Abstract
Electrical stimulation of the cerebral cortex (ESCC) has been used to treat intractable neuropathic pain for nearly two decades, however, no standardized approach for this technique has been developed. In order to optimize targeting and validate the effect of ESCC before placing the permanent grid, we introduced initial assessment with trial stimulation, using a temporary grid of subdural electrodes. In this retrospective study we evaluate the role of electrode location on cerebral cortex in control of neuropathic pain and the role of trial stimulation in target-optimization for ESCC. Location of the temporary grid electrodes and location of permanent electrodes were evaluated in correlation with the long-term efficacy of ESCC. The results of this study demonstrate that the long-term effect of subdural pre-motor cortex stimulation is at least the same or higher compare to effect of subdural motor or combined pre-motor and motor cortex stimulation. These results also demonstrate that the initial trial stimulation helps to optimize permanent electrode positions in relation to the optimal functional target that is critical in cases when brain shift is expected. Proposed methodology and novel results open a new direction for development of neuromodulation techniques to control chronic neuropathic pain.
Similar content being viewed by others
Introduction
Chronic pain is clinically identified as a disabling syndrome, which is relatively frequent across the general population, affecting 8% of adults in the United States, with an incidence of about 18 million people per year1. The International Association for the Study of Pain (IASP) defines chronic pain as pain experienced every day for 3 months over a period of 6 months or longer2. Chronic pain can be related to variety of medical conditions and commonly leads to a complex sensory and emotional experience with variety of features. Perception of chronic pain depends on the context and meaning of the pain, the physical, psychological, and psychosocial state of the patient3. The management of patients with chronic pain is considered to be one of the most difficult challenges in medicine4. Various chronic pain syndromes, such as post-stroke pain, trigeminal neuralgia, or phantom limb pain are highly resistant to pharmacological treatment4. Neuromodulation techniques have been increasingly used either as a substitute for surgical treatment or in addition to pharmacological therapy. Several conditions, such as essential tremor, Parkinson’s disease, dystonia, and psychiatric disorders have been successfully controlled with neuromodulation therapy5. Stimulation of brain structures for the treatment of chronic pain, however, have led to variable outcomes. Several trials reported successful neuromodulation of ventral posterior lateral nucleus (VC, Ventralis Caudalis) of thalamus, periventricular grey, and periaqueductal grey deep brain stimulation (DBS) with mean relief more than 50%6,7. Motor Cortex Stimulation (MCS) was introduced by Tsubokawa in 1991 as a treatment approach for patients with intractable pain, in response to growing frustration with inadequate DBS efficacy in this patient population8. Initial studies revealed that in contrast to stimulation of the of primary sensory cortex, which increases pain, stimulation of the motor cortex causes suppression of neuropathic pain8,9. Later MCS was found to be effective for various types of neuropathic pain (NP), such as trigeminal neuralgia, peripheral neuropathy, neuropathic pain after spinal cord injury (SCI), and others10,11. Over the last decade MCS has emerge as a promising alternative to pharmacological therapy and for patients with drug-resistant chronic pain syndromes5,12. Interestingly, until now, no standardized approach for MCS has been formed and there is no consensus regarding the surgical technique, electrode array implantation site, and optimal stimulation parameters13. The surgical technique, as well as target optimization varies between different centers with most of the institutions implanting epidural or subdural electrodes based on cortical anatomy or results of functional imaging. In order to identify cortical areas, which give the best clinical outcome with MCS and optimal initial stimulation settings, a trial stimulation was introduced at Mayo Clinic and was successfully used over last decade14,15. In this study we retrospectively evaluated the effect of ESCC in patients treated with the inclusion of a period of trial stimulation prior to permanent implant. The electrode locations with correction for brain shift were correlated with clinical outcome. In all cases the temporary electrodes were placed in the subdural space for initial trial stimulation and, then, were replaced with permanent electrode arrays for subsequent long-term stimulation. All procedures were performed off-label based on previously reported efficacy14. The outcome of motor and premotor cortex stimulation was analyzed and correlated with the position of the ‘most effective’ temporary electrode contacts and contacts on the permanent electrodes based on reconstruction of anatomical location.
Results
Medical records of nine subjects were acquired from Mayo Clinic database (Table 1 ) from patients who underwent cortical electrode implantation procedure to treat chronic pain (Fig. 1 )
Surgical intervention and mapping. (A) Surgery for temporary grid placement in order: craniotomy; 6 × 8 temporary grid placement; X-ray/CT-scan representative for temporary grid position. (B) Trial stimulation for target optimization, with following permanent grid implantation over identified area. X-ray/CT-scan representative for permanent grid position. (C) Further image processing to reveal electrodes position over exact cortex area according to Desican-Killiany atlas.
Algorithm of the image processing and post-implantation brain shift correction described in methods and outlined on Fig. 2.

Source: http://ielvis.pbworks.com/).
Algorithm of the image processing. (1) Processing of the T1-weighted MRI image using freesurfer(extraction of the pial surface and leptomeningeal surface, segmentation of brain structures); (2) Coregistration of the postimplant CT scan with processed MRI using iELVis coregistration tool; (3) Labeling of electrodes on coregistrated CT using Bioimage Suite 3. (4) Postimplantation brain shift correction using iELVis Yang, Wang et.al. tool. (5) Creating images of electrode locations. (
Trial stimulation
Retrospective review of programming records demonstrated that after surgical implantation of the permanent grid, all subjects in this study (n = 9) reported a "honeymoon effect" with significant improvement in pain during the first one to two days after surgery without active stimulation. Because of ‘honeymoon effect’, trial stimulation was delayed until the pain level exceeded 5/10 on the Numeric Rating Scale (NRS). Parameters of stimulation (frequency, pulse width, and stimulation intensity) were adjusted individually during trail stimulation and permanent stimulation and varied depending on pain reduction and side-effects. Initial settings were: 40 Hz, 450 µsec, 0–4 V. In two cases, stimulation frequency was increased up to 100 Hz (subjects 4 and 9), when the initial settings did not improve pain. Trial stimulation was started on day 1 (n = 3), 2 (n = 1), 3 (n = 3), or day 4 (n = 1) after temporary grid placement. In all subjects except one, the initial trial stimulation reduced the pain intensity in more than 50%, although one subject (subject 9) didn’t show reduction in pain and after trial stimulation his pain level was 8/10, comparing with 9–10/10 at baseline before implantation. The mean pain level by the end of the trial stimulation was 2.16 ± 2.39 (n = 9). Based on results of trial stimulation the areas of stimulation with the most effective pain control were identified as a target for permanent grid placement (Fig. 1). Trial stimulation was also helpful in evaluation of potential side effects of ESCC, i.e. problems with speech, muscles spasm or twitches, focal seizures, and in some cases increased pain (Table 2). For all cases, the final target for implantation of permanent grid was adjusted based on results of the trial stimulation.
Chronic stimulation
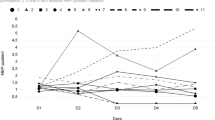
All subjects then underwent implantation of the permanent electrodes (2 × 8 spinal leads) centered over the most effective leads of the initial trial stimulation, except subject 9, who developed postoperative hematoma. After recovery from the 2nd surgery, they were evaluated and discharged with follow-up visits and evaluations based on regular reports. Based on results of retrospective analysis, patients with electrodes implanted over motor or motor and premotor areas (n = 6), yielded at least 70% pain reduction on the average and stated stable pain relief up to 0–4/10 on the NRS (Fig. 3). Patients with permanent electrodes placed over the premotor area (n = 2) yielded significant pain reduction and stated pain relief up to 0–1 on pain scale that was better compare to other patients (Figs. 3, 4). We identified one patient (Subject 9) with permanent electrodes covering the motor, pre-motor cortex, and also sensory cortex, who did not demonstrate significant pain relief (~ 5%). This patient had a postsurgical hematoma with the following cerebral edema, and, because of this surgical complication, permanent grid was implanted after hematoma evacuation without completion of the trial stimulation. Shifting of the electrode as a result of reduction in cerebral edema lead to abnormal electrode position. Six months to one-year follow-up after permanent electrodes implantation in this subject demonstrated that stimulation with permanent electrodes, pain increased up to the preoperative level (Fig. 4). In six subjects with electrodes located over premotor and motor area, pain reduction slightly decreased by 15%. However, two subjects with electrodes placed on premotor area did not show significant fluctuation in pain level over time (Table 2). At six-month follow up visit, all subjects who had a significant pain relief during trial stimulation demonstrated stable pain control (Fig. 4). In contrast, subjects, who had improvement less than 50% from the pre-operative level, demonstrated some level of decline in effect of ESCC to control chronic pain (Table 2). The analysis of the effect of ESCC to alleviate chronic pain in correlation with the location of permanent electrodes suggests that the neuromodulation of premotor cortical area may be as beneficial as, or better than primary motor cortex stimulation. The reconstruction of permanent electrodes location on average brain model demonstrated that subjects with significant pain relief have electrodes primarily on pre-motor cortex (Fig. 5).
Pain reduction effect of chronic subdural cortex stimulation. Reduction of pain with chronic subdural cortical stimulation presented here in three groups, based on electrodes location. 1st group: patients 1–6 with permanent grid implantation over both premotor and motor cortex; 2nd group: patients 7–8 with permanent grid implanted over premotor cortex; 3rd group: patient 9 for whom permanent grid implanted over motor, premotor and sensory cortex. Pie charts illustrate proportion of electrodes over particular cortex area. Line charts represent variation of pain level in patients after permanent grid implantation. PO—preoperation pain level; PI—permanent grid post-implantation pain level (PI1- within 2 weeks after permanent grid implantation; PI2—pain level during 6 months to 1-year period of observation).
Comparison of ESCC effects in three groups based on electrodes location. Blue—electrodes implanted over motor and premotor cortex (n = 6); Brown—electrodes implanted over premotor and prefrontal cortex (n = 2); Green—electrodes implanted over sensory, motor, and premotor cortex (n = 1). PO—preoperative pain level (NRS); TSS (trial stimulation start)—pain level when trial stimulation was started; TSE (trial stimulation end)—pain level by the end of trial stimulation; PI1 (post-implantation 1)—permanent grid post-implantation pain level within 2 weeks; PI2 (post-implantation 2)—permanent grid post-implantation pain level that had been achieved after adjustment of stimulation settings within 6–12 months of observation. Means ± standard deviation of the mean.
Location of permanent grid electrodes on average brain model. 1st group (blue dots): subjects 1–6 with permanent grid implantation over both premotor and motor cortex; 2nd group (brown dots): subjects 7–8 with permanent grid implanted over premotor cortex; 3rd group (red dots): subject 9 whom permanent grid implanted over sensory, motor, and premotor cortex.
Discussion
The surgical treatment of neuropathic pain with ESCC has been evolving over last decades, starting from the first implantation, reported by Tsubokawa and colleagues8. Most of the centres currently using image guidance with MRI to localize the region of the pain, i.e. in case of facial pain—anterior to the central sulcus at the level of the inferior frontal sulcus8. This information then loaded into a neuronavigation workstation to direct the most appropriate location for the incision and craniotomy. Typically, the specific region of motor cortex is identified through intraoperative somato-sensory evoked potentials (SSEP) and confirmed by trial intraoperative stimulation8,10. In addition, TMS can be useful for non-invasive trial stimulation and might potentially be considered as an assessment tool for cortical stimulation efficiency16. The surgical technique, however, varies significantly between institutions, with most centers placing electrodes in the epidural space. Epidural placement has several advantages including lower operative risk and shorter operative time, however, epidural electrode placement often leads to a scarring around the electrodes, resulting in higher electrode impedance and consequent declines in stimulation efficacy over time17,18. Epidural stimulation can also lead to less localized stimulation and may require a higher stimulus intensity, which usually results in shorter battery life. Epidural stimulation may also induce pain by direct activation of the dural pain fibers, limiting therapeutic effect of stimulation4,14,19. In a computer simulation study, Kim et al. compared the efficacy of both epidural and subdural placement and reported that the effective volume and depth of pulse penetration is significantly higher with subdural stimulation compared to epidural20,21. Placing stimulating electrodes in the subdural space routinely over last ten years we have shown consistent results with minimal surgical complication14,15.
Regardless of the long history of ESCC, the mechanisms responsible for alleviating of chronic pain are largely unknown. In animal studies it has been demonstrated that after deafferentation of reticular thalamic nucleus and ventral posterior lateral thalamus, normal thalamocortical circuitry activity shifts from high-threshold tonic firing to low-threshold theta-range oscillatory bursts22. This transformation further leads to a decrease in the excitatory input to the reticular thalamus and their subsequent hyperpolarization and low-frequency bursting that induces rhythmic discoordination of the thalamocortical loops in theta frequency band23. Based on these observations it was proposed that cortical stimulation may inhibit the hyperactivity of the thalamus and particularly the sensory nuclei of the thalamus that exhibit chronic pain-induced hyperactivity with increased spike density8. In fact, outcomes of multiple trials suggest that the mechanisms of neuropathic pain that respond to the cortical stimulation may have a final common pathway of deafferentation at the different levels of the sensory system. Later, it was shown that cortical stimulation may initially activate the axons that run horizontally in the precentral gyrus, parallel to the surface of the cortex. These cortico-cortical projections directed from primary motor to primary sensory cortex, and travel in Layer I, making them easily accessible for stimulation14. These results altogether support the commonly accepted hypothesis that during neuropathic pain, most of the cortical inputs tend to balance pathological thalamocortical oscillations and may also facilitate following reorganization in these areas, while cortical stimulation targeting the same mechanisms could further compensate pathological oscillations and related neuropathic pain. The brain areas with relatively higher degree of functional connectivity and plasticity, like premotor and motor cortical areas, could be particularly effective in compensation of central deafferentation. Multiple studies suggest that cortical stimulation can lead to reinforcement of intracortical GABAergic inhibition, increased secretion of endogenous opioids in various structures and more specifically, the cingulate cortex and periaqueductal gray matter (PAG)24,25. It was also found that the density of opioid receptors binding in the brain is correlated with postoperative pain relief with cortical stimulation in patients with chronic pain25. Another mechanism of ESCC could be related with activation of cortical and mesencephalic areas involved in the emotional appraisal of pain, particularly insula, cingulate, and orbitofrontal cortex26,27.
Only few studies have explored the effect of cortical stimulation on various pain syndromes (Supplementary Table 1) with total of over few hundreds participants evaluated4,8,10,24,28,30,31,32,34,52,53,54. Most of reports with MCS are focusing on trigeminal neuralgia and post stroke pain treatment. Trigeminal neuralgia generally showed a good response to cortical stimulation with more than a half of the patients receiving significant pain relief10,22,28. Several recent reviews indicate that 75–85% of patients have at least 50% reduction of trigeminal pain with motor cortex stimulation9,21. Results however, vary between the centers4,29. In contrast with trigeminal neuralgia, there is a lack of clinical trials on cortical stimulation for phantom limb pain with only few case-reports and mixed trials with small number of patients. Recently we reported an improvement of phantom limb syndrome with cortical stimulation in two subjects along with other studies demonstrating effect of cortical stimulation on phantom limb pain15,30,31. Currently, the implantable neuromodulation systems for MCS in patients with chronic pain are considered “off label” which significantly limits further exploration of this technique32. MCS can lead to several complications, typical for most craniotomies, such as infection and hemorrhage (Supplementary Table 1). Previously reported findings indicate on potential side effects directly related to the cortical stimulation, including intermittent dysarthria, aphasia, throat spasm, focal seizure, and intermittent facial twitching. In all cases these side effects were terminated with reducing intensity or termination of the stimulation. The seizure threshold does appear to respond to standard anticonvulsants (e.g. levetiracetam or fosphenytoin) that were used in this study26,30,33,34. Henderson et al. reported that seizures were associated primarily with stimulus rates between 70 and 90 Hz and patients who experienced seizures did not develop chronic epilepsy35. Other reported surgical complications include hematoma, as a consequence of subdural implantation, and a headache that could be related to craniotomy and liquorrhea.
Summarizing these results, we can outline two key outcomes of this study. The first outcome indicates on specific role of electrode location in the efficacy of cortical stimulation to control chronic neuropathic pain, and particularly the effect of pre-motor cortex stimulation. Most of the previous studies were focused on neuromodulation of the motor cortex, after initially negative experience with S1 stimulation8,10,20. The effect of premotor cortex stimulation was not studied and remained largely unknown. In contrast to a somatotopic organization (motor homunculus), premotor cortex is likely organized in a functional manner, where overlapped regions represent different motor patterns36,37,38. This organization has been supported by several studies in animals, and indicates that the areas of cerebral cortex controlling specific part of the body in pre-motor cortex may have more diffuse and overlapped representations compared to M1, i.e. related to complex sensorimotor functions with involvement of multiple body parts and may have higher cortical plasticity35,39. This specific organization was primarily suggested by animal studies and still needs to be confirmed in humans. Recent works suggest that premotor cortex along with primary motor cortex, primary sensory cortex, and prefrontal cortex are organized in a neuronal network responsible for complex control of movements and sensorimotor integration (Fig. 6)35,39,40,41. Specific mechanisms of this organization and functioning of this network still need to be investigated and may further facilitate development of new strategies for treatment of neuropathic pain.
The second outcome of this study indicates on importance of trial stimulation for target optimization. According to these results, initial reduction in pain, observed during trial stimulation, was consistent during chronic stimulation for most of the tested subjects. Those who did not respond to trial stimulation, in fact, did not improve, even after multiple adjustments. These findings suggest that trial stimulation provides important information for positioning of permanent electrodes and helps to assess individual response to initial stimulation settings. Trial stimulation started after the ‘honeymoon period’, when the pain is returning to the baseline, can help to avoid misinterpretation of results of trial stimulation. The main disadvantage of trial stimulation with temporary grids is surgical side effects, i.e. increased risk of hematoma and loss of CSF. In combination, this leads to the shifting from initially planned position and may result in displacement of the permanent grid that we observed in subject 9. Retrospective analysis of DBS implantations has been performed previously for evaluation of the electrode shift and did not find significant displacement with postoperative intracranial air volume up to 35 cm3, although, DBS electrodes are less affected by the brain shift due to their deeper locations42.
In conclusion, the results of this study demonstrate the first case-series report with subdural stimulation of the mixed motor and pre-motor cortical areas (n = 6) and solely pre-motor cortex (n = 2). Results demonstrate improvement in chronic neuropathic pain with pre-motor cortex stimulation from 8–9 to 0, compare to more moderated improvement with mixed motor and pre-motor cortex stimulation from 8–9 to 1–4 on NRS scale. The results of this study suggest that modulation of neural networks with subdural ESCC is beneficial and provides control of neuropathic pain when located on motor and particularly on pre-motor areas. Due to low number of subjects in the second group, these results will require future confirmation. The most important and novel finding of this study is that pre-motor cortex stimulation could be is at least as beneficial as motor cortex stimulation and can be considered for future therapy with ESCC. In cases of complex pain syndromes, i.e. thalamic pain, regional pain syndrome, or spinal cord injury-associated pain, pre-motor cortex stimulation could provide additional benefits through the coverage the wider functional area. Another important finding of this study is that trial stimulation for target optimization can significantly improve the outcome of ESCC. Future differentiation of specific mechanisms related to effect of ESCC could improve pain control in cases when access to the motor cortex is limited or the effect of stimulation is insufficient.
Methods
Patient selection
For trial stimulation subjects initially underwent implantation of a temporary grid of electrodes (6 × 6 or 8 × 6, based on expected size of testing area of the cortex) (Fig. 1A). After completing the craniotomy, the central sulcus was identified as well as the cortical region representing the primary sensory cortex. Preoperative functional MRI (fMRI) for tongue tapping and face brushing was implemented to aid in localization of these regions. Then, a temporary grid was placed in the subdural space. Data were merged with preoperative imaging in the neuronavigation workstation to guide the surgical procedure. Following placement of the chronic permanent electrode, the leads were tunneled, externalized, and connected to an external stimulus generator for trial stimulation monitoring in the intensive care unit (Fig. 1B). All patients underwent postoperative CT scan to localize the position of the grid. The initial assessment with trial stimulation using a temporary grid of subdural electrodes to optimize targeting and validate the effect of stimulation before placing the permanent electrodes was started after the initial ‘honeymoon period’ had worn off (generally in about 24 h), and when pain had increased at least to 6/10 on the NRS. The mean duration of the trial stimulation was usually 4–7 days, while patient was in the intensive care unit under continuous monitoring. Factors affecting the duration of trial stimulation included the duration of the ‘honeymoon period’, number of contacts on the temporary grid, latency to response with stimulation changes, stimulation-induced seizures, and clinical scheduling. Patients and/or nursing staff maintained an hourly pain journal to provide a guide for response to changes in stimulation parameters. Initial parameters for temporary grid trial stimulation at the motor and pre-motor cortex were: pulse width of 450 μs, rate of 40 Hz, intensity 0–4.0 V, selected as initial settings based on previous findings14,15. Subjects were maintained on seizure prophylaxis with fosphenytoin throughout trial stimulation (1000 mg once during surgery and 300 mg daily during trial stimulation) and following the permanent implantation (also at 300 mg/d) for one week. Each column of the implanted subdural electrode array was tested with cathodal stimulation. If successful with the patient reporting a 50% reduction in pain (or reduction below 5/10 with baseline 8–10/10), the patient returned to the operating room for implantation of permanent electrode array for chronic stimulation (2 × 8 spinal leads, Medtronic Leads 977C265, Medtronic USA Inc) (Fig. 1) connected to an internal pulse generator positioned in the (usually ipsilateral) subclavicular region. The final target for implantation of the permanent grid was adjusted based on the results of the trial stimulation. All subjects were evaluated every few weeks during the following up visits while chronically stimulated. All procedures were approved by the the Mayo Clinic Institutional Review Board (IRB).
Image processing for reconstruction of the electrode contact locations
As a part of each patient's routine presurgical evaluation, a preoperative T1-weighted volumetric MRI without contrast was acquired, typically a sagittal magnetization-prepared rapid gradient echo (MPRAGE) (voxel dimensions 0.86 × 0.86 × 0.9 mm, 3 T field strength), although additional coronal and axial MPRAGE and spoiled gradient recalled echo images with similar resolution were conducted as well. The preoperative MRI was converted using a DICOM to NIfTI conversion tool for Matlab and preprocessed in FreeSurfer to segment brain structures, extract the pial surface, extract the leptomeningeal surface (i.e., a smoothed pial surface), and map the patient's cortical surface to the FreeSurfer average cortical surface (Fig. 2)43,44. As part of this process, each patient's cortical surface was mapped to the Desikan-Killiany brain atlas, which assigns each neocortical vertex to 1 of 35 areas based on gyral morphology44. After grid implantation, high-resolution CT images were acquired to confirm electrode placement. The post-implant CT volume was co-registered to the preoperative MRI using the FMRIB's linear image registration tool algorithm (ct2mri.sh command line tool) included in FSL via a 6 degrees of freedom affine transformation that maximized the mutual information between the 2 volumes45,46. Co-registered image volumes were visually inspected for accuracy. After co-registration, electrodes were manually labeled at the coregistered CT volume imported into BioImage Suite 3.047. The cortical surface and electrode positions in the postimplant images were analyzed. Functional mapping (motor and sensory cortex) was not performed in this study due the absence of data on the importance of this approach in adjustment of the permanent cortical grid location. As presented earlier, the initial electrode position on CT images may shift due to loss of CSF during surgery, cerebral edema or surgical complications, such as hematoma48,49. To compensate for potential brain shift, subdural grid and strip electrode coordinates were projected to the leptomeningeal surface using an inverse gnomonic projection method described by Yang et al. (“yangWangElecPjct (‘subject’)” in Matlab command line)50. The surface under each grid was approximated as part of a larger sphere, and the algorithm iteratively adjusted the projection of the grid plane onto the sphere to minimize the difference between the projected and known electrode geometry. Subdural strip electrodes were assigned to the nearest leptomeningeal surface vertex. The brain shifting correction was done in MATLAB (Mathworks Inc., Natick, MA) via iELVis package51. iElVis was also used to identify the anatomical location of the permanent electrodes. The result of this processing is the grid location labeled at the hemisphere cortical surface mapped to the Desikan-Killiany brain atlas with defined cortex areas (“plotMgridOnPial (‘patient’)” in Matlab command line) (Fig. 2)44,50. All results are reported as means ± standard deviation of the mean.
Informed consent
Informed consent, including the discussion of off-label use of FDA approved devices, was obtained from all individual participants included in the study. Following our practice of subdural cortical stimulation performed off-label, the patients underwent a two-step procedure that involved implantation of a trial electrode array followed by permanent spinal leads and an internalized pulse generator.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This project was limited to a retrospective review of information from medical records within a group of patients who accepted MN research authorization. All data were analyzed anonymously with the approval of the Institutional Review Board (IRB) of our institution (ID# 18-006449) and in accordance to the usual policies and safeguards enforced by the Department of Health Science Research to protect the confidentiality of the patient record.
Data availability
Data collected for the study were deidentified and will be available with publication.
References
Jensen, M. P., Chodroff, M. J. & Dworkin, R. H. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 68, 1178–1182 (2007).
Merskey, N. Classificaiton of chronic pain; description of chronic pain syndromes and definitions of pain Terms. Task force Taxon. Int. Assoc. Study Pain 3, 41–43 (1994).
Bushnell, M. C., Čeko, M. & Low, L. A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 14, 502–511 (2013).
Delavallée, M., Abu-Serieh, B., De Tourchaninoff, M. & Raftopoulos, C. Subdural motor cortex stimulation for central and peripheral neuropathic pain: A long-term follow-up study in a series of eight patients. Neurosurgery 63, 101–105 (2008).
Cruccu, G. et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 14, 952–970 (2007).
Kumar, K., Toth, C. & Nath, R. K. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery 40, 736–747 (1997).
Hamani, C. et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain 125, 188–196 (2006).
Tsubokawa, T., Katayama, Y., Yamamoto, T., Hirayama, T. & Koyama, S. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin. Electrophysiol. 14, 131–134 (1991).
Levy, R., Deer, T. R. & Henderson, J. Intracranial neurostimulation for pain control: a review. Pain Phys. 13, 157–165 (2010).
Nguyen, J. P. et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch. Med. Res. 31, 263–265 (2000).
Fontaine, D., Hamani, C. & Lozano, A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature - Clinical article. J. Neurosurg. 110, 251–256 (2009).
Fiocchi, S., Chiaramello, E., Ravazzani, P. & Parazzini, M. Modelling of the current density distributions during cortical electric stimulation for neuropathic pain treatment. Comput. Math. Methods Med. 2018, (2018).
Honey, C. M., Tronnier, V. M. & Honey, C. R. Deep brain stimulation versus motor cortex stimulation for neuropathic pain: a minireview of the literature and proposal for future research. Comput. Struct. Biotechnol. J. 14, 234–237 (2016).
Thomas, L., Bledsoe, J. M., Sandroni, P., Gorman, D. & Lee, K. H. Motor cortex and deep brain stimulation for the treatment of intractable neuropathic face pain. Curr. Neurol. Neurosci. Rep. 9, 120–126 (2009).
Krushelnytskyy, M. D. et al. Chronic subdural cortical stimulation for phantom limb pain: report of a series of two cases. Acta Neurochir. (Wien) 161, 925–934 (2019).
André-Obadia, N. et al. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin. Neurophysiol. 117, 1536–1544 (2006).
Saitoh, Y. et al. Motor cortex stimulation for central and peripheral deafferentation pain. J. Neurosurg. 92, 150–155 (2000).
Velasco, F. et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain 147, 91–98 (2009).
Tronnier, V. & Rasche, D. Epidural and subdural stimulation. Handb. Clin. Neurol. 116, 343–351 (2013).
Kim, D., Jun, S. C. & Kim, H. I. Computational study of subdural and epidural cortical stimulation of the motor cortex. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 7226–7229 (2011) https://doi.org/10.1109/IEMBS.2011.6091826.
Monsalve, G. A. Motor cortex stimulation for facial chronic neuropathic pain: a review of the literature. Surg. Neurol. Int. 3, S290 (2012).
Steriade, M., Domich, L., Oakson, G. & Deschenes, M. The deafferented reticular thalamic nucleas generates spindle rhythmicity. J. Neurophysiol. 57, 260–273 (1987).
Steriade, M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Curr. Opin. Neurobiol. 3, 619–625 (1993).
Lefaucheur, J. P. et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 132, 1463–1471 (2009).
Maarrawi, J. et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 69, 827–834 (2007).
García-Larrea, L. et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 83, 259–273 (1999).
Garcia-Larrea, L. & Peyron, R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage 37, S71 (2007).
Ebel, H., Rust, D., Tronnier, V., Böker, D. & Kunze, S. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir. (Wien) 138, 1300–1306 (1996).
Parmar, V. K., Gee, L., Smith, H. & Pilitsis, J. G. Supraspinal stimulation for treatment of refractory pain. Clin. Neurol. Neurosurg. 123, 155–163 (2014).
Roux, F. E., Ibarrola, D., Lazorthes, Y. & Berry, I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case. Neurosurgery 48, 681–688 (2001).
Pereira, E. A. C., Moore, T., Moir, L. & Aziz, T. Z. Long-term motor cortex stimulation for phantom limb pain. Br. J. Neurosurg. 29, 272–274 (2015).
Raslan, A. M., Nasseri, M., Bahgat, D., Abdu, E. & Burchiel, K. J. Motor cortex stimulation for trigeminal neuropathic or deafferentation pain: an institutional case series experience. Stereotact. Funct. Neurosurg. 89, 83–88 (2011).
Machado, A. G., Mogilner, A. Y. & Rezai, A. R. Motor Cortex Stimulation for Persistent Non-cancer Pain. in Textbook of Stereotactic and Functional Neurosurgery 2239–2249 (Springer Berlin Heidelberg, 2009). https://doi.org/10.1007/978-3-540-69960-6_132.
Meyerson, B. A., Lindblom, U., Linderoth, B., Lind, G. & Herregodts, P. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir. Suppl. (Wien) 58, 150–153 (1993).
Buccino, G. et al. Action observation activates premotor and parietal areas in a somatotopic mannen: An FMRI study. in Social Neuroscience: Key Readings vol. 9780203496190 133–142 (Taylor and Francis, 2013).
The Cerebral Cortex of Man. A clinical study of localization of function. J. Am. Med. Assoc. 144, 1412 (1950).
Aflalo, T. N. & Graziano, M. S. A. Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array. J. Neurosci. 26, 6288–6297 (2006).
Graziano, M. S. A. Ethological action maps: a paradigm shift for the motor cortex. Trends Cogn. Sci. 20, 121–132 (2016).
Abe, M. & Hanakawa, T. Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav. Brain Res. 198, 13–23 (2009).
Graziano, M. S. A. & Aflalo, T. N. Mapping behavioral repertoire onto the cortex. Neuron 56, 239–251 (2007).
Hanakawa, T. Rostral premotor cortex as a gateway between motor and cognitive networks. Neurosci. Res. 70, 144–154 (2011).
Slotty, P. J. et al. The impact of brain shift in deep brain stimulation surgery: observation and obviation. Acta Neurochir. (Wien) 154, 2063–2068 (2012).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Joshi, A. et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics 9, 69–84 (2011).
Hastreiter, P. et al. Strategies for brain shift evaluation. Med. Image Anal. 8, 447–464 (2004).
Hill, D. L. G. et al. Sources of error in comparing functional magnetic resonance imaging and invasive electrophysiological recordings. J. Neurosurg. 93, 214–223 (2000).
Yang, A. I. et al. Localization of dense intracranial electrode arrays using magnetic resonance imaging. Neuroimage 63, 157–165 (2012).
Groppe, D. et al. iELVis: an open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. iELVis An open source MATLAB toolbox localizing Vis. Hum. intracranial electrode data 069179 (2016) https://doi.org/10.1101/069179.
Carroll, D. et al. Motor cortex stimulation for chronic neuropathic pain: a preliminary study of 10 cases. Pain 84, 431–437 (2000).
Rasche, D., Ruppolt, M., Stippich, C., Unterberg, A. & Tronnier, V. M. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain 121, 43–52 (2006).
Velasco, F. et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J. Neurosurg. 108, 698–706 (2008).
Acknowledgements
No financial and personal relationships with other people or organizations.
Funding
The work of TL and EM was supported by the Russian Science Foundation under grant 21-75-30024.
Author information
Authors and Affiliations
Contributions
I.L. and M.S. conceived the study and were the coordinating investigators. I.L., B.K., K.L. and M.S. designed the study protocol. T.L., I.L., and B.K. oversaw image quality assurance, and analyzed all available images and did the statistical analysis. I.L. and T.L. wrote the first draft of the article. I.L., T.L., E.M., B.L., P.S., M.S., and B.K. built a writing committee to work on the further drafts of the Article, which was reviewed by all authors. All authors contributed to data acquisition, management, and brain imaging analyses. All authors contributed to critical revision of the manuscript and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lavrov, I., Latypov, T., Mukhametova, E. et al. Pre-motor versus motor cerebral cortex neuromodulation for chronic neuropathic pain. Sci Rep 11, 12688 (2021). https://doi.org/10.1038/s41598-021-91872-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91872-2
This article is cited by
-
Brain imaging signatures of neuropathic facial pain derived by artificial intelligence
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.