Abstract
Autologous stem cell transplantation (ASCT) continues to be the standard treatment for transplant-eligible multiple myeloma (MM) patients. A portion of MM patients received ASCT in an isolation room with high-efficiency particulate air (HEPA) filtration. The effectiveness of the HEPA filtration on reducing treatment-related mortality (TRM) is controversial. We enrolled patients with newly diagnosed MM in Taiwan between 2000 and 2017. The primary endpoint of the study was TRM, which was defined as death within 100 days after ASCT. A total of 961 MM patients received ASCT. Of them, 480 patients (49.9%) received ASCT in an isolation room with HEPA filtration (HEPA group). The median overall survival from ASCT was 7.52 years for the HEPA group and 5.88 years for the remaining patients (non-HEPA group) (p = 0.370). The 100-day mortality rate was 1.5% and 1.0% for the HEPA and non-HEPA groups, respectively. In the multivariate analysis, the 100-day mortality had no difference between the HEPA and non-HEPA groups (adjusted hazard ratio 1.65, 95% CI 0.52–5.23). The median cost for ASCT inpatient care was $13,777.6 and $6527.6 for the HEPA and non-HEPA groups, respectively (p < 0.001). Although half of MM patients in Taiwan received ASCT in HEPA room, it didn’t affect 100-day mortality.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a hematologic neoplasm characterized by the clonal proliferation of plasma cells1. In the United States, the estimated new MM cases in 2019 were 32,110, which represented 1.6% of all cancers, and MM was the second most common hematologic malignancy2. The standardized incidence of MM was 17.0 per 10,000 person-years in Taiwan in 2016. MM therapy has remarkably changed in past decades with the introduction of novel agents3. The early mortality rate has been substantially reduced, and the survival rate has doubled4.
High-dose chemotherapy with autologous stem cell transplantation (ASCT) prolonged progression-free survival (PFS) and overall survival (OS) in newly diagnosed MM patients who were eligible for transplantation5,6,7. MM patients who received high-dose chemotherapy plus ASCT were often hospitalized in an isolation room with high-efficiency particulate air (HEPA) filtration8,9. Krüger et al. presented the results of a multi-center survey to the members of the European Group for Bone and Marrow Transplantation (EBMT) in 1999 and reported that 47.2% of the patients received ASCT in a special ward with HEPA filtration10. Another EBMT survey in 2008 revealed that HEPA-filtered rooms were used in 53% of ASCT conditioned without total body irradiation11. The latest survey conducted between 2014 and 2015 showed that the use of HEPA-filtered rooms was 68% for ASCT recipients.
The need for environmental HEPA filtration for patients receiving ASCT has not been established. IDSA guidelines suggest considering the use of HEPA-filtered rooms for ASCT recipients who develop prolonged neutropenia, which is the major risk factor of nosocomial aspergillosis12. Conversely, rapid engraftment with peripheral blood stem cells and the improvement of supportive care have made ASCT very safe, with a low treatment mortality rate13,14. Taiwan is located in southeastern Asia, with a warm humid climate, so ASCT patients in Taiwan might have a high rate of nosocomial infection15. Half of the MM patients in Taiwan received ASCT in an isolation room with HEPA filtration, although the efficacy of HEPA filtration for those patients has not been established. Therefore, we conducted a nationwide population-based study to evaluate the benefits and cost of the use of HEPA filtration for MM patients receiving ASCT.
Patients and methods
Study population
We used data from the Taiwan Cancer Registry, Cause of Death Data, and National Health Insurance Research Database (NHIRD). Data retrieval and analysis were carried out in the Health and Welfare Data Science Center (HWDC). Taiwan’s NHIRD provides nationwide population-based data for health research. All patients with severe diseases, of which cancers are included, are enrolled in the Registry for Catastrophic Illness Patients (RCIP) and receive copayment exemption under the National Health Insurance (NHI) program. The integration of multiple NHI databases, including RCIP, NHI enrollment files, inpatient and outpatient databases, provides comprehensive information on NHI enrollment and utilization of healthcare resources, including examinations and treatment16. Cancer stages and treatment plans are available in the Taiwan Cancer Registry. All the patients’ identification has been encrypted and can be analyzed only in the HWDC. This study has been approved by the Institutional Review Board of Taipei Veterans General Hospital (no. 2020-02-019AC). All methods for the study were performed in accordance with relevant guidelines and regulations of Taipei Veterans General Hospital in Taiwan. The institutional ethical committee waived the informed consent form.
Study cohort and study design
We enrolled patients with newly diagnosed MM in Taiwan between January 1, 2000 and December 31, 2017 from diagnosis codes according to the International Classification of Diseases, 9th revision, and Clinical Modification (ICD-9-CM) codes (203, 203.0X, and 203.1X) and 10th revision (ICD-10-CM) codes (C90.X). The diagnosis of MM had to be further verified by the RCIP, in which diagnosis was confirmed by pathologic reports. Patients under age 20, without ASCT, or receiving non-melphalan conditioning regimens were excluded. We identified MM patients who received ASCT in an isolation room with HEPA filtration as the HEPA group. Comparatively, those who received ASCT in a ward without HEPA filtration were identified as the non-HEPA group.
Endpoints
The primary endpoint of the study was treatment-related mortality, which was defined as death within 100 days following receiving ASCT, of which only the first ASCT was analyzed17. We used the National Cause of Death Data to identify the date and cause of death. The secondary endpoints included OS, medical expenditures within 100 days, length of stay in hospitals for the treatment course of ASCT, and emergency room visits and readmission within 14 days after discharge. Medical expenditures included all the expenditures of the treatment course of ASCT within 100 days.
Characteristics of the study population
The potential confounders considered in this study include age, sex, comorbidities, including hypertension, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease (CAD), heart failure, end-stage renal disease (ESRD), cerebrovascular accidents, liver cirrhosis, and autoimmune diseases, disease stage, and socioeconomic status. Patients’ socioeconomic status was categorized by degree of urbanization and level of monthly salary income stratified according to the previous work18.
Statistical analysis
Patients’ demographic and clinical characteristics were presented as the total number (n) and proportion (%) for categorical data, and medians and interquartile ranges (IQR) for continuous data. Patients’ demographic data were compared by using the chi-square test for categorical variables, and the Mann–Whitney U test for continuous variables.
In the survival analysis, the Kaplan–Meier method was used for estimation of cumulative incidence of mortality, and differences between groups were tested using a log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models, controlling for potential confounding factors in the multivariate model. All factors with p < 0.1 in the univariate analysis were included in the multivariate analysis. Sensitivity analyses were performed using different cutoffs (60 days and one year after ASCT) to evaluate the mortality risk between the HEPA and non-HEPA groups.
Furthermore, to eliminate bias in selection, propensity score matching at a 1:1 ratio using greedy matching techniques was performed to match the HEPA and non-HEPA groups. Propensity scores were calculated using age, sex, comorbidities, stage, degree of urbanization, income level, and medications in a logistic regression model. Data management and all statistical analysis were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) and STATA statistical software, version 15.1 (StataCorp, College Station, TX, USA). All statistically significant levels were set at p < 0.05.
Results
Clinical characteristics of the study population
We enrolled 7918 patients with newly diagnosed MM in Taiwan between January 1, 2000 and December 31, 2017. Patients under 20 years of age (n = 8), without ASCT (n = 6942), or receiving ASCT before MM (n = 7) were excluded. Finally, a total of 961 MM patients received ASCT during the 18-year study period (Fig. 1). The median age was 57 (range 28–76), and 54.9% were men. Hypertension (50.6%), diabetes mellitus (28.6%), chronic obstructive pulmonary disease (COPD) (27.1%), and CAD (25.8%) were the most common comorbidities. In regard to socioeconomic status, 59.4%, 33.4%, and 5.3% of the patients lived in urban, suburban, and rural areas, respectively. Of them, 480 patients (49.9%) received ASCT in the HEPA group. Age and having ESRD were different between the HEPA group and non-HEPA group. Patient characteristics are shown in Table 1.
Treatment-related mortality rate
The 100-day mortality rate was 1.5% and 1.0% for the HEPA and non-HEPA groups, respectively. The crude hazard ratio (HR) for the 100-day mortality HEPA group was 1.39 (95% confidence interval [CI] 0.44–4.37, p = 0.576) compared to the non-HEPA group. In the univariate analysis, CAD (HR 5.80) and ESRD (HR 5.46) were associated with 100-day mortality. After adjusting for the variables found in univariate analysis, the 100-day mortality rate still had no difference between the HEPA and non-HEPA groups (adjusted HR 1.65, 95% CI 0.52–5.23, p = 0.399). Additionally, CAD and ESRD were significant risk factors of 100-day mortality in the multivariate analysis (Table 2).
Overall survival, time to transplantation, length of stay, emergency room visits, and readmission rate
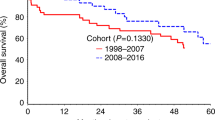
The median overall survival from ASCT was 7.52 years (95% CI 6.00–8.73) for the HEPA group, while it was 5.88 years (95% CI 4.99–8.46) for the non-HEPA group. There was no difference in the overall survival between the HEPA and non-HEPA group (p = 0.370, Fig. 2). The median time from MM diagnosis to ASCT was 7.5 (IQR 5.6–10.3) months and 7.0 (IQR 5.3–10.2) months for the HEPA and non-HEPA groups, respectively (p = 0.073). The median length of hospital stay for ASCT was longer for the HEPA group (24 [IQR 20–29] days for the HEPA group and 21 [IQR 18–26] days for the non-HEPA group, p < 0.001). There was no difference in emergency room visits within 14 days (8.3% and 6.4% for HEPA and non-HEPA, respectively) or the readmission rate within 14 days after discharge (6.5% and 4.2% for the HEPA and non-HEPA groups, respectively). The results of all the secondary endpoints are listed in Tables 3 and 4.
The healthcare cost
We further analyzed healthcare costs, including cost of hospitalization for ASCT and further treatment within 100 days, as well as outpatient services. The healthcare cost for ASCT inpatient care was $13,777.6 (IQR 11,772.1–16,696.7) and $6527.6 (IQR 4808.8–8601.4) for the HEPA and non-HEPA groups, respectively (p < 0.001). The cost of outpatient care was similar between the two groups (p = 0.249). Table 5 shows all the healthcare costs within 100 days of ASCT.
Sensitivity analysis
We conducted sensitivity analyses using 60 days and one year as the cutoffs for transplant-related mortality (TRM). The 60-day mortality rate was 0.8% and 1.0% for the HEPA and non-HEPA groups (adjusted HR 0.92, 95% CI 0.25–3.47, p = 0.905) and the one-year mortality rate was 7.1% and 7.3% for the HEPA and non-HEPA groups (adjusted HR 1.00, 95% CI 0.62–1.61, p = 0.991), respectively. The results are consistent with our primary definition of TRM (100-day mortality; adjusted HR 1.65, 95% CI 0.52–5.23, p = 0.399).
Propensity score–matched analysis
We conducted a propensity score–matched analysis to compare the HEPA and non-HEPA groups. We calculated the propensity scores for the likelihood of receiving ASCT in an isolation room with HEPA filtration using a multivariate logistic regression. We matched patients in the HEPA and non-HEPA groups with a 1:1 ratio (Supplemental Fig. S1). A total of 654 patients were matched. There was no statistically significant difference in the baseline characteristics between the two groups after matching (Supplemental Table S1). We further analyzed the primary endpoint and all secondary outcomes. There was also no statistically significant difference in the 100-day mortality rate (adjusted HR 1.83, 95% CI 0.54–6.27, p = 0.335) between the HEPA and non-HEPA groups. The 60-day, 1-year, and all-cause mortality rate, as well as emergency room visits and readmission within 14 days, were not statistically different (Supplemental Table S2). No overall survival difference between the HEPA and non-HEPA group were observed after matching (p = 0.089, Supplemental Fig. S2). However, the HEPA group had three more days of hospitalization and spent approximately $6500 more compared with the non-HEPA group (Supplemental Table S3).
Discussion
To the best of our knowledge, this is the first population-based study that compares early mortality rates of MM patients receiving ASCT in an isolation room with HEPA filtration (the HEPA group) to those in a standard ward (the non-HEPA group). Our study reveals no difference in 100-day mortality, OS, emergency room visits, or readmission rate between the HEPA and non-HEPA groups receiving ASCT. However, the healthcare cost was higher for the HEPA group, and the time from diagnosis to ASCT was marginally increased (p = 0.073). Our results may help hematologists and healthcare administrators more appropriately allocate HSCT resources.
We have systematically reviewed existing studies regarding ASCT in MM patients. We found many MM patients receiving ASCT in an isolation room with HEPA filtration8,9,19,20. Our study also reveals that half of the ASCT in MM patients was performed in an HSCT special ward with such facilities, which implies that many hematologists still believe that an isolation room with HEPA filtration can reduce infection and early-mortality rates in those patients. Our study discloses a very low mortality rate for MM patients receiving ASCT whether in an isolation room with HEPA filtration or not. The effect of HEPA filtration was to lower the nosocomial invasive fungal infection rate12. However, our previous study indicated low incidence of invasive fungal infection in MM patients15. Therefore, the insignificance of treatment-related mortality between the HEPA and non-HEPA group might be related to the low incidence of invasive fungal infection in MM patients.
In addition, we found that CAD and ESRD were independent risk factors of TRM among MM patients receiving ASCT. Tsakiris et al. reported that the median OS of MM patients receiving renal replacement therapy was only 0.91 years21. Lee et al. reported that MM patients with dialysis-dependent renal failure had a median survival of 3.4 years from ASCT, and 22.0% of the patients became dialysis independent after ASCT22. The 60-day, 100-day, and 1-year mortality rates of the ESRD patients in our cohort were 2.7%, 4.0%, and 8.7%, respectively.
ASCT should be performed with caution in MM patients with CAD. Stillwell et al. reported that TRM and one-year mortality were 5.6% and 15.3% in CAD patients receiving HSCT. Among them, 68.1% received ASCT23. Our study reveals that the 100-day mortality rate of CAD patients receiving ASCT was 3.2%, which was much higher than those without CAD. Saad et al. analyzed the records in the Center for International Blood and Marrow Transplant Research database and concluded that hematopoietic cell transplant comorbidity index (HCT-CI), including CAD and renal dysfunction, could predict survival in MM patients receiving ASCT24.
We found that the HEPA group had three more days of hospitalization and spent $7250 more for ASCT, in comparison with the non-HEPA group. However, all the short-term outcomes and long-term survival of the two groups had no difference. This population-based study shows that an isolation room with HEPA might not be necessary for MM patients receiving ASCT. Furthermore, ambulatory HSCT or even at-home HSCT, emphasizing on outpatient visits only or early discharge after stem cell transfusion, had been demonstrated to be feasible, safe, and cost-effective in several studies, especially for carefully selected MM patients undergoing ASCT25,26,27. A meta-analysis even showed a lower chance of febrile neutropenia and septicemia with outpatient ASCT28. The necessity of HEPA filtration for MM patients receiving ASCT should be re-examined.
Some studies have different definitions of early mortality and TRM in MM. Reece reported 100-day and one-year TRM mortality for patients receiving ASCT. Schmidt-Hieber, Kumar, Yin reported 100-day and one-year TRM for allogeneic transplantation32,33,34,35. Schmidt-Hieber, Kröger, Kuruvilla used one year as the cutoff for TRM for patients receiving allogeneic transplantation29,30,31. Bringhen, Augustson, Larocca used 60 days as the cutoff for early mortality in MM studies36,37,38. We conducted sensitivity analysis showing that neither 60-day, 100-day, nor one-year mortality had any statistical difference between HEPA and non-HEPA groups. These results consistently demonstrate that ASCT in MM is fairly safe whether the patients are treated in an isolation room with HEPA or not.
The non-HEPA group was older and had a higher proportion of ESRD. We conducted a propensity score–matched analysis. After matching, all the patient characteristics were similar. Both groups still had no difference in 60-day, 100-day, and one-year mortality, OS, emergency room visits, or readmission within 14 days. The HEPA group nevertheless had a higher medical expenditure and longer length of stay after matching. Saini et al. reported MM patients with t(11;14) had similar outcomes as those with normal cytogenetic and FISH studies in a propensity score–matched analysis39. Varma et al. reported that MM patients with 1q + /1p–were at significantly increased risk of progression or death compared to the propensity score–matched comparison group40. In the present study, the propensity score was defined as the conditional probability of receiving ASCT in an isolation room with HEPA. The calculated score was used to balance the covariates in the two groups and therefore reduced the bias41.
Our study has some limitations. Most of our patients didn’t receive genetic studies by FISH, which are required according to the Revised International Staging System42. Second, this study has inherent limitations by using administrative data that did not provide smoking status, performance status, disease status prior to ASCT, comprehensive medications, and some essential laboratory data. Finally, the HEPA and non-HEPA groups were not randomly assigned, so confounding factors might exist. However, there was no survival difference between the two groups, although the non-HEPA group was slightly older and more of them had ESRD.
In conclusion, some hematologists believe an isolation room with HEPA filtration can reduce complications of hematologic patients receiving ASCT. We found that about 50% of the MM patients in Taiwan received ASCT in an isolation room with HEPA filtration, and it didn’t affect 100-day mortality. This study may help clinicians and healthcare administrators utilize the limited resources of HSCT facilities. Further validation of our findings in other cohorts is warranted.
References
Manier, S. et al. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 14, 100–113. https://doi.org/10.1038/nrclinonc.2016.122 (2017).
American Cancer Society. Cancer Facts & Figures 2019 (American Cancer Society, 2019).
Kumar, S. K. et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 28, 1122–1128. https://doi.org/10.1038/leu.2013.313 (2014).
Kumar, S. K. et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 111, 2516–2520. https://doi.org/10.1182/blood-2007-10-116129 (2008).
Child, J. A. et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 348, 1875–1883. https://doi.org/10.1056/NEJMoa022340 (2003).
Palumbo, A. & Cavallo, F. Have drug combinations supplanted stem cell transplantation in myeloma?. Blood 120, 4692–4698. https://doi.org/10.1182/blood-2012-05-423202 (2012).
Palumbo, A. et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl. J. Med. 371, 895–905. https://doi.org/10.1056/NEJMoa1402888 (2014).
Cohen, Y. C. et al. Efficacy and safety of autologous hematopoietic cell transplantation in elderly patients with multiple myeloma: A retrospective national multi-site cohort study. Ann. Hematol. 96, 271–278. https://doi.org/10.1007/s00277-016-2882-9 (2017).
Aggarwal, M. et al. Autologous stem cell transplantation in first remission is associated with better progression-free survival in multiple myeloma. Ann. Hematol. 97, 1869–1877. https://doi.org/10.1007/s00277-018-3370-1 (2018).
Kruger, W. H. et al. Practices of infectious disease prevention and management during hematopoietic stem cell transplantation: A survey from the European group for blood and marrow transplantation. J. Hematother. Stem Cell Res. 10, 895–903. https://doi.org/10.1089/152581601317210999 (2001).
Hicheri, Y. et al. Environmental prevention of infection in stem cell transplant recipients: A survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Transplant Infect. Dis. 15, 251–258. https://doi.org/10.1111/tid.12064 (2013).
Dykewicz, C. A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 33, 139–144. https://doi.org/10.1086/321805 (2001).
Tricot, G. et al. Peripheral blood stem cell transplants for multiple myeloma: Identification of favorable variables for rapid engraftment in 225 patients. Blood 85, 588–596 (1995).
Vesole, D. H. et al. High-dose therapy for refractory multiple myeloma: Improved prognosis with better supportive care and double transplants. Blood 84, 950–956 (1994).
Tsai, C. K. et al. Risk and impact of invasive fungal infections in patients with multiple myeloma. Ann. Hematol. 99, 1813–1822. https://doi.org/10.1007/s00277-020-04125-z (2020).
Hsieh, C. Y. et al. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 11, 349–358. https://doi.org/10.2147/clep.s196293 (2019).
Garderet, L. et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: A prospective multicenter study. Haematologica 101, 1390–1397. https://doi.org/10.3324/haematol.2016.150334 (2016).
Hung, Y. P. et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: A nationwide population-based study. Psychooncology 22, 2227–2234. https://doi.org/10.1002/pon.3277 (2013).
Jaglowski, S. M. et al. The hematopoietic stem cell transplant comorbidity index can predict for 30-day readmission following autologous stem cell transplant for lymphoma and multiple myeloma. Bone Marrow Transplant. 49, 1323–1329. https://doi.org/10.1038/bmt.2014.155 (2014).
Yeshurun, M. et al. Antibacterial prophylaxis with ciprofloxacin for patients with multiple myeloma and lymphoma undergoing autologous haematopoietic cell transplantation: A quasi-experimental single-centre before-after study. Clin. Microbiol. Infect. 24, 749–754. https://doi.org/10.1016/j.cmi.2017.11.019 (2018).
Tsakiris, D. J. et al. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: An ERA-EDTA Registry study. Nephrol. Dial. Transplant. 25, 1200–1206. https://doi.org/10.1093/ndt/gfp679 (2009).
Lee, C. K. et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 33, 823–828. https://doi.org/10.1038/sj.bmt.1704440 (2004).
Stillwell, E. E. et al. Retrospective outcome data for hematopoietic stem cell transplantation in patients with concurrent coronary artery disease. Biol. Blood Marrow Transplant. 17, 1182–1186. https://doi.org/10.1016/j.bbmt.2010.12.698 (2011).
Saad, A. et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol. Blood Marrow Transplant. 20, 402-408.e401. https://doi.org/10.1016/j.bbmt.2013.12.557 (2014).
Fernández-Avilés, F. et al. Case-control comparison of at-home to total hospital care for autologous stem-cell transplantation for hematologic malignancies. J. Clin. Oncol. 24, 4855–4861. https://doi.org/10.1200/jco.2006.06.4238 (2006).
Martino, M. et al. Very low rate of readmission after an early discharge outpatient model for autografting in multiple myeloma patients: An Italian multicenter retrospective study. Biol. Blood Marrow Transplant. 20, 1026–1032. https://doi.org/10.1016/j.bbmt.2014.03.027 (2014).
Holbro, A. et al. Safety and cost-effectiveness of outpatient autologous stem cell transplantation in patients with multiple myeloma. Biol. Blood Marrow Transplant. 19, 547–551. https://doi.org/10.1016/j.bbmt.2012.12.006 (2013).
Owattanapanich, W., Suphadirekkul, K., Kunacheewa, C., Ungprasert, P. & Prayongratana, K. Risk of febrile neutropenia among patients with multiple myeloma or lymphoma who undergo inpatient versus outpatient autologous stem cell transplantation: A systematic review and meta-analysis. BMC Cancer 18, 1126. https://doi.org/10.1186/s12885-018-5054-6 (2018).
Schmidt-Hieber, M. et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br. J. Haematol. 130, 568–574. https://doi.org/10.1111/j.1365-2141.2005.05631.x (2005).
Kröger, N. et al. Deletion of chromosome band 13q14 as detected by fluorescence in situ hybridization is a prognostic factor in patients with multiple myeloma who are receiving allogeneic dose-reduced stem cell transplantation. Blood 103, 4056–4061. https://doi.org/10.1182/blood-2003-12-4435 (2004).
Kuruvilla, J. et al. Long-term outcome of myeloablative allogeneic stem cell transplantation for multiple myeloma. Biol. Blood Marrow Transplant. 13, 925–931. https://doi.org/10.1016/j.bbmt.2007.04.006 (2007).
Schmidt-Hieber, M. et al. Reduced-toxicity conditioning with fludarabine and treosulfan prior to allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 39, 389–396. https://doi.org/10.1038/sj.bmt.1705605 (2007).
Kumar, S. et al. Trends in allogeneic stem cell transplantation for multiple myeloma: A CIBMTR analysis. Blood 118, 1979–1988. https://doi.org/10.1182/blood-2011-02-337329 (2011).
Reece, D. E. et al. Autologous stem cell transplantation in multiple myeloma patients <60 vs >/=60 years of age. Bone Marrow Transplant. 32, 1135–1143. https://doi.org/10.1038/sj.bmt.1704288 (2003).
Yin, X. et al. Allogeneic stem-cell transplantation for multiple myeloma: A systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 18, 62. https://doi.org/10.1186/s12935-018-0553-8 (2018).
Bringhen, S. et al. Early mortality in myeloma patients treated with first-generation novel agents thalidomide, lenalidomide, bortezomib at diagnosis: A pooled analysis. Crit. Rev. Oncol. Hematol. 130, 27–35. https://doi.org/10.1016/j.critrevonc.2018.07.003 (2018).
Augustson, B. M. et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J. Clin. Oncol. 23, 9219–9226. https://doi.org/10.1200/jco.2005.03.2086 (2005).
Larocca, A. et al. Early mortality in elderly patients with newly diagnosed multiple myeloma treated with novel agents. Clin. Lymphoma Myeloma Leuk. 15, e42–e43. https://doi.org/10.1016/j.clml.2015.07.174 (2015).
Saini, N. et al. Impact of autologous transplantation in patients with multiple myeloma with t(11;14): A propensity-score matched analysis. Clin. Cancer Res. 25, 6781–6787. https://doi.org/10.1158/1078-0432.ccr-19-0706 (2019).
Varma, A. et al. Outcome of multiple myeloma with chromosome 1q gain and 1p deletion after autologous hematopoietic stem cell transplantation: Propensity score matched analysis. Biol. Bloodmarrow Transplant. 26, 665–671. https://doi.org/10.1016/j.bbmt.2019.12.726 (2020).
D’Agostino, R. B. Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17, 2265–2281. https://doi.org/10.1002/(sici)1097-0258(19981015)17:19%3c2265::aid-sim918%3e3.0.co;2-b (1998).
Palumbo, A. et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. 33, 2863–2869. https://doi.org/10.1200/jco.2015.61.2267 (2015).
Acknowledgements
This study is based on data from the National Health Insurance Research Database, provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Funding
This study was supported by grants from Taipei Veterans General Hospital (V105E10-002-MY2-1 and V105B-016), the Ministry of Science and Technology (MOST 104-2314-B-075-085-MY2, MOST 105-2314-B-418-003-MY3, and MOST 109-2314-B-075-079-MY2), the Taiwan Clinical Oncology Research Foundation, the Szu-Yuan Research Foundation of Internal Medicine, the Yen Tjing Ling Medical Foundation, and the Chong Hin Loon Memorial Cancer and Biotherapy Research Center at National Yang-Ming University. The funding sources had no role in the study design or conduct, or in the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
C.-J.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C.-J.L., C.-K.T., and Y.-C.H. designed the study. C.-J.L. and C.-M.Y. acquired the data and performed statistical analysis. C.-J.L., C.-K.T., and C.-M.Y. provided the final interpretation of the results. C.-J.L., C.-K.T., and Y.-C.H. drafted the manuscript. Y.-C.H. made critical revisions to the manuscript for important intellectual content. P.-M.C., J.-H.L., and C.-J.L. provided administrative, technical, and material support. J.-P.G. and J.-H.L. were the study supervisors. C.-J.L. and C.-K.T. act as guarantors and accept responsibility for the integrity of the work as a whole. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, CK., Yeh, CM., Hong, YC. et al. The influence of high-efficiency particulate air filtration on mortality among multiple myeloma patients receiving autologous stem cell transplantation. Sci Rep 11, 11789 (2021). https://doi.org/10.1038/s41598-021-91135-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91135-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.