence for FCT, but not for DT. Spearman's r (dose-ΔFTN) measured − 0.54 (P < 0.0001) and Spearman's r (dose-ΔLIC) measured − 0.32 (P = 0.02) for FCT formulation, while it was not significant for DT (Fig. 3C–E). This correlation was higher for patients affected by thalassaemia major [Spearman's r (dose-ΔFTN) measured − 0.69 (P < 0.0001) and Spearman's r(dose-ΔLIC) measured − 0.39 (P = 0.01) for FCT formulation], while it was not significant for the other diagnoses. Comparison of iron overload variation between patients that received an FCT dose lower than the mean (range 6.4–15.3 mg/kg/day; N = 28) and those that received a higher one (range 15.6–28.2 mg/kg/day; N = 21) resulted in a significant difference in terms of ΔFTN (P = 0.001), but not ΔLIC (Fig. 3F). Gender, age, state of pubertal activation or BMI did not alter DFX PD significantly in this group of patients (Suppl. Tab. 5). Mean compliance measured 89.3 ± 12.1% and 91.6 ± 10.4% for DT and FCT, respectively, showing a limited but significant increase for the latter.
Safety
Safety of DFX formulations was evaluated by the analysis of hepatic and renal function monitored during treatment. Baseline values were not different between formulations. Direct comparison of baseline to end-of-study values did not identify any relevant difference between formulations for both hepatic enzyme levels and sCr levels (Suppl. Fig. 5). No differences were observed considering each diagnosis separately. (Suppl. Tab. 6). Measuring AKI episodes based on eGFR reduction from baseline, we registered 1.06 ± 1.8 and 0.48 ± 1.9 episodes/patient/year during DT and FCT treatment, respectively (P = 0.02). Correlation of the number of AKI episodes with Cmax or AUC was significant for FCT, but not DT (Suppl. Tab. 7). Furthermore, to evaluate the risk related to long-term DFX administration, CKD risk measured at baseline was compared with the highest CKD risk class reached during treatment. A significant increase was registered for both DT (P < 0.0001) and FCT treatment (P = 0.0007) and no differences were observed between the two formulations in terms of probability of CKD risk class worsening during treatment when taking FCT compared to DT. The mean time to reach the worst score during treatment was not different (12 ± 12 weeks for DT and 9 ± 11 weeks for FCT formulation). Relevantly, no significant differences were observed between baseline and end-of-treatment risk of CKD (Fig. 4).
Chronic Kidney Disease (CKD) risk assessment. Sankey diagrams reproduce how patients were distributed among different CKD risk classes at baseline, at the highest risk reached during treatment and at the end of treatment considered in the study. For both DFX DT and FCT, the distribution within risk classes is not significantly different between the baseline and the end of treatment, but it is significantly shifted toward higher risk during treatment (P < 0.0001 (DT) and P = 0.0007 (FCT), one-sided test). The difference of probabilities of increasing CKD risk class during DFX treatment between DT and FCT formulation is not significant. ns: not significant.
Discussion
DFX is the most recent iron chelator available to treat hemosiderosis; firstly released in DT formulation, it was later substituted by FCT formulation. However, only a few reports currently exist about long-term effects of DFX and, specifically, no independent data offer a direct and extensive comparison of the two formulations of DFX. We present here the first study analyzing pharmacokinetics (PK), pharmacodynamics (PD) and safety of subsequent formulations of DFX DT and DFX FCT in everyday clinical practice of iron overload treatment in β-thalassaemia and other rare anaemias.
Primarily, we found that DFX FCT was superior to DT in terms of both Cmax and AUC, even at less than recommended dosage (− 41% compared to DT). Considering the indication of 30% lower dose of FCT compared to DT (or conversion factor 1.43) reported by the ECLIPSE study21, these results indicate an increased bioavailability and thus an exposure to the drug higher than expected for FCT when compared to DT formulation. We speculate that this difference could be likely due to different compositions between formulations. However, no additional data are available in this study to support this hypothesis and the absence of 24-h PK results from the ECLIPSE study limits a broader comparison. Interestingly, DFX FCT showed a higher correlation among dose and bioavailability parameters, namely Cmax and AUC, indicating a better correspondence between administered dose and serum concentration. In addition, the variation coefficients of the mean were lower for FCT than DT for both Cmax and AUC, in agreement with the results reported during the phase II study that led to FCT approval by FDA21. In terms of clinical practice, this supports the idea that less variability should be expected in the therapeutic effect observed when a selected dose is administered, allowing for more accurate regulation of the therapy, or scalability. Notably, since all the patients included in the study were subsequently treated with both formulations and matched, we speculate that the differences here reported are mostly due to inter-patient variability. In addition, the consistency of results among different diagnoses indicates a minor contribution of the underlying pathology in determining the response to the drug. For both formulations, measurements of DFX bioavailability at a given dose remained consistent regardless of gender or age, considered both as nominal age and as state of pubertal activation, potentially excluding the need of adjusting the dose according to these parameters. However, even if no differences were observed in the pharmacokinetic profile of the limited group of paediatric patients considered, recent reports20,30,31 highlighted the critical role of surveillance for this category.
Furthermore, the PD study provided data about the impact of DFX treatment under real-world conditions. To overcome a possible bias due to partially different conditions at baseline for the two study periods (namely, higher levels of iron overload measured by LIC, but not FTN, when starting FCT), the rate of variation instead of the total amount of iron overload was considered. As expected, no significant differences were observed when patients were treated with DFX DT or FCT, underlining an effective dynamic equilibrium among iron loading and elimination through chelation. The effect was confirmed to be dose-dependent for FCT formulation, but not DT, as demonstrated by the correlation between the administered dose and the efficacy parameters ΔFTN and ΔLIC. When considering subgroups of patients with the same diagnosis, this correlation was confirmed only for thalassaemia major, but the statistical analysis for the other groups was considerably limited by the small sample size. The moderate correlation coefficients measured even in the presence of a highly consistent pairing, however, indicate that the drug dose alone is not sufficient to predict its effect, suggesting a role of other unknown effect-modifiers. Further studies will be required to properly investigate and address the characteristics of these factors. The decrease in FTN observed in patients that took a FCT dose above the mean of all doses administered during the study (ΔFTN = − 504 ± 1216 ng/mL/year at mean dose of 20.2 mg/kg, N = 21) is consistent with the long-term results from patients that took part in the ECLIPSE study (ΔFTN = − 618 ± 1013 ng/ml in 12 months at mean dose of 21.3 mg/kg, N = 36)32. Altogether, these results indicate and confirm that DFX FCT is reliable in terms of dose adjusting and effects, thus suggesting the possibility of its better scalability compared to DT, as already observed in the PK analysis for this formulation.
Compliance to DFX DT was already close to optimal in the considered series and was slightly but consistently higher for FCT, confirming what was expected from previous reports about the acceptability of this formulation21,23,32,33. The evaluation of the impact of DFX FCT on compliance for patients with non-optimal adherence to therapy or a possible psychological role of novelty itself in long-term treatment is beyond the scope of this work.
Finally, the safety profile of DFX therapy was evaluated to assess the effects of long-term exposition to the drug. In agreement with previous results21,34, hepatic and renal functions at the end of study did not differ significantly from the levels registered at baseline. However, the analysis of renal function throughout the whole study time highlighted a consistent increased risk of CKD during treatment, comparable for both formulations. Retrospective assessment of CKD risk class in patients treated with DFX showed a consistent increase during treatment; nevertheless, the final distribution of patients in risk classes did not differ from the baseline. In addition, we report here that the number of AKI episodes retrospectively measured by laboratory findings was consistently lower for DFX FCT and correlated significantly with both DFX FCT Cmax and AUC, but not with the overall dose administered. On the one side, this result suggests a possible role of this formulation in limiting acute renal complications through a more reliable dose-scaling, as previously discussed. On the other side, it indicates that the evaluation of DFX PK profile provides a more accurate instrument to limit drug-related toxicity than considering the administered dose alone. It is indeed essential to consider that careful management of these patients allowed the rapid adaptation of the ongoing therapy to any relevant adverse event, adjusting the dose or even suspending it if necessary, thus limiting any possible toxicity. Taken together, these observations indicate that (1) DFX is associated with consistent renal function impairment during long-term treatment and (2) these alterations could be fully reversible when strict monitoring is maintained.
Evidence of both observations was already available for DT formulation, although limited to a few patients with a long follow-up time (up to 108 weeks)35 and a reversible, mild increase in sCr has been reported since the core registration trials19,34. Different studies are available for FCT and none of them extensively evaluated its effects on renal function in a direct confrontation with DT at the indicated corresponding dose. The ECLIPSE study was limited to 24 weeks21 and its long-term extension considered FCT only, evaluating sCr, creatinine clearance, and urinary protein/creatinine ratio for up to 2 years in a larger group of patients with very different diagnoses32, reporting no major concerns about renal safety. A single-centre study analyzed the switch from DFX DT to FCT in 74 patients with thalassaemia and other transfusion-dependent conditions from all ages and reported a modest rise in mean sCr, with 6 patients (8%) having a sCr increase of > 30% from baseline, and 5 out 6 returning to the normal range by dose adjustment22. A separate study conducted in patients with low iron overload did not show an effect on decreased renal function, considered as ≥ 2 × upper limits of sCr levels or eGFR estimated by EPI24. However, the presence of several cases of proximal tubular toxicity in paediatric and adult patients taking DFX30,31,36,37 underlined the limitations of sCr alone as a sufficient and reliable marker of kidney injury, drawing attention to the necessity of more sensitive evaluations30,31,36,37,38,39,40,41. Therefore, this study provides for the first time a long-term direct comparison of both formulations, confirming the overall safety profile of the newest and highlighting the need for constant and careful drug monitoring to avoid side effects associated with DFX FCT chronic administration.
Among the limitations of this study, we highlight that inclusion criteria did not consider patients that terminated DFX before completing ≥ 12 months of treatment due to any cause, including poor tolerability or drug-related adverse events. Since these occurrences were not considered in the present study, caution has to be taken when applying the results presented to a broader population. Also, we did not include in the study the evaluation of the clinical condition of patients in the time frame between each period considered for DT and FCT study. For this reason, we are not able to motivate or explain the possible differences observed at FCT baseline when compared to DT. In addition, evaluation of PD did not account for sources of iron intake other than blood transfusions, e.g. dietary iron absorption. Finally, the patient population showed some variability in terms of diagnosis, basal conditions, and time of observation: while on the one hand this mirrors the heterogeneity typical of a real-world setting, on the other it could limit the consistency of results. To this regard, confirming the analysis carried out so far on a larger sample of patients could be particularly beneficial for those affected by iron-loading conditions different from thalassaemia major, that are under-represented within this population. Altogether, considering the data and results here reported alongside the increasing and somehow contrasting reports related to DFX, we think that a broader evaluation of DFX safety and effectiveness besides the clinical trials would be necessary, to improve the appropriateness of its use in different pathologies and clinical settings, optimizing the ratio among effectiveness and toxicity and moving a step forward in treatment tailoring and personalization.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Gabutti, V. & Piga, A. Results of long-term iron-chelating therapy. Acta Haematol. 95, 26–36 (1996).
Borgna-Pignatti, C. et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89, 1187–1193 (2004).
Piga, A. et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica 91, 873–880 (2006).
Novartis Pharmaceutical. Prescribing information for Exjade (deferasirox). (2018).
Tanaka, C. Clinical pharmacology of deferasirox. Clin. Pharmacokinet. 53, 679–694 (2014).
Waldmeier, F. et al. Pharmacokinetics, metabolism, and disposition of deferasirox in beta-thalassemic patients with transfusion-dependent iron overload who are at pharmacokinetic steady state. Drug Metab. Dispos. 38, 808–816 (2010).
Bruin, G. J. M. et al. Pharmacokinetics, distribution, metabolism, and excretion of deferasirox and its iron complex in rats. Drug Metab. Dispos. 36, 2523–2538 (2008).
Skerjanec, A., Wang, J., Maren, K. & Rojkjaer, L. Investigation of the pharmacokinetic interactions of deferasirox, a once-daily oral iron chelator, with midazolam, rifampin, and repaglinide in healthy volunteers. J. Clin. Pharmacol. 50, 205–213 (2010).
Galanello, R. et al. Phase II clinical evaluation of deferasirox, a once-daily oral chelating agent, in pediatric patients with β-thalassemia major. Haematologica 91, 1343–1351 (2006).
Cappellini, M. D. et al. Aphase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood 107, 3455–3462 (2006).
Porter, J. et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): A 1-yr prospective study. Eur. J. Haematol. 80, 168–176 (2008).
Cermak, J. et al. A comparative study of deferasirox and deferiprone in the treatment of iron overload in patients with myelodysplastic syndromes. Leuk. Res. 37, 1612–1615 (2013).
Maggio, A. et al. Evaluation of the efficacy and safety of deferiprone compared with deferasirox in paediatric patients with transfusion-dependent haemoglobinopathies (DEEP-2): A multicentre, randomised, open-label, non-inferiority, phase 3 trial. Lancet. Haematol. 7, e469–e478 (2020).
Bollig, C. et al. Deferasirox for managing iron overload in people with thalassaemia. Cochrane database Syst. Rev. 8, 007476 (2017).
Chirnomas, D. et al. Deferasirox pharmacokinetics in patients with adequate versus inadequate response. Blood 114, 4009–4013 (2009).
Allegra, S. et al. Deferasirox pharmacogenetic influence on pharmacokinetic, efficacy and toxicity in a cohort of pediatric patients. Pharmacogenomics 18, 539–554 (2017).
Menaker, N. et al. Acute liver failure during deferasirox chelation: A toxicity worth considering. J. Pediatr. Hematol. Oncol. 39, 217–222 (2017).
Feldman, E. A., Miller, C. D., Wojnowicz, S. & Seabury, R. Significant hyperbilirubinemia and acute hepatocellular jaundice in a pediatric patient receiving deferasirox: A case report. J. Pediatr. Pharmacol. Ther. 23, 64–67 (2018).
Origa, R. et al. Renal safety under long-course deferasirox therapy in iron overloaded transfusion-dependent β-thalassemia and other anemias. Am. J. Hematol. 93, E172–E175 (2018).
Bird, S. T. et al. Effects of deferasirox dose and decreasing serum ferritin concentrations on kidney function in paediatric patients: An analysis of clinical laboratory data from pooled clinical studies. Lancet. Child Adolesc. Health 3, 15–22 (2019).
Taher, A. T. et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study. Am. J. Hematol. 92, 420–428 (2017).
Cheesman, S. et al. Real-world experience of switching from deferasirox dispersible to film-coated tablets: Impact on adherence to chelation therapy, iron overload and renal function. Blood 132, 1062–1062 (2018).
Quarta, A. et al. Switching from dispersible to film coated tablet formulation of deferasirox improves hemoglobin levels and transfusional interval in patients with transfusion-dependent-thalassemia. Br. J. Haematol. 189, e60–e63 (2020).
Scaramellini, N. et al. A holistic approach to iron chelation therapy in transfusion-dependent thalassemia patients with serum ferritin below 500 μg/L. Am. J. Hematol. 95, E230–E232 (2020).
De Francia, S. et al. A new HPLC UV validated method for therapeutic monitoring of deferasirox in thalassaemic patients. J. Chromatogr. B. 893–894, 127–133 (2012).
Piga, A. et al. Assessment and management of iron overload in β-thalassaemia major patients during the 21st century: A real-life experience from the Italian Webthal project. Br. J. Haematol. 161, 872–883 (2013).
Wood, J. C. Guidelines for quantifying iron overload. Hematology 2014, 210–215 (2014).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 120, c179–c184 (2012).
R Core Team. R: A Language and Environment for Statistical Computing. (2020).
Rheault, M. N., Bechtel, H., Neglia, J. P. & Kashtan, C. E. Reversible Fanconi syndrome in a pediatric patient on deferasirox. Pediatr. Blood Cancer 56, 674–676 (2011).
Dubourg, L. et al. Deferasirox-induced renal impairment in children: An increasing concern for pediatricians. Pediatr. Nephrol. 27, 2115–2122 (2012).
Tartaglione, I. et al. Two-year long safety and efficacy of deferasirox film-coated tablets in patients with thalassemia or lower/intermediate risk MDS: Phase 3 results from a subset of patients previously treated with deferasirox in the ECLIPSE study. Exp. Hematol. Oncol. 9, 20 (2020).
Taher, A. T. et al. Patient-reported outcomes from a randomized phase II study of the deferasirox film-coated tablet in patients with transfusion-dependent anemias. Health Qual. Life Outcomes 16, 216 (2018).
Cappellini, M. D. et al. Tailoring iron chelation by iron intake and serum ferritin: The prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica 95, 557–566 (2010).
Piga, A. et al. Deferasirox effect on renal haemodynamic parameters in patients with transfusion-dependent $β$ thalassaemia. Br. J. Haematol. 168, 882–890 (2015).
Naderi, M. et al. A prospective study of tubular dysfunction in pediatric patients with beta thalassemia major receiving deferasirox. Pediatr. Hematol. Oncol. 30, 748–754 (2013).
Capolongo, G. et al. Urinary metabolic profile of patients with transfusion-dependent β-thalassemia major undergoing deferasirox therapy. Kidney Blood Press. Res. 45, 455–466 (2020).
Rafat, C., Fakhouri, F., Ribeil, J.-A., Delarue, R. & Le Quintrec, M. Fanconi syndrome due to deferasirox. Am. J. kidney Dis. 54, 931–934 (2009).
Grangé, S., Bertrand, D. M., Guerrot, D., Eas, F. & Godin, M. Acute renal failure and Fanconi syndrome due to deferasirox. Nephrol. Dial. Transplant. 25, 2376–2378 (2010).
Yacobovich, J. et al. Acquired proximal renal tubular dysfunction in β-thalassemia patients treated with deferasirox. J. Pediatr. Hematol. Oncol. 32, 564–567 (2010).
Yui, J. C., Geara, A. & Sayani, F. Deferasirox-associated Fanconi syndrome in adult patients with transfusional iron overload. Vox Sang https://doi.org/10.1111/vox.13064 (2021).
Acknowledgements
The authors thank Prof. Micheal Mostert, MD and Keegan J. Brighton, BSc for their critical revision of the language and communicability of the manuscript.
Author information
Authors and Affiliations
Contributions
A.Piol. designed the study, collected data, performed the statistical analysis and wrote the manuscript; P.B. planned, performed and reviewed statistical analysis; S.A. and S.D.F. performed PK measurements of DFX; G.B.F. reviewed results and manuscript; A.Piga designed the study and reviewed results, F.L. designed the study, performed LIC measurements by SQUID, reviewed results and wrote the manuscript. All authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
No sponsorship was obtained for this study. APiga received institution funding by Acceleron Pharma, Inc (Massachusetts, USA) and Celgene Corporation (New Jersey, USA). APiol, PB, SA, SDF, GBF and FL declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piolatto, A., Berchialla, P., Allegra, S. et al. Pharmacological and clinical evaluation of deferasirox formulations for treatment tailoring. Sci Rep 11, 12581 (2021). https://doi.org/10.1038/s41598-021-91983-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91983-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.