Abstract
Pteris vittata is an arsenic (As) hyperaccumulator plant that accumulates a large amount of As into fronds and rhizomes (around 16,000 mg/kg in both after 16 weeks hydroponic cultivation with 30 mg/L arsenate). However, the sequence of long-distance transport of As in this hyperaccumulator plant is unclear. In this study, we used a positron-emitting tracer imaging system (PETIS) for the first time to obtain noninvasive serial images of As behavior in living plants with positron-emitting 74As-labeled tracer. We found that As kept accumulating in rhizomes as in fronds of P. vittata, whereas As was retained in roots of a non-accumulator plant Arabidopsis thaliana. Autoradiograph results of As distribution in P. vittata showed that with low As exposure, As was predominantly accumulated in young fronds and the midrib and rachis of mature fronds. Under high As exposure, As accumulation shifted from young fronds to mature fronds, especially in the margin of pinna, which resulted in necrotic symptoms, turning the marginal color to gray and then brown. Our results indicated that the function of rhizomes in P. vittata was As accumulation and the regulation of As translocation to the mature fronds to protect the young fronds under high As exposure.
Similar content being viewed by others
Introduction
Arsenic (As) is a toxic element that is usually found in soils and groundwater as a result of both natural and anthropogenic processes. Chronic exposure to inorganic As may lead to cancers of prostate, bladder and skin1. Pteris vittata is the first fern to be identified as an As hyperaccumulator that can accumulate As to > 22,000 mg/kg dry weight in fronds when grown in soil spiked with As at 1500 mg/kg2. As concentration in contaminated water can also be reduced by P. vittata to a level that is within the World Health Organization (WHO) guidelines3. Different from other remediation methods, phytoremediation of As-contaminated soils and water by P. vittata is more environmentally friendly. P. vittata is a unique model to study the interactions between plant and As.
Arsenate (AsV) and arsenite (AsIII) are two major inorganic As species in water, with AsV the more predominant form in aerobic water. In P. vittata, AsV is taken up by roots via phosphate transporters4,5, and then AsV is translocated from roots to rhizomes and reduced to AsIII6,7. Different from other plants, rhizomes are the unique underground stems that connect the roots and fronds in fern plants like P. vittata8,9. In many studies rhizomes and roots in P. vittata are not separated and are both recognized as roots10,11. Thus, there is little available information on As accumulation in rhizomes of P. vittata.
As (AsV and AsIII) in rhizomes is translocated to the fronds through the xylem in P. vittata12. In fronds, As accumulation and distribution have been well studied by several methods, including scanning electron microscope with energy dispersive X-ray spectrometer (SEM–EDX) and synchrotron X-ray microprobe analysis. These studies found that As was concentrated at the tip of the apical pinna and decreased toward the basal pinna of fronds13. Inside the pinna, As was found in the proximity of veins in the pinna surface, and most of the As was localized near the midrib, which indicated that As in the pinnae is contained in the apoplast rather than vacuoles14. Necrotic symptoms in the pinna margin were induced by high As exposure, and the As concentration in the pinna margin was 2.3 times higher than that in the midribs15. These techniques only provide static data and because of the detection limit, only high As exposure experiments were conducted. Therefore, As accumulation and distribution in fronds of P. vittata under various As conditions are still unclear.
The positron-emitting tracer imaging system (PETIS) is one of the most advanced radiotracer-based imaging methods available today; its principle is the same as that of positron emission tomography, which has been widely used for medical diagnosis16. PETIS is specially designed for studying plants and provides serial time-course images (i.e., animation) of the two-dimensional distribution of a radiotracer within a living plant without contact; it has been applied to many studies on plant nutrition over the last decade17.The transport of metals in plants, including Fe18, Mn19, Zn20,21 and Cd22,23 have been visualized using PETIS. As transport has not yet been visualized in the plant by PETIS.
In this study, we investigated As accumulation in rhizomes, and As accumulation in different stages of fronds in hydroponically grown P. vittata with high As exposure (30 mg/L) in a long term phytofiltration experiment. We successfully visualized As translocation and accumulation in living P. vittata from low to high As exposure by using PETIS and autoradiography with positron-emitting 74As labeled tracer and compared it with that in the non-accumulator model plant Arabidopsis thaliana. This study provides important insights into the behavior of As in plants as well as providing significant information to advance our understanding of As hyperaccumulation.
Results
Long term As phytofiltration with P. vittata
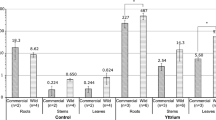
To understand the As accumulation in rhizomes and fronds of P. vittata with high As exposure, P. vittata was grown hydroponically for 16 weeks in phosphate (Pi) free hydroponic medium with 30 mg/L AsV. Within 16 weeks more than 60% of the AsV was taken up by P. vittata (Fern) when compared with the negative control (NC) (Fig. 1a). The image of the P. vittata shows that after 1 week the margin of pinnae became gray, and after 4 weeks the gray margin turned to brown and continued browning through to 16 weeks (Supplementary Fig. S1a). This finding was similar to those of several studies, but they only recorded the tissue necrosis of the brown margin pinna and ignored the gray margin pinna15,24. After 4 weeks, three plants were sampled and separated into fronds, rhizomes and roots; the As concentration in fronds was much higher than those in the rhizomes and roots (Fig. 1b) (p < 0.05). After 16 weeks the rest of the plants were sampled and separated into fronds, rhizomes and roots. When compared with plants at 4 weeks, the As concentrations in the 16-week plants were comparable between fronds and rhizomes (around 16,000 mg/kg) and much higher than that in the roots (Fig. 1c) (p < 0.05). This result showed that rhizomes and fronds accumulated the high levels of As, which differed from the results of a previous study that showed that the only function of rhizomes was AsV reduction and AsIII translocation to fronds8. The fronds were then separated into three different types: fronds brown margin (tissue necrosis), fronds gray margin (tissue necrosis) and fronds new (Supplementary Fig. S1b, c). The As concentration in fronds brown margin was higher than those in fronds gray margin and fronds new (Fig. 1d) (p < 0.05). Our results were supported by a previous study of high As concentration soil cultivation with P. vittata15, but our study showed a significant difference between fronds brown margin with fronds gray margin and fronds new, and much higher As concentrations in the fronds because the availability of As in hydroponic cultivation was higher than that in soil cultivation. On a dry weight basis, more than 95% of As was accumulated in fronds and rhizomes (Fig. 1e,f). The mass balance of As in plants and the medium was calculated; the As accumulated in the plant was the same as the declined in As observed in the medium (Fig. 1g). Our data indicated that the function of rhizomes in P. vittata was not only AsV reduction and AsIII translocation but also As accumulation, and the necrotic symptoms in the margin of pinna might be the key to high As accumulation in fronds of P. vittata.
As phytofiltration experiment with P. vittata over 16 weeks. (a) Time course of As removal in medium with P. vittata (Fern) and without P. vittata (NC) over 16 weeks. After 4 weeks As phytofiltration experiment, (b) As concentration in fronds, rhizomes and roots of P. vittata. After 16 weeks As phytofiltration experiment, (c) As concentration in fronds, rhizomes and roots of P. vittata. (d) As concentration in different stages of fronds (fronds brown margin, fronds gray margin, fronds new) of P. vittata. (e) The dry weight of fronds, rhizomes and roots of P. vittata. (f) As amounts in fronds, rhizomes and roots of P. vittata. (g) As mass balance between As uptake by P. vittata and As decline from the medium. Data are expressed as mean + standard deviation (n = 3).
Seven days of PETIS imaging for As translocation and accumulation in P. vittata and A. thaliana
As translocation and accumulation in P. vittata fed with modified Pi free 1/5 Hoagland solution was real-time imaged using PETIS. Unlike the long-term hydroponic experiments in Fig. 1, PETIS imaging was conducted over 7 days. Thus, to reproduce the phenomenon of the necrotic symptom margin of pinna in fronds of P. vittata within 7 days, P. vittata were exposed to a high As concentration of 2000 μM was applied for to conduct PETIS imaging. Lower As conditions (0.2, 20 and 200 μM AsV) were also tested.
Figure 2a shows four plants supplied with 0.2, 20, 200 and 2000 μM non-radioactive AsV (total As of 0.00019, 0.017, 0.21, 1.83 mg, respectively) including 2 kBq 74As-labeled AsV (0.0074 pmol) and subjected to PETIS for 7 days (168 h). Figure 2b and Supplementary video S1 show representative results of PETIS imaging of As translocation and accumulation in P. vittata. Time courses of the radioactivity of 74As over time within each region of interest (ROI) were generated through manual selection of ROI on the image data (Fig. 2c). The ROI of rhizomes and fronds were observed as the bright spot in the PETIS imaging after 168 h, which showed that As accumulation in P. vittata occurred in both fronds and rhizomes (Fig. 2b,c). Representative curves of AsV dynamics in the solution and As uptake into the roots and translocation to the rhizomes and fronds were shown in Fig. 2d–g. Over the 168 h of experiment, the volume of solution was maintained automatically by supplying the solution without AsV, therefore AsV absorption by roots would have decreased the amount of AsV in the solution. Therefore, the curve of AsV depletion in the solution directly reflected root absorption. It was clearly showed that in the 0.2 μM As treatment, all the supplemented As in the hydroponic solution was taken up by the roots and translocated to rhizomes and fronds within 24 h after As exposure and the As concentration in the solution decreased simultaneously. It took 48 h and 144 h to observe similar depletion of the As concentration in the solution in 20 μM and 200 μM As treatments, respectively. After 168 h of the exposure to 2000 μM As, some As remained in the solution. In the 0.2 and 20 µM treatments, As in roots was gradually decreased, corresponding to As translocation to rhizomes and subsequent translocation to fronds. However, As accumulation was not decreased in rhizomes like roots and reached a plateau after 24 h, with little increase after this time (Fig. 2d–g). This result is consistent with that of the phytofiltration experiment (Fig. 1c) which showed As accumulation in rhizomes.
Serial images of 74As behavior and time course of the amount of arsenic in different regions of P. vittata. (a) Photograph of test plants in the experimental apparatus. The solid red rectangles indicate the field of the PETIS imaging. (b) Serial images of P. vittata (0–168 h). Each frame was created from the integration of original images collected every 5 min. (c) Examined regions in the plants. The blue trapezoid indicates the region of the solution, the purple rectangle indicates the solution with the roots inside, the yellow-green rectangle indicates the roots above the solution, the black rectangle indicates the rhizomes and the green rectangle indicates the fronds. Time course of the amount of As in the regions shown in (c) fed with 0.2 μM AsV (d), fed with 20 μM AsV (e), fed with 200 μM AsV (f) and fed with 2000 μM AsV (g), all experiments included 2 kBq of 74As-labeled AsV.
To compare As accumulation kinetics of an As-hyperaccumulator and a non-As-accumulator, PETIS imaging was applied to a non-accumulator A. thaliana with 0.2 μM non-radioactive AsV including 2 kBq 74As-labeled AsV for 7 days (168 h) (Fig. 3a). Figure 3b and Supplementary video S2 show representative results of PETIS imaging of As translocation and accumulation in A. thaliana. Note that a rhizome does not exist in A. thaliana. The ROI of roots was observed as the bright spot in the PETIS imaging after 168 h (Fig. 3b,c). In A. thaliana, different from P. vittata, most of the As in the solution was retained in roots and there was little As translocation to shoots (Fig. 3d).
Serial images of 74As behavior and time course of the amount of As in different regions of A. thaliana. (a) Photograph of test plant in the experimental apparatus. The solid red rectangles indicate the field of view of the PETIS during the plant imaging. (b) Serial images of the A. thaliana (0–168 h). Each frame was created from the integration of original images collected every 5 min. (c) Examined regions in the plant. The blue rectangle indicates the region of the solution, the purple rectangle indicates the solution with the roots inside, the yellow-green rectangle indicates the roots above the solution and the green rectangle indicates the shoots. (d) The time course of the amount of As in the regions shown in (c) fed with 0.2 μM AsV including 2 kBq of 74As-labeled AsV.
Autoradiography analysis for P. vittata and A. thaliana
After 168 h of PETIS experiments, plants were dissected and subjected to autoradiography of 74As. This provided the static distribution of As in individual fronds, rhizomes and roots for P. vittata (Figs. 4 and 5) and individual shoots, stem and roots for A. thaliana (Fig. 6), which was supplementary to the above PETIS analysis.
Autoradiography of static 74As in fronds of P. vittata supplied with As for 168 h. (a) Fed with 0.2 μM AsV. (b) Fed with 20 μM AsV. (c) Fed with 200 μM AsV. (d) Fed with 2000 μM AsV. All experiments included 2 kBq of 74As-labeled AsV. Fiddlehead and apical pinna of young fronds (brown arrow), midrib (green arrow), rachis (yellow arrow) sporangia (blue arrow) and the gray margin pinna showing necrotic symptoms (red arrow).
Figure 4a–d shows the fronds from P. vittata supplied with 0.2, 20, 200, and 2000 μM non-radioactive AsV as well as 74As, respectively (from left to right, young fronds to mature fronds in every panel). After 7 days of 0.2 and 20 μM As exposure, fronds did not show any necrotic symptoms but strong accumulation of 74As was observed in the fiddlehead and apical pinna of young fronds (brown arrow). Accumulation of As in the middle-to-basal pinna of young fronds and whole mature fronds were observed in the midrib (green arrow), rachis (yellow arrow) and sporangia (blue arrow) (Fig. 4a,b). After 7 days of 200 μM As exposure, the As accumulation pattern in fronds was similar to those of the 0.2 and 20 μM As treatments. However, necrotic symptoms (gray margin) were observed in one pinna of mature fronds (red arrow) and As accumulation was higher than others in mature fronds (Fig. 4c). After 7 days of 2000 μM As exposure, more pinna with necrotic symptoms were found in mature fronds and young fronds started to show necrotic symptoms (red arrow). The sections of pinna showing necrotic symptoms showed strong As accumulation (Fig. 4d). The accumulation of As in pinna with necrotic symptoms (gray margin) in this experiment was consistent with the phytofiltration experiment (Fig. 1d and Supplementary Fig. S1c), which showed that high As exposure induced the necrotic symptom because of the As accumulated in the margin of the pinna.
Figure 5a–d show the rhizomes and roots from P. vittata exposed to 0.2, 20, 200 and 2000 μM non-radioactive AsV as well as 74As, respectively. When compared with roots (purple arrow), strong accumulation of 74As in rhizomes (red arrow) was observed. The pattern of As accumulation in rhizomes from 0.2 μM to 2000 μM was confirmed by the PETIS imaging at 168 h (Fig. 2b).
Figure 6 shows individual shoots, stem and roots of A. thaliana exposed to 0.2 μM non-radioactive AsV as well as 74As. Strong accumulation of 74As was observed in the primary root (blue arrow), lateral root (purple arrow) and shoots base (red arrow). The As accumulation in these tissues was higher than in the main-stem (yellow arrow) and shoots (green arrow) in A. thaliana. These results showed that a non-accumulator A. thaliana took up As from the solution through the roots and translocated As to shoots through a shoot base, but most of the As was retained in the roots.
Discussion
In this study, we used PETIS for the first time to obtain the noninvasive serial images of As behavior in living plants with positron-emitting 74As-labeled AsV. The As-hyperaccumulator P. vittata is efficient in AsV uptake, with the recent identification and investigation of a group of phosphate transporters in roots of P. vittata that were responsible for AsV uptake4,5. This is supported by the data of long term phytofiltration experiment and PETIS imaging presented here, which showed that AsV in solution was significantly decreased with different levels of AsV exposure (Figs. 1a and 2). After AsV enters the roots of P. vittata, most of it is translocated to rhizomes. AsV reductase in rhizomes played a critical role in reducing most of the AsV to AsIII within the rhizomes, and then most of the As (AsV and AsIII) was translocated to fronds via the xylem in P. vittata8. However, our data revealed that rhizomes in P. vittata not only translocated As to fronds but also accumulated the same high level of As as the fronds (Fig. 1c), and that As accumulation in rhizomes was not affected by the different concentrations of As applied (Figs. 2 and 5). Our data indicated that the function of rhizomes in P. vittata was As translocation and accumulation.
Pteris vittata fronds are well known for As accumulation. PETIS imaging and autoradiography analysis revealed that As accumulation in fronds shifted from young fronds to the gray margin of mature fronds, which contained high amounts of As with increasing As exposure (Figs. 2 and 4). This result was similar to that of a previous study of P. vittata cultivation in soil, which showed that under high As exposure, As concentrations in pinnae margins were higher than in the midrib, consistent with As-induced necrotic symptoms15. Unlike their work, our study showed that high As exposure induced necrotic symptoms in the pinna of P. vittata in two stages: gray margin and brown margin, where more As was accumulated in fronds brown margin than fronds gray margin and fronds new, and the As concentration in fronds brown margin was similar to that in rhizomes (Fig. 1c,d). These results indicated that after As was translocated from rhizomes to fronds, the As accumulation pattern in fronds was dependent on the level of As exposure, and increased As translocation to the margin of pinna in mature fronds might be regulated by rhizomes to protect the young fronds from high As exposure. In contrast, in the non-accumulator A. thaliana, most of the As was retained in the roots even with low As exposure (Figs. 3 and 6).
In this study, using a combination of a long term phytofiltration experiment and PETIS imaging and autoradiography analysis, we proposed the possible mechanism of As translocation and accumulation in P. vittata: After AsV is taken up by roots, most of the AsV is translocated to rhizomes and reduced to AsIII. Rhizomes in P. vittata keep accumulating As (AsV and AsIII) and regulating the As translocation to fronds. With low As exposure, most of the As is translocated and accumulated in young fronds. With high As exposure, As accumulation is shifted from young fronds to the mature fronds, especially in the margin of the pinna and induces the progression of necrotic symptoms from gray to brown.
Methods
Plant materials
Pteris vittata used in the present study was procured from Fujita Co., Ltd. (Tokyo, Japan). Arabidopsis thaliana ecotype Columbia (Col-0 accession) was kindly provided from Prof. Tomonobu Kusano (Graduate School of Life Science, Tohoku University).
Pre-cultivation of P. vittata seedling was conducted as described previously25. Three-month-old fern seedlings were individually transplanted into a hydroponic tank with surface area of 30 cm × 30 cm filled with 15 L of modified Hoagland nutrient solution (16 seedlings per tank). After four months of hydroponic cultivation, the 16-week As phytofiltration experiments including negative control (NC) were started (Supplementary Fig. S1a). Growth chamber conditions were set as a 14-h light period with a light intensity of 70 μmol m−2 s−1 and a 10-h dark period; 25 °C.
For the PETIS experiment, the size of the plant needs to be controlled, so three-month-old P. vittata seedlings were individually transplanted into a 250-mL vessel wrapped in aluminum foil containing 1/5 modified Hoagland nutrient solution. Plants were grown hydroponically for 2 months before the experiment. Seeds of A. thaliana were germinated, and then each plant was hydroponically grown for 2 months in a 250-mL vessel with 1/5 modified Hoagland solution in growth chamber with same condition for P. vittata before the experiment. Studies complies with local and national regulations.
As phytofiltration experiment
The AsV concentration was adjusted to 30 mg/L by adding sodium arsenate (Na2HAsO4, Wako Pure Chemical, Osaka, Japan) into 15 L Pi-free 1/5 modified Hoagland solution. Solution samples were collected at 0, 1, 2, 3, 6, and 16 weeks after the As exposure and the As concentration was measured. To investigate As accumulation in the fern, three ferns were collected (one plant from each tank) after 4 weeks of the exposure, and after 16 weeks of the phytofiltration experiment, the rest of the plants were harvested, and separated into fronds, rhizomes and roots.
As concentrations in the hydroponic solution and digested plant tissue samples were determined using an inductively coupled plasma mass spectrometer (ICP-MS) (ELAN 9000 or NexION 300, Perkin Elmer, Massachusetts, USA) as described previously1.
The values reported in both text and figures are the mean ± SE (standard error of the mean). The statistical significance (at 95% confidence) was tested using ANOVA.
74As tracer
74As radioisotope (T1/2 = 17.7 d) was produced as previously desccribed26. Ga2O3 powder(0.1 g) was prepared as a 10 mm diameter pellet. It was covered with aluminum foil (10 μm thickness) and used as a target. The target was irradiated with an alpha beam (30 MeV, 1.5 pμA) for 387 min from an AVF cyclotron at the Cyclotron and Radioisotope Center (CYRIC) in Tohoku University. The following processes for tracer preparation were started after the byproducts such as 71As (T1/2 = 65 h) and 72As (T1/2 = 26 h) decayed out at least 4 weeks after the irradiation. The irradiated Ga2O3 was dissolved in 1 mL of 12 M NaOH by heating up to 80 °C. After all the Ga2O3 was dissolved, about 2 mL of 6 M hydrochloric acid (HCl) was added to adjust the pH to ~ 6. The solution became a white suspension. The suspension was solvent extracted with 5 mL of 1 M Di-(2-ethylhexyl) phosphoric acid organic solution with hexane by shaking for 1 h. Ethanol was added to the aqueous phase after solvent extraction with hexane (about 3 mL) to make up the volume to about 50 mL, and sodium chloride (NaCl) in the solution was precipitated. For anion exchange column chromatography, 2 mL of Muromac 1 × 8 (100–200 mesh, Cl– form) changed to OH– form was used. The supernatant was charged into this column. Ethanol and contaminants were washed off by flushing 20 mL of H2O on the column. The As tracer was eluted with 8 mL of 0.1 M HCl. The eluate was neutralized with 0.8 mL of 1 M KOH (pH ~ 7). After that, the AsIII/AsV ratio after the dissolution of the target in H2O was determined by thin layer chromatography27. Ten μL of the solution was spotted about 1 cm from the end of a Si-60 thin layer on the Al plate. The plate was developed for 10 min by a mixture of 0.01 M aqueous sodium hydrogen L-tartrate /methanol solution in a ratio of 3/1 as mobile phase. The locations of the origin, AsIII, and AsV on the developed plate were then identified by Electronic Autoradiography (Instant-Imager, Packard Instruments). The retention factors (Rf) of 0.6 and 0.9 were used to specify AsIII and AsV, respectively. Only AsV was detected (Supplementary Fig. S2). 74As-labeled AsV was dissolved in an appropriate volume of the culture solution containing a designated concentration of nonradioactive As. Finally, 2 kBq 74As-labeled AsV (0.0074 pmol) with nonradioactive AsV was fed to each test plant.
Whole-plant PETIS imaging
The PETIS imaging experiments were conducted following the methods of Fujimaki et al. and Suzui et al. with some modifications16,21. All imaging experiments were conducted in a clean booth (2 m × 2 m × 2 m) under controlled temperature and humidity conditions with continuous light at a density of 70 μmol m−2 s−1; 25 °C.
For P. vittata, the roots of intact P. vittata plants were inserted in an acrylic square container (KGS 1407-D01-00, Kumikouki Co., Gunma, Japan), and the fronds were sandwiched between 2 mm thick acrylic boards to maximize the annihilation of positrons. The acrylic board, which held four plants at a time, was placed in the field of view of the PETIS (PPIS-4800 positron imaging system; Hamamatsu Photonics, Hamamatsu, Japan) (Fig. 2a). Each container was supplied with 10 mL of Pi-free 1/5 Hoagland solution containing 2 kBq of 74As-labeled AsV, plus the different concentrations of non-radioactive AsV (as Na2HAsO4: 0.2, 20, 200, 2000 μM). The behavior of 74As in the plants, including the roots, rhizomes and fronds, was monitored by the PETIS every 5 min for 168 h. The solution was continuously stirred with gentle aeration to maintain a uniform composition in each compartment of the container.
Based on the PETIS imaging results of P. vittata, the PETIS imaging of A. thaliana was conducted with 0.2 μM AsV. The experimental conditions and preparation were the same as the PETIS imaging of P. vittata.
Qualitative and quantitative analyses of PETIS data
In the PETIS imaging experiments, the indicated amounts of nonradioactive As were mixed with measured activities of pure 74As at a certain time before plant exposure. Therefore, the amount of total As (i.e. sum of radioactive and nonradioactive As) corresponding to the radioactivity of 74As at a given time can be easily determined. All the graphs and PETIS images shown in this paper indicated the determined amounts of total As, not just the intensities of the 74As signal. The time course data of the As amount (mg) in the regions of interest in the images were calculated by the values of the signal intensity (cps) extracted using the NIH Image J 1.50 software (http://rsb.info.nih.gov/ij/), counting efficiency of the system (cps Bq−1) and specific radioactivity (Bq mol−1).
Autoradiography
The test plant was dissected and placed on paper sheets after the PETIS imaging was finished and then placed in contact with imaging plates (Fujifilm, Tokyo, Japan) in cassettes. After 2 days of exposure, the imaging plates were scanned using a bio-imaging analyzer (BAS-1500; Fujifilm, Tokyo, Japan) to obtain the auto-radiographic images of 74As in plant.
References
Huang, Y., Hatayama, M. & Inoue, C. Characterization of As efflux from the roots of As hyperaccumulator Pteris vittata L. Planta 234, 1275–1284 (2011).
Ma, L. Q. et al. A fern that hyperaccumulates arsenic—A hardy, versatile, fast growing plant helps to remove arsenic from contaminated soils. Nature 409, 579 (2001).
Natarajan, S., Stamps, R. H., Saha, U. K. & Ma, L. Q. Phytofiltration of arsenic-contaminated groundwater using Pteris vittata L.: effect of plant density and nitrogen and phosphorus levels. Int. J. Phytoremed. 10, 222–235 (2008).
Sun, D. et al. Expression of new Pteris vittata phosphate transporter PvPht1;4 reduces arsenic translocation from the roots to shoots in tobacco plants. Environ. Sci. Technol. 54, 1045–1053 (2020).
Feng, H. Y. et al. Expressing phosphate transporter PvPht2;1 enhances P transport to the chloroplasts and increases arsenic tolerance in Arabidopsis thaliana. Environ. Sci. Technol. 55, 2276–2284 (2021).
Wang, J. R., Zhao, F. J., Meharg, A. A., Feldmann, J. & McGrath, S. P. Mechanisms of arsenic hyperaccumulation in Pteris vittata uptake kinetics, interactions with phosphate, and arsenic speciation. Plant. Physiol. 130, 1552–1561 (2002).
Poynton, C. Y., Huang, J. W., Blaylock, M. J., Kochian, L. V. & Elless, M. P. Mechanisms of arsenic hyperaccumulation in Pteris species: Root As influx and translocation. Planta 219, 1080–1088 (2004).
Li, X. Y. et al. Efficient arsenate reduction in As-hyperaccumulator Pteris vittata are mediated by novel arsenate reductases PvHAC1 and PvHAC2. J. Hazard. Mater. 399, 122895 (2020).
Foster, F. G. Ferns to Know and Grow (Timber Press, 1985).
Han, Y. H. et al. Mechanisms of efficient As solubilization in soils and As accumulation by As-hyperaccumulator Pteris vittata. Environ. Pollut. 227, 569–577 (2017).
Su, Y. H., McGrath, S. P., Zhu, Y. G. & Zhao, F. J. Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol. 180(2), 434–441 (2008).
Mathews, S., Ma, L. Q., Rathinasabapathi, B., Natarajan, S. & Saha, U. K. Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. Bioresour. Technol. 101, 8024–8030 (2010).
Sakai, Y. et al. Influence of arsenic stress on synthesis and localization of low-molecular-weight thiols in Pteris vittata. Environ. Pollut. 158(12), 3663–3669 (2010).
Rupali, D. et al. Evidence for exocellular Arsenic in Fronds of Pteris vittata. Sci. Rep. 7, 2839 (2017).
Han, Y. H. et al. Arsenic accumulation and distribution in Pteris vittata fronds of different maturity: Impacts of soil As concentrations. Sci. Total Environ. 715, 135298 (2020).
Fujimaki, S. et al. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 152, 1796–1806 (2010).
Ishikawa, S. et al. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting107Cd tracer. BMC Plant Biol. 11, 172 (2011).
Tsukamoto, T. et al. 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): Evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol. 50, 48–57 (2009).
Tsukamoto, T. et al. 52Mn translocation in barley monitored using a positron-emitting tracer imaging system. Soil Sci. Plant Nutr. 52, 717–725 (2006).
Suzuki, M. et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J. 48, 85–97 (2006).
Suzui, N., Yin, Y. G., Ishii, S., Sekimoto, H. & Kawachi, N. Visualization of zinc dynamics in intact plants using positron imaging of commercially available 65Zn. Plant Methods 13, 40 (2017).
Fontanili, L. et al. Kinetic analysis of zinc/cadmium reciprocal competitions suggests a possible Zn-insensitive pathway for root-to-shoot cadmium translocation in rice. Rice 9, 16 (2016).
Hu, P. J. et al. Nitrate facilitates cadmium uptake, transport and accumulation in the hyperaccumulator Sedum plumbizincicola. Environ. Sci. Pollut. Res. 20, 6306–6316 (2013).
Lombi, E., Zhao, F. J., Fuhrmann, M., Ma, L. Q. & McGrath, S. P. Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol. 156, 195–203 (2002).
Huang, Y., Miyauchi, K., Inoue, C. & Endo, G. Development of suitable hydroponics system for phytoremediation of arsenic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 80(3), 614–618 (2016).
Ikeda, H. et al. Production of arsenic RI tracer from gallium oxide target by alpha beam irradiation. RIKEN Accel. Prog. Rep. 52, 206 (2019).
Shehata, M. M., Scholten, B., Spahn, I., Coenen, H. H. & Qaim, S. M. Separation of radioarsenic from irradiated germanium oxide targets for the production of 71As and 72As. J. Radioanal. Nucl. Chem. 287, 435–442 (2011).
Acknowledgements
We thank Catherine Dandie, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript. We are grateful to the referees for useful comments. 74As tracer was supplied through Supply Platform of Short-lived Radioisotopes, supported by JSPS Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 16H06278. This research was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 19H01158 (Grant-in-Aid for Scientific Research (A)).
Author information
Authors and Affiliations
Contributions
Y.H.T.K. and C.I. designed the research. K.M. and G.E. performed the growth chamber treatments and phytofiltration experiment. Y.H.T.K. and M.F.C. performed ICP-MS for As analysis. Y.H.T.K. and Z.J.Q. performed the PETIS analysis and Autoradiography analysis. N.S., Y.-G.Y. and N.K. analyzed the PETIS and Autoradiography data. H.I., H.W. and H.K. produced 74As tracer. N.K. supplied the P. vittata seedlings and technical support for cultivation. Y.H.T.K., C.I., N.S., Y.-G.Y. and N.K. interpreted the data. Y.H.T.K. wrote the paper with contributions from N.S., H.W. and C.I. All authors reviewed and edited the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohda, Y.HT., Qian, Z., Chien, MF. et al. New evidence of arsenic translocation and accumulation in Pteris vittata from real-time imaging using positron-emitting 74As tracer. Sci Rep 11, 12149 (2021). https://doi.org/10.1038/s41598-021-91374-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91374-1
This article is cited by
-
Perspectives on the Potentials of Phlorotannins in Enhancing Phytoremediation Performance
Journal of Plant Growth Regulation (2023)
-
Ionomics and metabolomics analysis reveal the molecular mechanism of metal tolerance of Pteris vittata L. dominating in a mining site in Thai Nguyen province, Vietnam
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.