Abstract
This study sought to assess the feasibility of design, adherence, satisfaction, safety and changes in outcomes followed by a home-based foot–ankle exercise guided by a booklet in individuals with diabetic peripheral neuropathy (DPN). 20 participants were allocated usual care [control group (CG)] or usual care plus home-based foot–ankle exercises [intervention group (IG)] for 8 weeks. For feasibility, we assessed contact, preliminary screening and recruitment rates, adherence, and using a 5-point Likert scale to satisfaction and safety of the booklet. In the IG, we assessed preliminary changes in DPN symptoms, DPN severity (classified by a fuzzy model) and foot–ankle range of motion between baseline and Week 8. In the first 20 weeks, 1310 individuals were screened for eligibility by phone contact. Contact rate was 89% (contacted participants/20w), preliminary screening success 28% (participants underwent screening/20w), and recruitment rate 1.0 participants/week (eligible participants/20w). The recruitment rate was less than the ideal rate of 5 participants/week. The adherence to the exercises programme was 77%, and the dropout was 11% and 9% for the IG and CG, respectively. In the IG, participants’ median level of satisfaction was 4 (IQR: 4–5) and perceived safety was 3 (IQR: 3–5). IG significantly decreased the DPN severity (p = 0.020), increased hallux relative to forefoot (first metatarsal) range of motion (ROM) (p < 0.001) and decreased maximum forefoot relative to hindfoot (midfoot motion) dorsiflexion during gait (p = 0.029). The home-based programme was feasible, satisfactory, safe and showed preliminary positive changes in DPN severity and foot motion during gait.
Trial Registration ClinicalTrials.gov, NCT04008745. Registered 02/07/2019. https://clinicaltrials.gov/ct2/show/NCT04008745.
Similar content being viewed by others
Introduction
Diabetic peripheral neuropathy (DPN) is the most common chronic complication of diabetes affecting up to 50% of the diabetic population, which has as a major outcome the formation of ulcers and amputation1. Complications including progressive loss of vibratory, thermal, tactile and proprioception sensitivities2; loss of motor units and motor axons3; skin breakdown4; and lower limb musculoskeletal complications disrupt the daily living activities and quality of life of people with diabetes5. Musculoskeletal complications include muscles and locomotor alterations, such as progressive atrophy of the foot–ankle extrinsic and intrinsic muscles leading to common foot deformities6; an increased proportion of fat tissue within foot muscles7 and altered mechanical properties of the calcaneal tendon8. Altered locomotion mechanics include impairments in the lower limbs’ muscle activation magnitude and timing9,10; decreased foot–ankle range of motion (ROM)6,11; high plantar pressure and changes in foot rollover temporal parameters12,13.
Therapeutic supervised exercises targeting the foot–ankle may be beneficial for people with DPN and were recommended for the first time in the most recent guidelines of the International Working Group on the Diabetic Foot14. It has been shown that supervised therapeutic foot–ankle exercises improve DPN signs and symptoms15,16,17,18; foot rollover and pressure-related variables while walking17,19,20,21,22; gait temporal parameters21,23,24; foot–ankle muscle strength16,23 and foot–ankle joint mobility17,25. However, not all positive effects are retained at follow-up26,27 suggesting the need for a continuous educational process for improving self-management and self-care.
Home-based exercise/rehabilitation is a self-management approach for people with chronic diseases. For most of this population, self-management is a lifetime taskl28. Recent clinical trials and reviews have shown that home-based exercises are safe, have a good compliance and presents efficacy for chronic diseases and conditions, such as cancer29, Parkinson’s disease30, cardiac and pulmonary diseases31, dementia32, stroke33 and complications of old age34. Although we did not find any evidence for the efficacy of a home-based exercise programme focusing on foot–ankle deficits in people with diabetes and DPN, Dadgostar et al.35 found positive results using an educational booklet that included resistance and aerobic exercises in people with diabetes. However, the general adherence of people with diabetes to exercise is poor36, which may compromise the efficacy of a home-based autonomous programme. Therefore, assessing the adherence of people with diabetes and DPN to a home-based, booklet-guided foot exercises programme prior to the development of a larger trial is pivotal because the efficacy of a therapeutic intervention is dependent on participants’ compliance.
Physiotherapy interventions using foot–ankle exercise have not yet been implemented worldwide to prevent the progression of musculoskeletal deficits in people with diabetes and DPN. For this reason, it is still unclear whether recruitment for a trial that tests the efficacy of these physiotherapy programmes would be feasible in this population. Thus, the feasibility of a preventive home-based therapeutic programme should first be studied to determine the best training progression in terms of exercises, frequency and intensity and the outcomes used to assess the success of the intervention.
At present, we are conducting a single-blinded, randomised controlled superiority trial with two parallel arms called the FOotCAre (FOCA) trial II to study the efficacy of an 8-week home-based therapeutic foot–ankle exercise programme using an educational booklet in people with DPN. The present study is a first step to investigating the feasibility of the trial in terms of contact, preliminary screening and recruitment rates, adherence to the home-based programme, participant safety and satisfaction and estimated preliminary changes in clinical aspects of DPN and biomechanical outcomes during gait. Our hypotheses are as follows: (H1) the programme will be feasible, (H2) the home-based intervention will show preliminary changes in the DPN symptoms, DPN severity and foot–ankle ROM during gait after 8 weeks.
Results
Feasibility outcomes
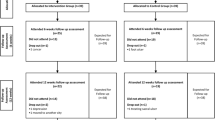
Recruitment was collected from April 23th to September 5th of 2019, totalling 20-week period from a database of 1310 individuals with diabetes mellitus, who were screened for eligibility by phone contact. We were not able to contact 142 candidates (11%) before the recruitment period ended, resulting in a contact rate of 89%. From the 1168 contacted individuals, 323 (28%) met the eligibility criteria as determined over the phone and were scheduled for further screening at the laboratory. Thus, the preliminary screening success was 28%. Further screening of the remaining 323 individuals showed that only 20 (6.2%) had moderate DPN (fuzzy score ≥ 2) and were thus eligible for the study, resulting in a recruitment rate of 1.0 participants/week. Twenty eligible individuals (17 female) were included in the baseline assessment. The challenges of the recruitment process and more details about participant recruitment are described in Fig. 1.
Adherence to the home-based programme was 77.7%, i.e., seven participants out of 9 completed more than 75% of the 24 sessions (at least 18 sessions), and five complete all 24 sessions. The mean (SD) number of sessions performed by all participants that completed the programme was 21.6 (3.5). Of the 20 participants enrolled, two (one each from the CG and IG) dropped out before the T8 assessment, resulting in dropout rates of 9.1% (CG) and 11.1% (IG), respectively. The reasons for dropout were personal reasons and distance from home to the laboratory.
The satisfaction with the programme was excellent to great, with a median score of 4 (IQR: 4–5). Overall, 44.1% considered the programme to be excellent (score of 5 on the Likert scale), 51.9% great (score of 4) and 11.1% good (score of 3). None of the participants rated the programme as poor (score of 2) or terrible (score of 1). For question 7, ‘Would you recommend using the booklet to others with diabetes?’, all participants answered ‘for sure’ (score of 5). Participants considered the booklet to be reasonably safe, with a median score of 3 (IQR: 3–5). No adverse events were reported.
Most of the participants performed the first 10 sessions while sitting, and the volume progressively increased, achieving its peak during session 11 (mean volume = 290) (Fig. 2). The progression of the exercises based on the posture adopted decreased between the sessions 11 and 13. For these sessions, 90% of the participants performed all exercises standing on both legs and 10% standing on one leg, but the overall volume was reduced by 20%, probably because of the increased intensity caused by the posture adopted. In the last four sessions, almost all participants performed all exercises on one leg, and the overall mean volume was 94. The mean number of repetitions performed for all exercises was 25.0 ± 4.5.
Changes in clinical and biomechanical outcomes
At the baseline, the groups were similar for all characteristics and outcomes assessed (Table 1). After 8 weeks, we observed a decrease in DPN severity (fuzzy score), increase in the hallux relative to forefoot (first metatarsal joint) ROM and decrease in the maximum forefoot relative to hindfoot (midfoot motion) dorsiflexion during gait in the IG. There was also an increase trend in the maximum hallux dorsiflexion relative to forefoot (first metatarsal joint), but the difference was not statistically significant (Table 2; Fig. 3).
Ensemble averaged kinematics of the hallux (HXFFA), forefoot (FFHFA) and hindfoot (HFTBA) joints in degrees (deg) relative to the proximal segment from de IG participants. Royal blue color indicates baseline assessment (T00), dark blue color indicates Week 8 assessment (T08). Error bars represent standard error. FS indicates foot strike, vertical lines (FO) indicate foot off event in the gait cycle.
Discussion
This feasibility study provides preliminary data from a limited sample of DPN participants to determine contact, preliminary screening and recruitment rates, adherence, satisfaction and safety of a home-based intervention.
The recruitment rate was far below laboratory capacity and what was initially anticipated. However, the recruitment of 20 participants in this study was reasonable for starting the RCT. The primary challenge to recruitment was the inclusion criteria. In addition to the age range and health conditions, participants had to have a score ≥ 2 for DPN severity. Mattishent et al.37 found a similar low recruitment rate in their feasibility study of individuals with diabetes. Based on this result, new strategies for maximising recruitment are needed. We are developing other strategies, such as advertising on social media and in public places (e.g., metro and bus stations) that this population frequents and broadening our recruitment centres to include primary care centres and secondary and tertiary hospitals. We are continuing to contact potential participants by telephone because this significantly increases recruitment success38.
Adherence to the home-based exercise programme exceeded expectations. This could be related to the high satisfaction with the programme, safety of the programme and absence of adverse events. Home-based exercise programmes usually result in a high compliance for several chronic diseases and in older adult populations29,30,31,32,33,34. Supportive elements incorporated into home-based designs, like printed materials, exercise equipment, telephone support, home visits and technology, can be utilised alone or in combination to encourage adherence39. Some of the reasons given by our participants for the high compliance and satisfaction and low dropout were the possibility of performing the exercises at home at any time (convenience), thus avoiding using crowded public transportation or heavy traffic. The programme was built to offer autonomy to the participant, with no need for supervision from a health professional when performing the exercises. Considering the high satisfaction and adherence, we feel confident that the purpose of the booklet was successfully achieved.
A low dropout rate among the IG was achieved (11%), which differs from other behavioural trials involving participants with diabetes. In a systematic review, Trivedi et al.40 had a mean retention rate of 77.5% (range 36–100%), defined as the ratio between the number of participants retained at follow-up and the number of participants enrolled. Our retention rate of 89% is highly superior.
The exercise volume in each session and progression were acceptable. After 8 weeks of intervention, approximately 95% of the participants who continued to perform the exercises were able to progress to the maximum level of difficulty. However, there was a decrease in the volume of sessions, which could be explained by the progressive increase in the intensity (body posture) of the exercises. In rehabilitation interventions, decreasing the volume and increasing the intensity are fundamental for gaining muscle strength, which is one of the objectives of the full RCT. Thus, this feasibility study showed that was not necessary to modify the initial exercise protocol included in the booklet.
The preliminary changes in outcomes should be interpreted with caution. The DPN severity decreased in the IG, representing an improvement in clinical aspects of DPN. The educational aspects of the programme may have contributed to changing the participants’ health care habits, resulting in better control of diabetes and thus DPN symptoms41. Our results corroborate with Sartor et al.16, who showed a reduction in DPN symptoms after 12 weeks of a foot–ankle exercise programme. Another aspect that may have contributed in the improvement in DPN severity was subtle changes in the tactile and vibratory sensitivity, which are accounted for the fuzzy score calculation. Kanchanasamut and Pensri17 showed changes in vibration perception after 8 weeks of weight-bearing exercise, and Chang et al.18 showed improvements in DPN symptoms after 1 year of a Buerger exercise programme.
The intervention improved first metatarsophalangeal joint ROM in the sagittal plane in late stance. A difference of 3.2 degrees was observed, suggesting an improved mobility at the propulsion phase. A greater metatarsophalangeal joint extension may be associated with metatarsophalangeal joint deformity42 and may increase the risk of metatarsal heads exposure on the plantar surface repeatedly during gait, increasing plantar pressures at the forefoot43. However, our result can be considered positive because according to Deschamps et al.44, individuals with DPN present significantly smaller sagittal ROM of the first metatarsophalangeal joint during the forefoot push-off phase. We believe that the increased ROM in this joint is functional, as it was not accompanied by any increase in plantar pressures at the forefoot (will be measured in the full RCT), but it definitely increased the motion of the impaired joint. In addition to the hallux motion, the forefoot dorsiflexion peak was reduced after the intervention, suggesting an increased stiffness in the midfoot excursion during propulsion. The exercises focused on strengthening of the intrinsic foot muscles, such as quadratus plantaris, related to the talonavicular and calcaneocuboid joints. This can lead to increased stiffness of the midfoot during push-off, favouring the midtarsal joint locking mechanism, resulting in a changeover from a flexible foot during weight acceptance to a rigid foot structure during propulsion45.
This study’s strengths include its rigorous RCT methodology; customised physiotherapeutic approach guided by a booklet, focusing autonomy of the participant; high satisfaction and adherence rates; and low dropout rate. This is the first study to evaluate the clinical, functional and biomechanical outcomes specifically related to DPN losses using a specialised foot-training protocol guided by a booklet.
This study was limited because participants’ glycaemic and glycated haemoglobin levels were not controlled, so their oscillations may have influenced DPN symptoms. The recruitment strategy needs to be adjusted for the larger trial46, and amendment to the original protocol needs to performed. Because this was the only adjustment in the larger trial, the data from participants in this feasibility study will not be excluded from the final analysis.
Conclusion
This feasibility study showed that the proposed foot–ankle home-based exercise programme is feasible based on the high adherence, satisfaction and perceived safety of the programme, although recruitment strategies need to be improved. The programme showed positive preliminary changes in DPN severity and hallux and forefoot motion during gait, which encourages the development of the larger RCT46.
Methods
Study design
This feasibility study was part of a series of two clinical trials—the FOot CAre (FOCA) trial I (SOPeD intervention) and FOCA trial II (booklet intervention) and its reporting was based on the Consolidated Standards of Reporting Trials (CONSORT) 2010: extension to randomised pilot and feasibility trials. The trial was approved by the research ethics committee of the School of Medicine of the University of Sao Paulo (CAAE: 90331718.4.0000.0065) and was registered at ClinicalTrials.gov, date of registration is 02/07/2019 and registration numbers is NCT04008745 under the name “Effect of Educational Booklet for Foot-related Exercises for Prevention and Treatment in People with Diabetic Neuropathy (FOCA-II)”. The main trial is currently recruiting, and, for this feasibility study, participants were enrolled from April 23th to September 5th of 2019, totalling 20 weeks of recruitment at the Endocrinology outpatient ambulatory clinic of the Hospital das Clínicas, School of Medicine, University of São Paulo.
The research protocol was published elsewhere46. The main trial, if feasible, is designed as a parallel group, two-arm, superiority trial with a 1:1 allocation ratio. All methods were carried out in accordance with relevant guidelines and regulations.
The main trial started and the feasibility study (internal pilot analysis) was carried out with the first 20 enrolled participants from the pre-calculated sample size of the trial. The advantage of this type of design is that it allows us to carry out a prior analysis on the feasibility of recruitment, treatment protocol, adherence, safety, and make amendments (if necessary) without increasing the time required for conducting the complete trial. Thus, the data from participants will be not excluded from final analyses, only if major amendments were necessary to the protocol after the feasibility analysis.
Assessments at baseline (T0) and 8 weeks after the intervention start (T8) were performed at the Physical Therapy department of the School of Medicine, University of São Paulo.
Participants
A sample size of the first 20 participants of both sexes who were between the ages of 18 and 65 years, had a clinical diagnosis of type 1 or 2 DM has been selected in accordance with published guidance for pilot feasibility studies47 who were being treated at the Endocrinology Outpatient Clinic of the Hospital das Clínicas were recruited through telephone contact. Eligibility criteria were DPN score ≥ 2 confirmed by the Decision Support System for Classification of Diabetic Polyneuropathy (http://www.usp.br/labimph/fuzzy)10, ability to walk independently for at least 10 m and a maximum of one amputated toe (not being the hallux). Participants were not included if they had an ulcer; a history of surgical procedure to the knee, ankle or hip or indication of surgery or arthroplasty; use of a walking aid such as walker or cane; diagnosis of other neurological disease besides DPN; dementia or inability to give consistent information; were receiving any physiotherapy intervention or using offloading devices during the intervention period; diagnosis of major vascular complications and/or severe retinopathy, as determined from medical files; or had a score of 12-21 (probable depression) on the Hospital Anxiety and Depression Scale. The principal investigator explained to each eligible participant all stages of the study, possible risks, and expected benefits. Upon agreeing to participate, they were asked to sign the Free and Informed Consent Form.
Randomisation, allocation and blinding
Block randomisation was performed using ClinStat software47. The randomisation sequence was organized into blocks with a 1:1 ratio and to prevent blind assessors from predicting allocation, we use more than one block size so that the treatment assignments appear to be more like a simple randomization scheme, and are less likely to be predicted accurately. The block sizes varied randomly between 4 and 8. The randomisation sequence was kept in opaque and sealed envelopes that were numbered sequentially, and the random allocation was performed after acquiring baseline data. An independent researcher not involved with the study assigned the participants to the intervention group (IG; n = 11) or control group (CG; n = 9). Only the physiotherapist responsible for the intervention was aware of group allocation. A physiotherapist and an occupational therapist, both blinded to treatment allocation, were responsible for all clinical and biomechanical outcome assessments. Participants were instructed not to reveal their treatment allocation to the physiotherapists who conducted the assessments. The study statistician was blind to treatment allocation until this preliminary analysis was completed.
Intervention protocol
CG participants received the usual care, including pharmacological treatment and self-care guidelines of the International Working Group on the Diabetic Foot14. All of these usual care orientations were included in a flyer given and explained to the participants of CG and IG by the physiotherapist who conducted the study during the baseline session.
IG participants received the usual care, including pharmacological treatment, plus an 8-week physiotherapeutic home-based foot–ankle exercise programme guided by an educational booklet. This booklet consisted of an informative session and an exercise programme session (see46 for more details). The informative session provides information about autonomous footcare, DPN, footwear and the benefits of exercising the foot–ankle. The exercise programme includes six exercises with three difficulty levels. The first exercise session intervention was supervised by the main researcher at the outpatient clinic of the Laboratory of Biomechanics of Human Movement and Posture, School of Medicine, University of São Paulo to instruct the participants on how to perform the exercises using the booklet and to deliver an exercise kit containing materials needed to perform the exercises (cotton balls, a towel, a pencil, mini elastic bands, balloons, light and moderate resistance elastic bands, a massage ball and finger separators). Participants were instructed to perform the exercise programme at home three times a week for 8 consecutive weeks (24 sessions total).
The physiotherapeutic exercise programme strengthens the intrinsic and extrinsic foot–ankle muscles and increase the ROM of the interphalangeal, metatarsophalangeal and ankle joints. It includes three warm-up exercises, four exercises targeting the intrinsic foot muscles and joints and two exercises targeting the extrinsic foot–ankle muscles and joints (Fig. 4A). Participants could change the difficulty level based on a perceived effort scale (Fig. 4B). The participants were encouraged to record the current difficulty level and number of repetitions of each exercise in the booklet (Fig. 4C). This booklet is an unsupervised exercise programme in which both groups received telephone calls every week from the main researcher to check on their adherence, adverse events, and allowing the participants to take questions about the programme, the disease and the booklet, considering this stage part of the learning process.
A sample of pages from the booklet. (A) exercise programme, (B) level of difficulty and (C) monthly-calendar. The booklet was created using Adobe Photoshop cs5 Windows (https://www.adobe.com/br/products/photoshop.html) and Microsoft PowerPoint to Microsoft 365 version 2104 and the pictures that make up the figure are from the authors' personal collection.
Outcomes
The intervention was considered feasible if participants could be recruited and retained based on the following criteria: (a) participant recruitment rate ≥ to the laboratory availability for assessments (five participants/week); (b) > 75% adherence to the programme48; (c) participant dropout ≤ 30%49, (d) participant satisfaction with the intervention ≥ 3.75 on a 5-point Likert scale (75% of 5-point scale); and (e) participant safety perception ≥ 3.75 on a 5-point Likert-scale (75% of 5-point scale). Within-group preliminary changes in DPN severity and foot–ankle ROM during gait were assessed for the IG at baseline and T8.
In the full RCT, the primary outcome is the DPN severity given by the fuzzy decision support system. Secondary outcomes are tactile and vibratory sensitivities, foot health and functionality, toe and hallux isometric muscle strength, plantar pressure distribution during gait and foot–ankle kinematics during gait.
The feasibility outcomes were presented for all groups; however, the clinical and biomechanical outcomes for estimating potential changes due to the exercise programme were only reported for IG participants to avoid potential changes in the physiotherapist behavior responsible for providing the interventions, which might affect the reliability of the trial results.
Feasibility outcomes
Recruitment
Recruitment was assessed in terms of contact rate and preliminary screening success as well as recruitment rate. Contact rate is the ratio between the participants contacted at the recruitment period (20 weeks) and the research population universe, expressed in percentage. Preliminary screening success is the ratio between the number of participants who underwent screening and number of the contacted individuals in the recruitment period, expressed as individuals per week. Recruitment rate is the ratio between the eligible participants and the recruitment period.
Adherence and dropout
The participants used the monthly-calendar (Fig. 4C) to report the number of sessions they performed and also to describe the exact number of repetitions and sets performed of each exercise (exercise dose). Then, adherence was determined based on data filled in the monthly-calendar regarding number of sessions performed (max 24). The adherence to the home-based programme was the percentage of participants who self-reported completing more than 75% of the 24 sessions (at least 18 sessions) during the 8-week period. The dropout rate was the proportion of participants who completed the therapeutic programme and then dropped out of the study, expressed as a percentage.
Participant satisfaction
Participant satisfaction was evaluated at T8 using a seven-item structured questionnaire with a 5-point Likert scale, with higher scores indicating greater participant satisfaction. The questions were: (1) How satisfied were you with the exercise presentation and clarity? (2) How satisfied were you with the opportunity to express your opinion about the booklet? (3) How satisfied were you with the possibility of performing the exercises at flexible and convenient times? (4) How satisfied were you with your feet health after using the booklet? (5) How satisfied were you with the booklet’s ability to encourage the continuation of exercise? (6) What was your general satisfaction with the booklet? (7) How likely are you to recommend the booklet to other individuals with diabetes?
Participant safety
In the absence of a systematic instrument to analyse participant safety, safety was assessed at T8 based on the participants’ answer to the question ‘How safe did you feel when performing the exercises at home?’ using a 5-point Likert scale. Furthermore, participants reported any adverse events during weekly calls with the main researcher, as previously recommended50. Cramps, moderate to intense pain, fatigue or any other condition that exposed the participant to discomfort were considered.
Exercise dose
The exercise dose was measured to assess the feasibility of the protocol in terms of participants’ tolerable exercise volume and intensity. The exercise progression in the booklet is tailored to each participant’s perceived level of effort (Fig. 4B), and the maximum daily volume is six exercises per session with two sets of each exercise and 30 repetitions in each set. The level of difficulty or exercise intensity is based on the posture adopted for the exercises: sitting, standing or standing on one leg. The participants were asked to use the monthly-calendar in the booklet (Fig. 4C) to annotate the exact number of sets and repetitions of each exercise, and the level of difficulty of the exercise (position performed). The exercise volume of each session was the number of exercises performed times the number of repetitions times the number of sets, for a maximum possible total of 360 units [exercise dose = number of exercises (max 6) × number of sets (max 2) × number of repetitions (max 30)]. In each session, general intervention progress was measured as the percentage of participants who performed each posture.
Clinical and biomechanical outcomes for estimating potential changes
DPN severity
DPN severity was defined by the Decision Support System for Classification of DPN10 publicly available at http://www.usp.br/labimph/fuzzy. This decision was based on fuzzy logic with the input variables as signs and symptoms extracted from the MNSI, as well as tactile (through the number of non-touch areas using a 10-g monofilament) and vibration sensitivities (measured by vibrating a tuning fork at 128 Hz), characterized as absent, present, or diminished. The fuzzy software gives a score from 0 to 10, with higher scores indicating more severe DPN.
DPN symptoms
The Brazilian version of the Michigan Neuropathy Screening Instrument (MNSI)51 was used to assess DPN symptoms. Participants answered 15 questions about the sensitivity of their legs and feet. Confirmatory answers for questions 1, 2, 3, 5, 6, 8, 9, 11, 12, 14 and 15 received a score of 1. A negative answer for questions 7 and 13 also scored 1. Question 4 measures circulatory deficit and question 10 measures general asthenia and was excluded from the score. The total score ranged from 0 to 13 (13 representing the worst DPN condition).
Foot–ankle ROM during gait
ROM and peak angles from hindfoot relative to tibia (ankle joint), forefoot relative to hindfoot (midfoot motion) and hallux relative to forefoot (first metatarsal joint) in the sagittal plane were assessed by three-dimensional (3D) displacements of passive reflective markers (9.5 mm in diameter) tracked by eight infrared cameras at 100 Hz (VERO, Vicon Motion System Ltd., Oxford Metrics, UK). Forty-two markers were placed on both limbs of the subject (pelvis, thigh, leg, ankle, and foot) according Plug-In Gait and Oxford Foot Model53 setup protocols. Participants walked barefoot at a comfortable, self-selected speed on a 10-m walkway. After habituation to the laboratory environment, 10 valid steps on each side were acquired. Automatic digitisation, 3D reconstruction of the markers’ positions and filtering of kinematic data were performed using NEXUS software (version 2.10.3, Vicon Motion System Ltd., Oxford Metrics, UK). Kinematic data were processed using a zero-lag second-order low-pass filter with cut-off frequency of 6 Hz. Kinematics were computed with the open-source Python package pyCGM2 (http://www.pycgm2.github.io) replicating the Vicon Plug-In Gait protocol and Plug-In of Oxford Foot Model.
Statistical analyses
The present study showed the results for the 20 participants who were recruited, within the predefined recruitment period of 20 weeks. It is recommended that the statistical analysis of any type of pilot or feasibility study should be primarily descriptive52 and focus on estimating confidence intervals53. There is disagreement regarding whether pilot and feasibility studies should be analysed using hypothesis testing54 because it would be inappropriate to assign undue significance, as no formal calculation of power is performed. Because of the small sample, it is likely that there was an imbalance in the pre-randomisation covariates, which would require adjustments to the analysis. In addition, the confidence interval is likely to be inaccurate, even when there are significant differences. The results of any hypothesis test should therefore be treated as preliminary, and analyses within groups should be favoured.
Since this feasibility study consists of a preliminary analysis (internal pilot analysis) of the randomized controlled trial, we chose not to present the CG data at this phase of the trial, in order to avoid possible changes in the behavior of the therapist responsible for providing the interventions, which might affect the trustworthiness of the final results.
Thus, we reported means and standard deviations or medians and interquartile ranges (IQRs) according to data distribution (Shapiro–Wilk test; p > 0.05). In order to not compromise the reliability of the final results of the trial, only the results of the IG are presented and discussed. We estimated the changes in outcomes between baseline and T8 by reporting within-group differences or medians of differences and their 95% confidence intervals.
Due to conceptual problems with using bilateral data from two legs, Menz55 recommended against data pooling in foot–ankle research. Because DPN is a symmetrical disease56, we chose one side randomly for biomechanical gait analysis (i.e. the left one).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Armstrong, D. G., Boulton, A. J. M. & Bus, S. A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 376, 2367–2375 (2017).
Shun, C. T. et al. Skin denervation in type 2 diabetes: Correlations with diabetic duration and functional impairments. Brain 127, 1593–1605 (2004).
Allen, M. D. et al. Increased neuromuscular transmission instability and motor unit remodelling with diabetic neuropathy as assessed using novel near fibre motor unit potential parameters. Clin. Neurophysiol. 126, 794–802 (2015).
Goodfield, M. J. D. & Millard, L. G. The skin in diabetes mellitus. Diabetologia 31, 567–575 (1988).
Riandini, T. et al. Functional status mediates the association between peripheral neuropathy and health-related quality of life in individuals with diabetes. Acta Diabetol. 55, 155–164 (2018).
Martinelli, A. R. et al. The foot muscle strength and ankle mobility for the gait parameters in diabetic neuropathies. Foot 23, 17–21 (2013).
Chey, V., Hastings, M., Commean, P., Ward, S. & Mueller, M. Intrinsic foot muscle deterioration is associated with metatarsophalangeal joint angle in people with diabetes and neuropathy. Clin. Biomech. 28, 1055–1060 (2014).
Guney, A. et al. Biomechanical properties of achilles tendon in diabetic vs. non-diabetic patients. Exp. Clin. Endocrinol. Diabetes 123, 428–432 (2015).
Gomes, A. A. et al. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve 44, 258–268 (2011).
Watari, R. et al. Effect of diabetic neuropathy severity classified by a fuzzy model in muscle dynamics during gait. J. Neuroeng. Rehabil. 11, 11 (2014).
Sacco, I. C. N. et al. Alterations in the lower limb joint moments precede the peripheral neuropathy diagnosis in diabetes patients. Diabetes Technol. Ther. 17, 405–412 (2015).
Malindu, F. et al. Plantar pressure in diabetic peripheral neuropathy patients with active foot ulceration, previous ulceration and no history of ulceration: A meta-analysis of observational studies. PLoS One 9, 1–10 (2014).
Bacarin, T. A., Sacco, I. C. N. & Hennig, E. M. H. Plantar pressure distribution patterns during gait in diabetic neuropathy patients with a history of foot ulcers. Clinics 64, 113–120 (2009).
Schaper, N. C. et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes. Metab. Res. Rev. 36, 1–10 (2020).
Sartor, C. et al. Effects of a combined strengthening, stretching and functional training program versus usual-care on gait biomechanics and foot function for diabetic neuropathy: A randomized controlled trial. BMC Musculoskelet. Disord. 13, 1–10 (2012).
Sartor, C. D. et al. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: Results of a randomized controlled trial. BMC Musculoskelet. Disord. 15, 1–13 (2014).
Kanchanasamut, W. & Pensri, P. Effects of weight-bearing exercise on a mini-trampoline on foot mobility, plantar pressure and sensation of diabetic neuropathic feet; a preliminary study. Diabet. Foot Ankle 8, 1–10 (2017).
Chang, C.-F., Chang, C.-C., Hwang, S.-L. & Chen, M.-Y. Effects of Buerger exercise combined health-promoting program on peripheral neurovasculopathy among community residents at high risk for diabetic foot ulceration. Worldviews Evid.-Based Nurs. 12, 145–153 (2015).
Goldsmith, J. R., Lidtke, R. H. & Shott, S. The effects of range-of-motion therapy on the plantar pressures of patients with diabetes mellitus. J. Am. Podiatr. Med. Assoc. 92, 483–490 (2002).
Fayed, E. E., Mohamed Badr, N., Mahmoud, S. & Hakim, S. A. Exercise therapy improves planter pressure distribution in patients with diabetic peripheral neuropathy. Int. J. PharmTech Res. 9, 151–159 (2016).
De León Rodriguez, D. et al. Biofeedback can reduce foot pressure to a safe level and without causing new at-risk zones in patients with diabetes and peripheral neuropathy. Diabetes Metab. Res. Rev. 29, 139–144 (2013).
York, R. M., Perell-Gerson, K. L., Barr, M., Durham, J. & Roper, J. M. Motor learning of a gait pattern to reduce forefoot plantar pressures in individuals with diabetic peripheral neuropathy. PM R 1, 434–441 (2009).
Francia, P. et al. Diabetic foot prevention: The role of exercise therapy in the treatment of limited joint mobility, muscle weakness and reduced gait speed. Ital. J. Anat. Embryol. 120, 21–32 (2015).
Mueller, M. J. et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: A randomized controlled trial Michael. Arch. Phys. Med. Rehabil. 94, 1–7 (2013).
Cerrahoglu, L., Kosan, U., Sirin, T. C. & Ulusoy, A. Range of motion and plantar pressure evaluation for the effects of self-care foot exercises on diabetic patients with and without neuropathy. J. Am. Podiatr. Med. Assoc. 106, 189–200 (2016).
Allet, L. et al. An exercise intervention to improve diabetic patients’ gait in a real-life environment. Gait Posture 32, 185–190 (2010).
Iunes, D. H. et al. Self-care associated with home exercises in patients with type 2 diabetes mellitus. PLoS One 9, 1–13 (2014).
Lorig, K. R. & Holman, H. R. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 26, 1–7 (2003).
Kim, J. Y. et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: A randomized controlled trial. Support. Care Cancer 27, 2933–2940 (2019).
Flynn, A., Allen, N. E., Dennis, S., Canning, C. G. & Preston, E. Home-based prescribed exercise improves balance-related activities in people with Parkinson’s disease and has benefits similar to centre-based exercise: A systematic review. J. Physiother. 65, 189–199 (2019).
Meyer, M. et al. Current state of home-based exercise interventions in patients with congenital heart disease: A systematic review. Heart 106, 333–341 (2020).
Almeida, S. I. L. de, Gomes da Silva, M. & Marques, A. S. P. de D. Home-based physical activity programs for people with dementia: Systematic review and meta-analysis. Gerontologist XX, 1–9 (2019).
Gelaw, A. Y., Janakiraman, B., Gebremeskel, B. F. & Ravichandran, H. Effectiveness of home-based rehabilitation in improving physical function of persons with Stroke and other physical disability: A systematic review of randomized controlled trials. J. Stroke Cerebrovasc. Dis. 29, 1–8 (2020).
Kis, O., Buch, A., Stern, N. & Moran, D. S. Minimally supervised home-based resistance training and muscle function in older adults: A meta-analysis. Arch. Gerontol. Geriatr. 84, 1–9 (2019).
Dadgostar, H. et al. Supervised group-exercise therapy versus home-based exercise therapy: Their effects on Quality of Life and cardiovascular risk factors in women with type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 10, 30–36 (2016).
Qiu, S. H., Sun, Z. L., Cai, X., Liu, L. & Yang, B. Improving patients’ adherence to physical activity in diabetes mellitus: A review. Diabetes Metab. J. 36, 1–5 (2012).
Mattishent, K. et al. Continuous glucose monitoring in older people with diabetes and memory problems: A mixed-methods feasibility study in the UK. BMJ Open 9, 1–8 (2019).
Harris, T. J., Carey, I. M., Victor, C. R., Adams, R. & Cook, D. G. Optimising recruitment into a study of physical activity in older people: A randomised controlled trial of different approaches. Age Ageing 37, 659–665 (2008).
Lopez, C. et al. Variability and limitations in home-based exercise program descriptions in oncology: A scoping review. Support. Care Cancer 28, 4005–4017 (2020).
Trivedi, R. B. et al. Recruitment and retention rates in behavioral trials involving patients and a support person: A systematic review. Contemp. Clin. Trials 36, 307–318 (2013).
Picon, A. P., Ortega, N. R. S., Watari, R., Sartor, C. & Sacco, I. C. N. Classification of the severity of diabetic neuropathy: A new approach taking uncertainties into account using fuzzy logic. Clinics 67, 151–156 (2012).
Zellers, J. et al. Multi-system factors associated with metatarsophalangeal joint deformity in individuals with type 2 diabetes. J. Clin. Med. 9, 1012 (2020).
Lavery, L. A., Armstrong, D. G., Vela, S. A., Quebedeaux, T. L. & Fleischli, J. G. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch. Intern. Med. 158, 157–162 (1998).
Deschamps, K. et al. Clinical Biomechanics Comparison of foot segmental mobility and coupling during gait between patients with diabetes mellitus with and without neuropathy and adults without diabetes. Clin. Biomech. 28, 813–819 (2013).
Blackwood, C. B., Yuen, T. J., Sangeorzan, B. J. & Ledoux, W. R. The midtarsal joint locking mechanism. Foot Ankle Int. 26, 1074–1080 (2005).
Silva, E. Q. et al. Effect of an educational booklet for prevention and treatment of foot musculoskeletal dysfunctions in people with diabetic neuropathy: The FOotCAre (FOCA) trial II, a study protocol of a randomized controlled trial. Trials 21, 1–13 (2020).
Altman, D. G. & Bland, J. M. How to randomise. Br. Med. J. 319, 703–704 (1999).
Mickle, K. J., Caputi, P., Potter, J. M. & Steele, J. R. Efficacy of a progressive resistance exercise program to increase toe flexor strength in older people. Clin. Biomech. 40, 14–19 (2016).
Lopienski, K. Retention in clinical trials–keeping patients on protocols. Fort Res. Elsevier 1, 2015 (2015).
Lima, L. O. & Rodrigues-de-Paula, F. Recruitment rate, feasibility and safety of power training in individuals with Parkinson’s disease: A proof-of-concept study. Braz. J. Phys. Ther. 17, 49–56 (2013).
Sartor, C., Oliveira, M., Campos, V., Ferreira, J. & Sacco, I. Brazilian Journal of Cross-cultural adaptation and measurement properties of the Brazilian Version of the Michigan Neuropathy Screening Instrument. Braz. J. Phys. Ther. 22, 222–230 (2018).
Lancaster, G., Dodd, S. & Williamson, P. Design and analysis of pilot studies: Recommendations for good practice. J. Eval. Clin. Pract. 10, 307–312 (2002).
Burrows, R. F., Gan, E. T., Gallus, A. S., Wallace, E. M. & Burrows, E. A. A randomised double-blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thrombolic events after caesarean section: A pilot study. Br. J. Obstet. Gynaecol. 108, 835–839 (2001).
Ross-McGill, H. et al. Antenatal home blood pressure monitoring: A pilot randomised controlled trial. Br. J. Obstet. Gynaecol. 107, 217–221 (2000).
Menz, H. B. Two feet, or one person? Problems associated with statistical analysis of paired data in foot and ankle medicine. Foot 14, 2–5 (2004).
Stevens, M. & Shakher, J. Update on the management of diabetic polyneuropathies. Diabetes Metab. Syndr. Obes. Targets Ther. 4, 289–305 (2011).
Acknowledgements
The authors acknowledge Professor Ana Claudia Latronico Xavier and Endocrinology departament of the Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo for all support in the recruitment of the patients throughout the study, and Professor Marcos Duarte from Universidade Federal do ABC for all support with the Phython programme for data analysis.
Funding
This work is supported by National Council for Scientific and Technological Development, Brazil (CNPq) [28/2018 FOCA Trial 407252/2018-5]. Silva and Verissimo are awarded by Agency Coordination of Improvement of Higher Education Personnel (CAPES, financial code 001). Beteli is awarded by CNPq [119178/2019-2]. Cruvinel-Júnior, Santos, Ferreira, Monteiro and Suda are awarded by Sao Paulo Research Foundation (FAPESP) [2019/06405-9, 2019/02624-8, 2019/02522-0, 2017/17848-3, 2017/15449-4, respectively]. Sacco is a fellow of the CNPq (Process: 304124/2018-4). The funders do not have any role in the study and do not have any authority over any study activity or in the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all three of sectsions (1), (2) and (3): The conception and design of the study, or acquisition of data, or analysis and interpretation of data (2) drafting the article or revising it critically for important intellectual content (3) final approval of the version to be submitted. In this feasibility study, the following roles were played by the authors: E.Q.S., R.L.M., J.S.S.P.F. and I.C.N.S. were responsible for the conception and design of the study; E.Q.S., D.P.S., R.I.B., R.L.M., J.S.S.P.F., J.L.V., R.H.C.J. and A.D. were responsible for data acquisition and data processing, E.Q.S., D.P.S., R.L.M., J.S.S.P.F., J.L.V., R.H.C.J., E.Y.S. and I.C.N.S. for data analysis and interpretation; and E.Q.S. and I.C.N.S. for drafting the paper. I.C.N.S. revised the manuscript critically. All authors read, provided feedback and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, É.Q., Santos, D.P., Beteli, R.I. et al. Feasibility of a home-based foot–ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: randomised controlled FOotCAre (FOCA) Trial II. Sci Rep 11, 12404 (2021). https://doi.org/10.1038/s41598-021-91901-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91901-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.