Abstract
Excess glucocorticoids (GCs) with either endogenous or exogenous origins deteriorate skin barrier function. GCs bind to mineralocorticoid and GC receptors (MRs and GRs) in normal human epidermal keratinocytes (NHEKs). Inappropriate MR activation by GCs mediates various GC-induced cutaneous adverse events. We examined whether MR antagonists can ameliorate GC-mediated skin barrier dysfunction in NHEKs, reconstructed human epidermis (RHE), and subjects under psychological stress (PS). In a preliminary clinical investigation, topical MR antagonists improved skin barrier function in topical GC-treated subjects. In NHEKs, cortisol induced nuclear translocation of GR and MR, and GR and MR antagonists inhibited cortisol-induced reductions of keratinocyte differentiation. We identified 7,3’,4’-trihydroxyisoflavone (7,3’,4’-THIF) as a novel compound that inhibits MR transcriptional activity by screening 30 cosmetic compounds. 7,3’,4’-THIF ameliorated the cortisol effect which decreases keratinocyte differentiation in NHEKs and RHE. In a clinical study on PS subjects, 7,3',4'-THIF (0.1%)-containing cream improved skin barrier function, including skin surface pH, barrier recovery rate, and stratum corneum lipids. In conclusion, skin barrier dysfunction owing to excess GC is mediated by MR and GR; thus, it could be prevented by treatment with MR antagonists. Therefore, topical MR antagonists are a promising therapeutic option for skin barrier dysfunction after topical GC treatment or PS.
Similar content being viewed by others
Introduction
Psychological stress (PS) negatively affects epidermal barrier function1,2 and aggravates many cutaneous dermatoses, such as atopic dermatitis (AD) and psoriasis3,4,5,6,7,8. PS inhibits the proliferation and differentiation of keratinocytes and decreases the production and secretion of lamellar bodies and the density of corneodesmosomes, compromising both permeability barrier homeostasis and stratum corneum (SC) integrity1,9. PS-induced alterations in epidermal barrier homeostasis are mediated by increased cortisol, the endogenous glucocorticoids (GCs)10. The source of cortisol under PS is not only the activation of the hypothalamus–pituitary–adrenal (HPA) axis11,12 but also elevated 11β-hydroxysteroid dehydrogenase type I (11β-HSD1) in the skin13, which converts inactive cortisone into cortisol14. Likewise, systemic or topical administration of exogenous GC disrupts the skin barrier through a similar mechanism15.
The mineralocorticoid receptor (MR) and GC receptor (GR) are members of the same nuclear receptor superfamily16. Owing to their high structural similarity, not only the mineralocorticoid hormone, aldosterone, but also cortisol, can bind to MR with high affinity17,18. The binding of cortisol to MR is controlled at the pre-receptor level by 11β-hydroxysteroid dehydrogenase type II (11β-HSD2), which converts cortisol into inactive cortisone19. However, the epidermis is a well-recognized 11β-HSD2-deficient tissue20,21; thus, MR can be inappropriately activated by increased GCs19,22,23,24. Recent studies have highlighted that inappropriately activated MR caused by excess GC is involved in generating GC-mediated cutaneous side effects, such as delayed wound healing, epidermal atrophy, and skin aging. Furthermore, such side effects can be prevented by topical MR blockade25,26,27,28.
Therefore, we hypothesized that MR inappropriately activated by GC mediates skin barrier dysfunction caused by exogenous GC or PS, and thus, topical MR antagonism could prevent their detrimental effects on the skin barrier.
First, we conducted a brief preliminary clinical investigation to verify whether topical MR antagonists could prevent topical GC-induced skin barrier dysfunction. Second, we examined whether cortisol activates both GR and MR, and whether the receptors are inhibited by their own antagonists in normal human epidermal keratinocytes (NHEKs). To gain mechanistic insights into how MR antagonists improve the skin permeability barrier, the mRNA expression of epidermal differentiation markers was analyzed in NHEKs and immunohistochemical staining for epidermal differentiation markers was conducted in reconstructed human epidermis (RHE) after treatment with either cortisol or an antagonist of both GR and MR. Third, to find a novel compound that can be utilized as a cosmetic ingredient, we screened 30 compounds, selected one, and validated it as a novel MR antagonist. Finally, we conducted a clinical investigation to verify the effect of our newly developed MR antagonist in improving skin barrier function in PS participants.
Results
Topical application of spironolactone, an MR antagonist prevents topical GC-induced skin barrier impairment
To preliminarily investigate the effect of an MR antagonist in improving skin barrier function that is impaired by GC, a small-sample clinical experiment was conducted using topical spironolactone, a proven MR antagonist, and topical GC. In 11 healthy participants, 0.05% clobetasol propionate ointment (Dermovate ointment; GlaxoSmithKline, Uxbridge, UK) was applied to both sides of the forearms, with 5% topical spironolactone cream (S5 cream, Shanghai Sunshine Technology, Shanghai, China) or Cetaphil moisturizing lotion (Galderma Laboratories, Fort Worth, TX, USA) as a control vehicle, on each side. After 5 days of topical application, the skin barrier function of the clobetasol + spironolactone-treated and clobetasol + vehicle-treated sides were measured and compared (Fig. 1a–e). Basal transepidermal water loss (TEWL), SC hydration, skin surface pH, and barrier recovery rate were not significantly different between the two sides. However, co-application of topical spironolactone resulted in significantly lower delta TEWL (p = 0.019). SC integrity was measured as the delta TEWL before and after tape-stripping 15 times on the same site on the skin. Each tape stripping removed the outer layer of the SC. Thus, the lower the value, the firmer the structure of the SC. In other words, SC integrity was improved by co-application with topical spironolactone.
Topical application of spironolactone, a mineralocorticoid receptor antagonist, improves skin barrier function in clobetasol-treated skin and increases the amount of stratum corneum (SC) lipids. Comparison of basal transepidermal water loss (TEWL) (a), SC integrity (b), SC hydration (c), skin surface pH (d), and barrier recovery rate (e) between clobetasol + spironolactone-treated sides and clobetasol + vehicle-treated sides in healthy participants. Quantitative analysis of SC lipids, ceramides (f), cholesterol (g), and fatty acids (h) using tape-stripped skin samples from the participants. Data are expressed as mean ± SD (N = 11). Wilcoxon signed-rank test was used.
To investigate the effect of MR antagonists on permeability barrier homeostasis, SC lipids were quantified using tape-stripped skin samples. Co-application of topical spironolactone significantly increased the amounts of cholesterol (p < 0.001) and to a much lesser extent of ceramides (p = 0.003) and fatty acids (FAs; p = 0.056), albeit without statistical significance (Fig. 1f–h). In summary, topical application of spironolactone, a proven MR antagonist, improved skin barrier function and increased the amounts of ceramides, cholesterol, and fatty acids in the SC of topical GC-treated skin.
GC activates nuclear translocation of GR and MR in NHEKs
To determine whether cortisol activates both GR and MR, and whether the receptors are inhibited by their own antagonists, we examined the nuclear translocation of GR and MR in NHEKs treated with mifepristone (a GR antagonist) or eplerenone (an MR antagonist) in the presence or absence of cortisol (Fig. 2 and S1). Cortisol induced the nuclear translocation of both GR and MR. Mifepristone decreased the cortisol-induced nuclear signals of GR and eplerenone decreased the cortisol-induced nuclear signals of MR. Treatment with both mifepristone and eplerenone decreased the nuclear signals of both receptors. In the basal state, mifepristone slightly decreased the nuclear signals of GR, albeit without statistical significance (Fig. S1). However, eplerenone did not decrease those of MR. Co-treatment with mifepristone and eplerenone decreased the nuclear signals of GR, even in the basal state. These results indicate that basal level nuclear translocation of GR is present even without exogenous cortisol treatment. In contrast, basal level nuclear translocation of MR is relatively minimal compared to that of GR.
Cortisol activates nuclear translocation of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) in NHEKs. NHEKs were treated with mifepristone (GR antagonist, 1 μM) or eplerenone (MR antagonist, 1 μM) in the presence of cortisol (10 μM) for 24 h. Cells were stained with antibodies against GR (red) and MR (green) and observed under confocal microscopy. Nuclei were stained with DAPI (blue). (a) Representative images. Scale bars, 10 μm. (b, c) Quantification of mean fluorescence intensity (MFI) of GR and MR in nucleus. Data are expressed as mean ± SD of three independent experiments.
GR and MR antagonists improve keratinocyte differentiation under GC treatment
To examine the effect of cortisol on keratinocyte differentiation, and whether it is inhibited by antagonists of GR and MR, the mRNA levels of keratinocyte differentiation markers were analyzed in NHEKs treated with mifepristone and eplerenone in the presence or absence of cortisol. Cortisol reduced the mRNA levels of Filaggrin (FLG), Loricrin (LOR), Desmocollin-1 (DSC1), Keratin 1 (KRT1), KRT10 and (Fig. 3a–e). Compared to the cortisol-only treatment group, co-treatment with mifepristone increased the mRNA levels of FLG, LOR, and DSC1, whereas co-treatment with eplerenone resulted in a remarkable increase in KRT1 and KRT10, and a slight increase in FLG and DSC1. Co-treatment with both mifepristone and eplerenone potentiated the increases in the mRNA levels of FLG, LOR, and DSC1. However, the increased mRNA levels of KRT1 and KRT10 after co-treatment with eplerenone were offset by further co-treatment with mifepristone. In addition, we analyzed the effect of mifepristone and eplerenone on keratinocyte differentiation in the basal state (Fig. S2). Mifepristone increased the mRNA levels of FLG and LOR but decreased those of KRT1, KRT10, and DSC1. In contrast, eplerenone had little effect on the mRNA levels of keratinocyte differentiation. Taken together, mifepristone increased the mRNA levels of FLG and LOR in both cortisol-treated and basal states; however, eplerenone showed a significant increase in the mRNA levels of KRT1 and KRT10 in cortisol-treated NHEKs.
Effect of cortisol, GR antagonist, and MR antagonist on mRNA expression of keratinocyte differentiation markers in NHEKs. (a–e) NHEKs were treated with mifepristone (GR antagonist, 1 μM) or eplerenone (MR antagonist, 1 μM) in the presence of cortisol (10 μM) for 4 days, and the mRNA expression of differentiation markers, FLG, LOR, DSC1, KRT1, and KRT10 was analyzed by RT-qPCR. (f–j) NHEKs were transfected with control (non-targeting; NT), GR, or MR siRNAs. (f, g) mRNA level of GR and MR in siRNA-treated NHEKs. (h–j) mRNA level of FLG, LOR, and KRT1 in siRNA-treated NHEKs treated with cortisol (10 μM) for 4 days. mRNA levels were normalized to that of RPL13A. Data are expressed as mean ± SD of three independent experiments.
To further elucidate the roles of GR and MR in keratinocyte differentiation, we introduced small interfering RNAs (siRNAs) against GR and MR into the NHEKs and examined the subsequent mRNA levels of keratinocyte differentiation markers (Fig. 3f–j). The reduction in GR and MR mRNA levels by each siRNA was validated (Fig. 3f, g). However, the siRNAs against GR and MR each also slightly decreased the mRNA levels of the other receptor. Cortisol reduced the mRNA expression of FLG, LOR, and KRT1 in non-targeting (NT) siRNA-treated NHEKs (Fig. 3h–j). GR siRNA-treated NHEKs showed higher mRNA levels of keratinocyte differentiation markers than NT siRNA-treated NHEKs in the basal state, whereas those of MR siRNA-treated NHEKs were similar. In GR siRNA-treated NHEKs, cortisol did not decrease the mRNA levels of FLG, LOR, and KRT1, but rather increased them. In contrast, in MR siRNA-treated NHEKs, cortisol decreased the mRNA levels of FLG, LOR, and KRT1. These results indicate that GR in involved in regulating keratinocyte differentiation in the basal status and the cortisol-induced decreases in the mRNA levels of keratinocyte differentiation markers mainly result from the activation of GR rather than MR by cortisol.
Screening compounds with MR antagonizing properties
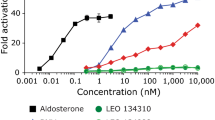
To find a compound with MR antagonist properties, which can be utilized as a cosmetic ingredient, we screened 30 single compounds derived from soybeans, green tea, and Korean ginseng. To select a compound that reacts selectively to MR, MR transcriptional activity was measured and compared among the 30 compounds. We first evaluated the expression of MR and GR in MDA-MB-453, CV-1, and MDA KB2 cells (Fig. S3a). Both GR and MR genes were expressed in MDA-MB-453 cells, and MR and GR genes were expressed in CV-1 cells and MDA-KB2 cells, respectively. Therefore, we transfected CV-1 cells, which expressed MR rather than GR, with an MMTV-luciferase reporter plasmid with hexanucleotide 5'-TGTTCT-3' as the enhancer element sequence and examined the effect of the 30 compounds on MR transcriptional activity using a luciferase reporter gene assay (Fig. S3c–h, and Table S1). The MR transcriptional activity values of the compounds were lowest (in ascending order) in epicatechin gallate, epicatechin, L-theanine, and 7,3',4'-trihydroxyisoflavone (7,3',4'-THIF). Considering the cell toxicity and stability of these cosmetic formulations, we selected 7,3′4,'-THIF for further study from among these four compounds with the lowest MR transcriptional activity values. 7,3’,4’-THIF is a hydroxyisoflavone that is daidzein-substituted by a hydroxyl group at position 3’ (Fig. S3b).
To validate 7,3’,4’-THIF as an MR antagonist in NHEKs, we analyzed the nuclear translocation of MR and GR in NHEKs treated with 7,3’,4’-THIF in the presence or absence of cortisol (Fig. 4 and Fig. S4). In cortisol-treated NHEKs, 7,3',4'-THIF inhibited the nuclear translocation of MR by cortisol. In addition, the nuclear translocation of GR by cortisol was also inhibited by 7,3',4'-THIF, but was less inhibited than that of MR (Fig. 4). In the basal state, 7,3’,4’-THIF treatment did not affect the nuclear translocation of either MR or GR (Fig. S4). These results indicate that, in NHEKs that express both MR and GR, 7,3’,4’-THIF inhibited the nuclear translocation of not only MR but also GR.
7,3’,4’-THIF inhibited cortisol-induced nuclear translocation of MR and GR in NHEKs. NHEKs were treated with 7,3’,4’-THIF (1 ppm) in the presence of cortisol (10 μM) for 24 h. Cells were stained with antibodies against GR (red) and MR (green) and observed under confocal microscopy. Nuclei were stained with DAPI (blue). (a) Representative images. Scale bars, 10 μm. (b, c) Quantification of mean fluorescence intensity (MFI) of GR and MR in nucleus. Data are expressed as mean ± SD of three independent experiments.
7,3',4'-THIF inhibits the cortisol-induced decreased expression of keratinocyte differentiation markers
To determine the effect of 7,3',4'-THIF on skin barrier function, the mRNA levels of keratinocyte differentiation markers such as KRT1, KRT10, FLG, and LOR were analyzed after treatment with 7,3',4'-THIF in the presence or absence of cortisol. In cortisol-treated NHEKs, 7,3’,4’-THIF inhibited the cortisol-induced reduction in the mRNA levels of KRT1, KRT10, FLG, and LOR in a dose-dependent manner (Fig. 5a–d). In the basal state, 7,3',4'-THIF increased the mRNA levels of FLG and LOR, but not KRT1 (Fig. S5).
Effect of 7,3’,4’-THIF on mRNA expression of keratinocyte differentiation markers in NHEKs. (a–d) NHEKs were treated with 7,3’,4’-THIF (0.01, 0.1, 1 ppm) in the presence of cortisol (10 μM) for 4 days, and the mRNA levels of KRT1, KRT10, FLG, and LOR were analyzed by RT-qPCR. (e, f) NHEKs were transfected with control (non-targeting; NT), GR, or MR siRNAs. mRNA expression of FLG and LOR in siRNA-expressing NHEKs treated with 7,3’,4’-THIF (1 ppm) in the presence of cortisol (10 μM) for 4 days. mRNA levels were normalized to that of RPL13A. Data are expressed as mean ± SD of three independent experiments.
To elucidate whether 7,3’,4’-THIF inhibits the cortisol effect via GR or MR, we examined the effect of 7,3’,4’-THIF in GR or MR siRNA-treated NHEKs in the presence of cortisol (Fig. 5e and f). In GR siRNA-treated NHEKs, the mRNA expression of FLG and LOR was further increased by co-treatment with cortisol and 7,3',4'-THIF, compared to when only cortisol was used. In contrast, in MR siRNA-treated NHEKs, there was no significant difference between 7,3’,4’-THIF + cortisol and cortisol only. As GR siRNA-treated NHEKs showed remarkably diminished GR expression (Fig. 3f), cortisol is more likely to activate MR than GR in GR siRNA-treated NHEKs. These results suggest that, in GR siRNA and cortisol-treated NHEKs, co-treatment with 7,3’,4’-THIF increased FLG and LOR mRNA levels by inhibiting of MR activation. This highlights the role of MR activation by cortisol in cortisol-induced decreases of keratinocyte differentiation, and the role of MR antagonists in preventing it.
We further examined the effect of 7,3’,4’-THIF on cortisol-induced skin barrier dysfunction in an RHE (Fig. 6). 7,3′4’-THIF (1 ppm), eplerenone (1 μM), or vehicle (0.01% DMSO in phosphate-buffered saline) were applied topically to an RHE in the presence or absence of cortisol (10 μM) every other day for 6 days. Cortisol induced a thinner and less dense epidermal structure (Fig. 6a). The topical application of 7,3’,4’-THIF and eplerenone ameliorated cortisol-induced skin barrier impairment and improved the protein levels of epidermal differentiation markers decreased by cortisol. In the RHE without cortisol treatment, 7,3’,4’-THIF and eplerenone also increased the protein expression of Filaggrin and Keratin 10 (Fig. S6). In addition, the cortisol-induced decrease in the mRNA levels of epidermal differentiation markers was also recovered by 7,3’,4’-THIF (Fig. 6b–e). In summary, 7,3’,4’-THIF treatment ameliorated cortisol-induced decreases in keratinocyte differentiation marker expression and increased their expression in the basal state, in a similar manner to eplerenone.
7,3’,4’-THIF ameliorated skin barrier impairment by cortisol in a reconstituted human epidermis (RHE). A RHE was topically treated with 7,3′4’-THIF (1 ppm) or eplerenone (MR antagonist, 1 μM) in the presence of cortisol (10 μM) for 6 days. (a) Immunohistochemistry analysis using relevant antibodies. H&E, Hematoxylin and eosin staining. Scale bars, 100 μm. Quantitative RT-PCR analysis of (b) KRT1, (c) KRT10, (d) FLG, and (e) LOR mRNA levels in the RHE. mRNA levels were normalized to that of RPL13A. Data are expressed as mean ± SD of three independent experiments.
Topical application of 7,3',4'-THIF prevents PS-induced skin barrier dysfunction
To verify the effect of 7,3',4'-THIF in improving skin barrier function impaired by PS, clinical experiments were conducted. Twenty-five healthy male medical students were recruited for this study. The mean age of the participants was 19.8 ± 0.9 years (mean ± SD). They applied a 7,3’,4’-THIF (0.1%)-containing cream or vehicle on each side of both forearms for 10 days until the middle of their exam period. Examination stress is an established model of PS that disrupts skin barrier function9,13,29,30. The experiments were approved by the Institutional Review Board of Wonju Severance Christian Hospital and were carried out in accordance with their guidelines and regulations. Informed consent was obtained from all participants.
After 10 days of topical application, in the middle of the examination period, the skin barrier function of each forearm was measured and compared. Topical application of the 7,3’,4’-THIF-containing cream significantly lowered skin surface pH (p = 0.014) and improved barrier recovery (p = 0.040) compared to the vehicle cream (Fig. 7a–e). Skin surface pH plays a crucial role in maintaining skin barrier function, and a lowered skin surface pH indicates improved of skin barrier function31. Basal TEWL, SC integrity, and SC hydration did not differ between the two sides. The SC cortisol levels in tape-stripped samples were significantly lower in the 7,3’,4’-THIF group than in the vehicle group (p < 0.001) (Fig. 7f).
Topical application of 7,3',4'-trihydroxyisoflavone (7,3’,4’-THIF), a novel mineralocorticoid receptor antagonist, improves psychological stress (PS)-induced barrier impairment and increases the amounts of stratum corneum (SC) lipids. Comparison of basal transepidermal water loss (TEWL) (a), SC integrity (b), skin surface pH (c), SC hydration (d), and barrier recovery rate (e) between 7,3’,4’-THIF-treated sides and vehicle-treated sides in 25 medical students under PS. Quantitative analysis of cortisol (f), total ceramides (g), ceramides with specific chain length (h), cholesterol (i), total fatty acids (j), and saturated and unsaturated fatty acids (k) using tape-stripped skin samples from the participants. Data are expressed as mean ± SD (a-f; N = 25, g-k; N = 6). Paired t-test and Wilcoxon signed-rank test were used as appropriate.
To investigate the effect of 7,3’,4’-THIF on permeability barrier homeostasis, the amounts of SC lipids were analyzed. Using tape-stripped SC samples from both sides, three main SC lipids (ceramides, cholesterol, and fatty acids) were quantified. Topical application of 7,3’,4’-THIF significantly increased the amount of ceramides (p = 0.008) and cholesterol (p = 0.012). In particular, very long-chain ceramides, such as C24:1, C24:0, C26:1, and C26:0, were preferentially increased. Unsaturated FAs were increased (p < 0.05), whereas total FAs were not (Fig. 7g–k). In summary, topical application of 7,3’,4’-THIF in PS participants improved skin barrier functions, such as skin surface pH and barrier recovery rate, which were attributed to increased SC lipids.
Discussion
Robust evidence supports that exogenous GC and endogenous GC under PS negatively affect the skin barrier function. Among various psychological stressors, examination stress is a validated model of PS, as shown in previous studies9,13,29,30. PS upregulates the HPA axis to stimulate systemic stress hormone production12 and 11β-HSD1 activity in keratinocytes14. An elevated concentration of cortisol in the skin is a key mediator of PS-induced skin barrier dysfunction. It causes decreased production of epidermal lipids and structural proteins, increased skin surface pH, and decreased corneodesmosome density, eventually deteriorating the skin barrier function characterized by decreased SC integrity and delayed barrier recovery1,10,13. In this study, we aimed to demonstrate the action of cortisol inside keratinocytes, which is hypothesized to be mediated by GR and MR. Furthermore, we investigated whether MR antagonists can ameliorate GC-induced skin barrier dysfunction in NHEKs, an RHE, and clinical studies.
Previous studies regarding MR antagonism against GC excess have demonstrated that it prevents delayed wound closure and epidermal atrophy by enhancing keratinocyte proliferation, migration, and differentiation25,26. Our results can be understood in the same context. Skin barrier function is organized by epidermal proliferation and differentiation, which begins at the basal layer32,33. In this study, we suggest further evidence of inappropriate GC-induced MR activation by illustrating that GC increases the nuclear translocation of MR, as well as GR. GR and MR are ligand-inducible transcription factors19. After steroid hormones are bound, these receptors translocate from the cytoplasm into the nucleus and exhibit their transactivation potential.
We observed that mifepristone and eplerenone decreased the nuclear translocation of their respective receptors in cortisol-treated NHEKs (Fig. 2). In addition, co-treatment of mifepristone or eplerenone with cortisol offset the cortisol-induced reduction in the mRNA levels of keratinocyte differentiation markers (Fig. 3a–e). However, in the basal state, single treatment with mifepristone increased the mRNA levels of FLG and LOR (Fig. S2). Such remarkable changes were not observed with eplerenone. In addition, in siRNA studies, treatment with GR siRNA alone enhanced the mRNA expression of FLG, LOR, and KRT1, which was not observed with MR siRNA (Fig. 3h–j). In GR siRNA-treated NHEKs, cortisol treatment did not decrease the mRNA levels of FLG, LOR, and KRT1. Furthermore, in GR siRNA- and cortisol-treated NHEKs, 7,3’,4’-THIF further increased the mRNA levels of FLG and LOR, which was not observed in MR siRNA-treated NHEKs (Fig. 5e and f). This could be attributed to 7,3’,4’-THIF inhibiting the MR activation by cortisol in the absence of GR. Taken together, GR is involved in regulating keratinocyte differentiation in both basal and cortisol-excess states. In contrast, MR is activated when excess cortisol is administered, and is involved in the cortisol-induced suppression of keratinocyte differentiation.
7,3’,4’-THIF is a compound with the property of an MR antagonist, selected after screening 30 compounds, according to their inhibition of MR transcriptional activity (Table S1 and Fig. S3). It is a metabolite of daidzein, a representative isoflavone found in soybeans. A recent study suggested that 7,3’,4’-THIF suppresses α-melanocyte-stimulating hormone-induced melanogenesis by targeting melanocortin 1 receptor in B16F10 cells34.
The inhibition of epidermal lipid synthesis is one of the key deleterious effects of GC, which deteriorates permeability barrier homeostasis. Topical application of physiologic SC lipids, comprising equimolar ceramides, cholesterol, and free FAs, prevents barrier disruption in both PS and topical GC-treated mice1,15,35. In this study, SC lipids were analyzed in two clinical studies. Topical application of spironolactone, an MR antagonist, increased the amounts of ceramides and cholesterol in clobetasol propionate-treated skin (Fig. 1f–h). Topical application of a 7,3’,4’-THIF-containing cream increased the amounts of ceramides and cholesterol in PS participants (Fig. 7g–k). In particular, the levels of long chain ceramides (C24 to C26), which are essential for skin barrier function36,37,38, were increased, while those of ceramides with shorter carbon chains were not significantly changed (Fig. 7h).
Skin barrier function was improved by topical application of spironolactone (Fig. 1a–e) and 7,3’,4’-THIF-containing cream (Fig. 7a–e), which was attributed to increased keratinocyte differentiation and SC lipids32,39. Although topical spironolactone only improved SC integrity and the 7,3’,4’-THIF-containing cream improved only the pH of the skin surface and barrier recovery rate, not all the measured parameters, these changes are important for the improvement of skin barrier function. An acidic skin surface pH plays a crucial role in maintaining permeability barrier homeostasis31. SC integrity and barrier recovery rate are challenge tests for skin barrier function, measuring the extent to which the permeability barrier is damaged after 15 consecutive tape-stripping cycles and how much it recovers after 2 h40. However, basal TEWL and SC hydration were measured when the skin barrier was not challenged. The participants had no skin diseases. Increased basal TEWL and decreased SC hydration are common findings in patients with impaired skin barriers or severe dry skin, which is one of the exclusion criteria of our clinical trials.
Another method of regulating MR activation by GC is through the pre-receptor regulating the activity of 11β-HSD119. Under various stimuli, including ultraviolet irradiation, aged skin, and exogenous GC and PS, the activation of 11β-HSD1 increases the local production of the active GC cortisol, thus stimulating MR downstream13,41,42. The inhibitory intervention of 11β-HSD1 or MR yielded similar results in augmenting wound healing and preventing skin atrophy, aging, or skin barrier dysfunction43,44. Therefore, the simultaneous inhibition of both effectors may provide more effective prevention of GC-mediated cutaneous adverse effects under various stimuli. However, the inhibition of 11β-HSD1 may also have negative effects, depending on the condition of the skin. In an oxazolone-induced mouse model of AD, inhibition of 11b-HSD1 aggravated the development of AD and increased the serum cytokine levels associated with AD45. Therefore, the potential negative effects of 11β-HSD1 inhibition may induce in AD. In this study, we tried to improve skin response by suppressing the stress response using MR antagonists in healthy people, not in those with inflammatory skin diseases such as AD. We also confirmed that 7,3’,4’-THIF did not convert cortisone to cortisol in NHEKs (Fig. S7).
Further investigation is required to address the alteration in the anti-inflammatory effects of GC by MR antagonists. When canrenoate, an MR antagonist, is co-applied with clobetasol in an irritated human skin equivalent model, among the anti-inflammatory properties of GC, the inhibition of COX-2 and upregulation of the anti-inflammatory cytokine interleukin (IL)-10 are preserved, whereas the inhibition of pro-inflammatory cytokines, such as IL-6 and IL-1α, is not46. Applying MR antagonists to inflamed skin (e.g., AD) could raise concerns about modulating the anti-inflammatory property of GCs or perhaps aggravating skin inflammation. However, in this study, the skin conditions we aimed to treat were those of topical GC-treated or PS subjects who had weakly increased cortisol and no apparent signs of inflammation.
For patients with cutaneous dermatoses where PS acts as an aggravating factor, an investigation into whether the MR blockade can prevent aggravation by PS is needed. We observed that the keratinocyte differentiation markers increased by co-treatment with mifepristone were FLG, LOR, and DSC1, but not KRT1 and KRT10, which were improved by co-treatment with eplerenone. FLG and LOR are late differentiation markers, and KRT1 and KRT10 are early differentiation markers47. Therefore, further investigation into the involvement of GR and MR in late and early keratinocyte differentiation is needed.
A limitation of our study is that the participants were all healthy male volunteers. In the preliminary clinical trial, spironolactone was used as an MR antagonist because topical spironolactone has been commercialized as a product, so called ‘S5 cream’, for the treatment of pattern hair loss and acne. However, eplerenone was used in the NHEKs and RHE studies because of its higher MR selectivity than spironolactone26,48. This difference in the chemicals used in the clinical and laboratory studies resents another limitation. In addition, to demonstrate the efficacy of 7,3’,4’-THIF in improving skin barrier function in clinical studies, it was compared to a control vehicle, not to a pre-existing MR antagonist, such as topical spironolactone.
In conclusion, under PS or topical GC treatment, excess GC activates MR as well as GR, resulting in skin barrier dysfunction. Topical application of MR antagonists significantly prevented the deleterious effects of PS or exogenous GC on the skin barrier by improving keratinocyte differentiation and SC lipid synthesis. This could represent a promising therapeutic option to ameliorate skin disorders caused by PS or skin fragility caused by the prolonged use of systemic or topical GCs.
Materials and methods
Human studies
The experiments were approved by the Institutional Review Board of Wonju Severance Christian Hospital (CR319140 and CR317125) and were carried out in accordance with the appropriate guidelines and regulations. Informed consent was obtained from all subjects.
Application of topical GC and spironolactone in healthy participants
A total of 11 healthy young adults were recruited. The same sites on both forearms of each participant were treated with 0.05% clobetasol propionate ointment (Dermovate, GlaxoSmithKline, Uxbridge, UK), 5% spironolactone cream (Shanghai Sunshine Technology, Shanghai, China), or 0.05% clobetasol propionate ointment + Cetaphil lotion (Galderma Laboratories, Fort Worth, TX, USA) for five consecutive days. Skin barrier functions and the amounts of SC lipids in the tape-stripped samples were measured on each forearm.
Application of a novel MR inhibitor, 7,3',4'-THIF, in humans under PS
A total of 25 medical students were recruited for this study. All participants were male medical students of the same grade and were all in good health. An examination model was adopted as the type of PS. The same sites on both forearms of each participant were treated with 7,3’,4’-THIF (0.1%)-containing cream or vehicle moisturizer for 10 consecutive days before the examination period. During the examination period, skin barrier functions and the amount of SC lipids in the tape-stripped samples were measured on each forearm.
Cell culture
NHEKs (Lonza, Basel, Switzerland) within two or three passages were cultured in KBM-Gold medium supplemented with a KGM-Gold Bullet Kit (Lonza) at 37 °C and 5% CO2. Hydrocortisone, one of the components of the KGM-Gold Bullet kit, was not added to the medium for the assay. NHEKs were treated with cortisol (10 μM) in the presence or absence of 7,3’,4’-THIF (Indofine Chemical Co., Inc., Hillsborough, NJ, USA), mifepristone (Tocris, Bristol, UK), or eplerenone (Tocris), and harvested 4 days later for further analysis.
Immunocytochemistry and immunohistochemistry
NHEKs were treated with cortisol with or without mifepristone, eplerenone, or 7,3’,4’-THIF, stained with anti-GR antibody (BD Bioscience, San Jose, CA, USA) or anti-MR antibody (Abcam, Cambridge, MA, USA), and analyzed under a confocal microscope (LSM 700; Carl Zeiss, Jena, Germany). The images obtained were quantified for mean fluorescence intensity (MFI) using ImageJ software (https://imagej.nih.gov/ij). For immunohistochemical analysis, replicate sections from an RHE were stained with the following antibodies: anti-keratin 10 (Biolegend, San Diego, CA), anti-keratin 1 (Biolegend), and anti-filaggrin (Abcam). The detailed protocols for the immunological analyses are described in the Supplementary Information.
Quantitative real-time PCR
Total RNA was prepared using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was synthesized using a Revertaid RT kit (Thermo Fisher Scientific). Quantitative real-time PCR was performed using TaqMan probes (described in the Supplementary Information; Thermo Fisher Scientific) and the 7500 Fast Real-Time PCR system (Thermo Fisher Scientific). The RPL13A gene was used to normalize the amount of cDNA. Relative differences in gene expression were calculated from the threshold cycle (Ct) values. At least three independent experiments were assessed to calculate mean values ± SD.
siRNA experiments
NHEKs were transfected with 50 nM of non-targeting control siRNA or siRNAs against GR or MR (Dharmacon, Lafayette, CO, USA), using Lipofectamine RNAimax transfection reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
MR transactivation assay
CV-1 cells were seeded into 24-well plates and cultured for 24 h before transfection. Prior to transfection, the medium was replaced with 10% charcoal dextran-treated FBS–DMEM. After 4 h, a DNA mixture containing an MMTV-luciferase reporter plasmid (pGL4.36[luc2P/MMTV/Hygro] vector, 0.3 μg, Promega) with (TGTTCT)6 as the enhancer element sequence, and an internal control plasmid pRL-SV-40 (5 ng), was transfected using the TransFast reagent (Promega, Madison, WI, USA). After transfection, cells were treated with 10 μg/mL compounds (samples) or 1 μM spironolactone. After 2 h, the treated cells were further treated with 1 nM dexamethasone and incubated for an additional 24 h. The luciferase activities of the cell lysates were measured using the Dual-Luciferase Reporter Assay System, according to the manufacturer’s instructions (Promega). The relative luciferase activity was normalized to the corresponding Renilla luciferase activity to determine transfection efficiency.
Reconstructed human epidermis
A RHE was purchased from MatTek (Ashland, MA, USA) and maintained according to the manufacturer’s instructions. The RHE was systemically treated with cortisol (10 μM), and topically treated with 7,3’,4’-THIF (1 ppm), eplerenone (1 μM), or vehicle (0.01% DMSO in phosphate-buffered saline) every other day for 6 days.
Abbreviations
- PS:
-
Psychological stress
- 7,3’,4’-THIF:
-
7,3',4'-Trihydroxyisoflavone
- TEWL:
-
Transepidermal water loss
- SC:
-
Stratum corneum
- GR:
-
Glucocorticoid receptor
- MR:
-
Mineralocorticoid receptor
- GC:
-
Glucocorticoid
- FA:
-
Fatty acid
- 11β-HSD1:
-
11β-Hydroxysteroid dehydrogenase type I
- 11β-HSD2:
-
11β-Hydroxysteroid dehydrogenase type II
References
Choi, E. H. et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J. Invest. Dermatol. 124, 587–595. https://doi.org/10.1111/j.0022-202X.2005.23589.x (2005).
Maarouf, M., Maarouf, C. L., Yosipovitch, G. & Shi, V. Y. The impact of stress on epidermal barrier function: an evidence-based review. Br. J. Dermatol. 181, 1129–1137. https://doi.org/10.1111/bjd.17605 (2019).
Baum, A. Stress, intrusive imagery, and chronic distress. Health Psychol. 9, 653–675. https://doi.org/10.1037//0278-6133.9.6.653 (1990).
Fortune, D. G. et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch. Dermatol. 139, 752–756. https://doi.org/10.1001/archderm.139.6.752 (2003).
Kabat-Zinn, J. et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom. Med. 60, 625–632. https://doi.org/10.1097/00006842-199809000-00020 (1998).
Kodama, A. et al. Effect of stress on atopic dermatitis: investigation in patients after the great hanshin earthquake. J. Allergy Clin. Immunol. 104, 173–176. https://doi.org/10.1016/s0091-6749(99)70130-2 (1999).
Koblenzer, C. S. & Koblenzer, P. J. Chronic intractable atopic eczema. Its occurrence as a physical sign of impaired parent-child relationships and psychologic developmental arrest: improvement through parent insight and education. Arch. Dermatol. 124, 1673–1677. https://doi.org/10.1001/archderm.124.11.1673 (1988).
Ullman, K., Moore, R. W. & Reidy, M. Atopic eczema: a clinical psychiatric study. J. Asthma Res. 14, 91–99. https://doi.org/10.3109/02770907709098955 (1977).
Garg, A. et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 137, 53–59. https://doi.org/10.1001/archderm.137.1.53 (2001).
Choi, E. H. et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am. J. Physiol. Regul. Int. Comput. Physiol. 291, R1657-1662. https://doi.org/10.1152/ajpregu.00010.2006 (2006).
Slominski, A. A nervous breakdown in the skin: stress and the epidermal barrier. J. Clin. Invest. 117, 3166–3169. https://doi.org/10.1172/JCI33508 (2007).
Slominski, A., Wortsman, J., Tuckey, R. C. & Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell. Endocrinol. 265–266, 143–149. https://doi.org/10.1016/j.mce.2006.12.012 (2007).
Choe, S. J. et al. Psychological stress deteriorates skin barrier function by activating 11beta-hydroxysteroid dehydrogenase 1 and the HPA Axis. Sci. Rep. 8, 6334. https://doi.org/10.1038/s41598-018-24653-z (2018).
Terao, M. et al. 11beta-Hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PLoS ONE 6, e25039. https://doi.org/10.1371/journal.pone.0025039 (2011).
Kao, J. S. et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J. Invest. Dermatol. 120, 456–464. https://doi.org/10.1046/j.1523-1747.2003.12053.x (2003).
Farman, N. et al. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon?. Exp. Dermatol. 19, 100–107. https://doi.org/10.1111/j.1600-0625.2009.01011.x (2010).
Farman, N. & Nguyen, V. T. A novel actor in skin biology: the mineralocorticoid receptor. Exp. Dermatol. 25, 24–25. https://doi.org/10.1111/exd.12888 (2016).
Hellal-Levy, C. et al. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett 464, 9–13. https://doi.org/10.1016/s0014-5793(99)01667-1 (1999).
Frey, F. J., Odermatt, A. & Frey, B. M. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr. Opin. Nephrol. Hypertens. 13, 451–458. https://doi.org/10.1097/01.mnh.0000133976.32559.b0 (2004).
Kenouch, S. et al. Human skin as target for aldosterone: coexpression of mineralocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab. 79, 1334–1341. https://doi.org/10.1210/jcem.79.5.7962326 (1994).
Smith, R. E. et al. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J. Clin. Endocrinol. Metab. 81, 3244–3248. https://doi.org/10.1210/jcem.81.9.8784076 (1996).
Farman, N. & Rafestin-Oblin, M. E. Multiple aspects of mineralocorticoid selectivity. Am. J. Physiol. Renal. Physiol. 280, F181-192. https://doi.org/10.1152/ajprenal.2001.280.2.F181 (2001).
Mihailidou, A. S., Loan Le, T. Y., Mardini, M. & Funder, J. W. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 54, 1306–1312. https://doi.org/10.1161/HYPERTENSIONAHA.109.136242 (2009).
Jaisser, F. & Farman, N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol. Rev. 68, 49–75. https://doi.org/10.1124/pr.115.011106 (2016).
Maubec, E. et al. Topical mineralocorticoid receptor blockade limits glucocorticoid-induced epidermal atrophy in human skin. J. Invest. Dermatol. 135, 1781–1789. https://doi.org/10.1038/jid.2015.44 (2015).
Nguyen, V. T. et al. Re-epithelialization of pathological cutaneous wounds is improved by local mineralocorticoid receptor antagonism. J. Invest. Dermatol. 136, 2080–2089. https://doi.org/10.1016/j.jid.2016.05.101 (2016).
Slominski, A. T. & Zmijewski, M. A. Glucocorticoids inhibit wound healing: novel mechanism of action. J. Invest. Dermatol. 137, 1012–1014. https://doi.org/10.1016/j.jid.2017.01.024 (2017).
Stojadinovic, O., Lindley, L. E., Jozic, I. & Tomic-Canic, M. Mineralocorticoid receptor antagonists-a new sprinkle of salt and youth. J. Invest. Dermatol. 136, 1938–1941. https://doi.org/10.1016/j.jid.2016.07.025 (2016).
Fukuda, S., Baba, S. & Akasaka, T. Psychological stress has the potential to cause a decline in the epidermal permeability barrier function of the horny layer. Int. J. Cosmet. Sci. 37, 63–69. https://doi.org/10.1111/ics.12169 (2015).
Kotter, T., Wagner, J., Bruheim, L. & Voltmer, E. Perceived Medical School stress of undergraduate medical students predicts academic performance: an observational study. BMC Med. Educ. 17, 256. https://doi.org/10.1186/s12909-017-1091-0 (2017).
Choi, E. H. Aging of the skin barrier. Clin. Dermatol. 37, 336–345. https://doi.org/10.1016/j.clindermatol.2019.04.009 (2019).
Segre, J. A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Invest. 116, 1150–1158. https://doi.org/10.1172/JCI28521 (2006).
Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H. & Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x (2008).
Kim, J. H. et al. 7,3’,4’-trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses alpha-melanocyte-stimulating hormone-induced melanogenesis by targeting melanocortin 1 receptor. Front Mol. Biosci. 7, 577284. https://doi.org/10.3389/fmolb.2020.577284 (2020).
Ropke, M. A. et al. Effects of glucocorticoids on stratum corneum lipids and function in human skin-A detailed lipidomic analysis. J. Dermatol. Sci. 88, 330–338. https://doi.org/10.1016/j.jdermsci.2017.08.009 (2017).
Gupta, R., Dwadasi, B. S. & Rai, B. Molecular dynamics simulation of skin lipids: effect of ceramide chain lengths on bilayer properties. J. Phys. Chem. B 120, 12536–12546. https://doi.org/10.1021/acs.jpcb.6b08059 (2016).
Pullmannova, P. et al. Permeability and microstructure of model stratum corneum lipid membranes containing ceramides with long (C16) and very long (C24) acyl chains. Biophys. Chem. 224, 20–31. https://doi.org/10.1016/j.bpc.2017.03.004 (2017).
Skolova, B. et al. Ceramides in the skin lipid membranes: length matters. Langmuir 29, 15624–15633. https://doi.org/10.1021/la4037474 (2013).
Steinert, P. M. & Marekov, L. N. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol. Biol. Cell 10, 4247–4261. https://doi.org/10.1091/mbc.10.12.4247 (1999).
Antonov, D., Schliemann, S. & Elsner, P. Methods for the assessment of barrier function. Curr. Probl. Dermatol. 49, 61–70. https://doi.org/10.1159/000441546 (2016).
Tiganescu, A. et al. UVB induces epidermal 11beta-hydroxysteroid dehydrogenase type 1 activity in vivo. Exp. Dermatol. 24, 370–376. https://doi.org/10.1111/exd.12682 (2015).
Tiganescu, A., Walker, E. A., Hardy, R. S., Mayes, A. E. & Stewart, P. M. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. J. Invest. Dermatol. 131, 30–36. https://doi.org/10.1038/jid.2010.257 (2011).
Tiganescu, A. et al. Increased glucocorticoid activation during mouse skin wound healing. J. Endocrinol. 221, 51–61. https://doi.org/10.1530/JOE-13-0420 (2014).
Tiganescu, A. et al. 11beta-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J. Clin. Invest. 123, 3051–3060. https://doi.org/10.1172/JCI64162 (2013).
Lee, N. R. et al. Role of 11beta-hydroxysteroid dehydrogenase type 1 in the development of atopic dermatitis. Sci. Rep. 10, 20237. https://doi.org/10.1038/s41598-020-77281-x (2020).
Boix, J., Nguyen, V. T., Farman, N., Aractingi, S. & Perez, P. Mineralocorticoid receptor blockade improves glucocorticoid-induced skin atrophy but partially ameliorates anti-inflammatory actions in an irritative model in human skin explants. Exp. Dermatol. 27, 185–187. https://doi.org/10.1111/exd.13473 (2018).
Sakai, Y. & Demay, M. B. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141, 2043–2049. https://doi.org/10.1210/endo.141.6.7515 (2000).
Garthwaite, S. M. & McMahon, E. G. The evolution of aldosterone antagonists. Mol. Cell Endocrinol. 217, 27–31. https://doi.org/10.1016/j.mce.2003.10.005 (2004).
Funding
This work was supported by AMOREPACIFIC and the National Research Foundation of Korea grant funded by the Korean government (NRF-2018R1A2B2005002).
Author information
Authors and Affiliations
Contributions
HL conducted the clinical experiments, participated in designing research studies, data analysis, interpretation, and writing of the manuscript; EJC conducted the in vitro studies and participated in designing research studies, data analysis, interpretation, and writing of the manuscript; EJK conducted the clinical experiments and participated in writing the manuscript; EDS participated in designing research studies, data analysis, interpretation, and conducting in vitro studies; HJK, WSP, YK, JK, and SNK participated in conducting in vitro studies; KS and KP conducted the lipid analysis and participated in writing the manuscript; EHC was the principal investigator of the clinical experiments and participated in designing research studies, data analysis, interpretation, and writing the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
EJ Choi, ED Son, HJ Kim, and WS Park are employees of AMOREPACIFIC. The remaining authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H., Choi, EJ., Kim, E.J. et al. A novel mineralocorticoid receptor antagonist, 7,3',4'-trihydroxyisoflavone improves skin barrier function impaired by endogenous or exogenous glucocorticoids. Sci Rep 11, 11920 (2021). https://doi.org/10.1038/s41598-021-91450-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91450-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.