Abstract
Self-propelled microscopic organisms are ubiquitous in water. Such organisms’ motility depends on hydrodynamic and physical factors related to the rheology of the surrounding media and biological factors depending on the organisms’ state and well-being. Here we demonstrate that the swimming speed of Paramecium aurelia, a unicellular protozoan, globally found in fresh, brackish, and salt waters, can be used as a measurable frugal indicator of the presence of pollutants in water. This study establishes a significant and consistent relationship between Paramecia’s swimming speed and the presence of five different organic and inorganic contaminants at varying concentrations centered around drinking water thresholds. The large size and ubiquity of the targeted microorganism, the avoidance of reagents or specialized tools for the measurement, and the simple data collection based on an object tracking algorithm enable the automatization of the assessment and real-time results using globally available technology.
Similar content being viewed by others
Introduction
Biomonitoring—that is, inferring the condition of an ecological system based on the observation of its organisms—is a central tool for ecosystem conservation, management, and restoration1. Most biomonitoring sampling methodologies were developed in the middle of the twentieth century, based on the then-existing ecological and technological knowledge. While considerable efforts are being made to modernize such measurements2, nowadays, most of those methods still consist of ecological censuses and taxonomic identifications, recording the diversity and abundance of multicellular organisms3. Despite the importance of this information to evaluate the impact and risks to humans and the environment, the data based on population changes do not necessarily capture the current state of the ecosystem but its past development.
Recording the population of microorganisms that are more sensitive to environmental changes and have shorter generation lengths than vertebrates, enables the use of methods such as the traditional half-maximal inhibitory concentration (IC50) to measure the effect of pollutants in water ecosystems in a few days4. However, as we progress in our understanding of environmental impact, the study of exogenous factors has moved beyond their influence on whole populations to include their effect on individual behaviors. Some of these abnormal behaviors resulting from the presence of pollutants have paved the way for the development of biomonitoring methods. The use of clams aperture is a recognized and extensively used technique to monitor in real-time the presence of heavy metals in water5. In the past few decades, other effects, such as fish assemblage6, movement and vocalization of birds7, or flying speeds in bees8, have been reported as possible air and water quality indicators.
While behavioral indicators enable the real-time acquisition of information9, their use is still hindered by their ambiguous nature, which might make their measurement using traditional biomonitoring methods extremely tedious, prone to error, or even impossible. However, as we develop new technologies, materials, and production models focused on their environmental impact10, obtaining more detailed and immediate information on their impact on the surrounding ecosystem is becoming even more critical11. On the other hand, the use of technology to obtain such field information on a global scale will necessitate the use of frugal engineering (i.e., avoiding specialized or expensive tools or consumables), such that the method is generalizable to resourceless, underdeveloped regions of the world12.
Here we demonstrate that the movement of Paramecium aurelia is a possible indicator for indirect measurement of chemical and physical properties of fluids, specifically for providing real-time information on the presence of pollutants in water. A simple object tracking algorithm enables us to obtain robust information in a few minutes. We demonstrate this correlation for several common organic and inorganic contaminants in the range of their permissible levels or limits (PL) in drinking water. Given the low technological requirements, the ubiquitous presence of Paramecium cells in fresh and sea water13,14, and the rapid output, requiring only a few minutes of video recording, the method developed is suitable for implementation in a broad range of situations and ecosystems.
Results and discussion
Experimental design
This study aims to identify the suitability of swimming patterns of the ubiquitous Paramecia to investigate the deviation in contaminated water due to the presence of pollutants. With a focus on the general applicability of the process, the approach is based on a frugal implementation that enables the measurement of the overall quality of water as a convolution of the impact of different additives on the swimming speed of aquatic microorganisms. Additionally, we also explore a scenario of constant chemistry and variable viscosity to use the changes in Paramecium’s swimming speed to indirectly quantify and document the environment’s rheology. Considering that the relation between the viscosity of an environment and the swimming speed of Paramecia has been manually measured multiple times in the past, here we use this magnitude to correlate the new automated methodology with previous results before its application to measure water contamination.
The overall illustration of the study is shown in Fig. 1A. Samples containing free-swimming Paramecia were modified by the addition of Methylcellulose (MC) or with set concentrations of commonly occurring organic (i.e., erythromycin, tetrachloroethylene, trichloroethylene) and inorganic (i.e., zinc chloride and copper sulfate) pollutants. The movement of Paramecia was recorded for three minutes using a simple object tracking system based on a Kalman filter reported earlier15 (Supplementary video 1). The large size of Paramecia, reaching several hundreds of microns, enables the acquisition of information using low magnification lenses (i.e., 5x), by supplementing the consumer phone cameras with additional commercially available lens using ambient light16. The output of the Paramecia the tracking system (i.e., the position of each Paramecium cell in each video frame) recorded at 15 fps (i.e., 2,700 data points per Paramecium cell in the sample) was used to measure the distance traveled by and speed of each Paramecium.
Automated tracking of Paramecium and measurement of viscosity. (A) The movement of Paramecia Aurelia cells in water samples is recorded for three minutes. Each cell is identified and followed through the video frames by an object tracking system. Their path is converted to cartesian coordinates, their speed calculated and correlated with changes in viscosity or presence of pollutants. Examples of path tracking are included in the Supplementary Fig. 1 and video 1. (B) Characteristics examples of the path followed by Paramecia for 3 min showing the whole field of view of the experiment conducted in water (top, green), 0.01% Methylcellulose (middle, blue), and 0.5% Methylcellulose (bottom, red). (C) Speed vs. viscosity for Paramecia. The red line is the fitting of the data to a power-law function for the whole range of viscosities tested (1–40 cP, R2 = 0.93). The blue line is the same fitting for the range of moderate viscosities (1–10 cP; R2 = 0.95). Error bars represent the standard deviation for the paths collected at each set of conditions (i.e., 30 Paramecia and paths per represented datapoint). Cumulative paths are presented in Supplementary Fig. 4.
It is noteworthy that similar studies to determine the migration of mammalian cells in environments of different mechanical characteristics concluded that path persistence is more significantly linked to the properties of the encapsulating environment than the migration speed17. In this case, the system was viewed on a 5 × objective lens to cover the geometrical constraints (i.e., a 1.1 × 1.3 mm chamber), necessary to fit Paramecia under a common charge-coupled device (CCD) field of view, forbid the calculation of those parameters as the collisions of Paramecia against the walls strongly affect those secondary observations. While those parameters might provide more granular information at the expense of more complex setups, as we demonstrate below, the use of swimming speed alone suffices for the quantitative measurement of viscosities and the qualitative presence of common pollutants, enabling practical application without any specialized devices.
Indirect measurement of viscosity
In addition to the advantages of its ubiquity on water bodies of different nature, Paramecium cells possess three physical characteristics that make them a unique bioindicator from the perspective of technical implementation; as small (~ 0.05–0.32 mm) ciliated organisms, their swimming speed is strongly affected by the viscosity of the environment18 but not the temperature in the most common range of natural water (i.e., 5–20 °C)19, they are large enough to be easily observed with resource-poor microscopy setups20, and they swim independently of each other based only on hydrodynamic factors21.
The swimming speed of Paramecia in different viscosities has previously been measured manually several times22,23. While this study focuses on the first characterization of the influence of pollutants, the existence of previous literature on its relation with viscosity enables the comparison of our automated collection with other independent studies, and the evaluation of the robustness of Paramecia’s swimming speed for its use as a global indicator of water quality.
As observed in previous studies, our study corroborates that in the range of the low viscosity of 1–5 cP, Paramecia’s swimming speed is strongly influenced by the environment’s viscosity, showing in that range an average relative drop of 20.3% of the swimming speed at 1 cP per incremented cP (Figs. 1B and C, Supplementary Fig. 3, Supplementary video 2). Beyond 5 cP, Paramecia’s movement is hindered, losing significance at viscosities higher than ~ 10 cP. Fitting the data to the generally accepted power-law function24,25 provides an order of scaling of -0.732 (i.e., s = 1.9517v-0.732, where s is swimming speed and v is viscosity), which is significantly higher than that reported before. This discrepancy might be due to the consideration here of a more extensive range of viscosities. If only the 1–10 cP data are considered, the order of scaling decreases to -0.811, similar to the values obtained in previous experiments, which only consider that range of viscosities22. Therefore, the assumption of a power-law functional relationship between Paramecium’s swimming speed and a medium’s viscosity is more accurate in the low viscosity range (1–10 cP; R2 = 0.95), than when extreme cases are included (1–40 cP; R2 = 0.93). This can be easily explained due to the existence of a maximum viscosity when Paramecia movement is inhibited, a situation (i.e., swimming speed = 0) outside the boundaries of a finite power-law function.
The consistency of the data in the literature regarding the swimming speed of Paramecia and their dependence on the viscosity of the environment are noteworthy and suggest the suitability of the indicator for general use. Additionally, due to the self-motility and small size of Paramecia, this unconventional approach to obtain rheological information is particularly interesting for the measurement of microliter size, low viscosity biocompatible samples, a particularly challenging measure by conventional methods due to the inability to be inquired by regular rheometers or nanoindentations.
Water pollutant detection
The correlation between viscosity and the swimming speed of Paramecia is a notable demonstration of the indirect measurement of the environment based on microorganisms’ behavior. The next step of the research evaluated the swimming speed variation of Paramecia with the chemical contamination of the environment. From the IC50 measurements, it is known that a microorganism’s reproductive capabilities are severely affected by pollution. Still, the few studies that focused on the effect of pollutants on swimming speed, performed in water tunnels with vertebrates, have demonstrated only a weak correlation between their exhaustion and water contamination26. In this study, the limitations of those previous works were circumvented by using an organism that is rapidly and largely affected by environmental changes and a parameter that can be immediately measured outside the laboratory conditions without human intervention (i.e., swimming speed). We analyzed Paramecia’s movement in various common water pollutants, such as families of heavy metals, chemical and pharmaceutical wastes, and volatile organic compounds (VOCs). We set the concentrations around the concentration limits in the country of this study (Singapore), which are similar to those prescribed by the World Health Organization27. To ensure that the response of the Paramecia is primarily biological and not physical/mechanical, we performed all the experiments at standard conditions (STP) and confirmed the limited impact in the viscosity of the pollutants, which reached a 9% increase when all five pollutants tested were simultaneously maxed at five times their PL (Supplementary table 4).
The presence of heavy metals immediately impacts Paramecia’s movement, showing a significant decrease in their speed even when zinc chloride (Figs. 2A) and copper sulfate (Fig. 2B) concentrations are only half of those considered unsafe for drinking water. This change happens in a time frame that enables real-time observations (Supplementary video 3). The average swimming speed immediately drops in both scenarios to almost half than in the unaltered media (to 66% for ZnCl2 and 59% for CuSO4). Further increasing the pollutant concentration does not proportionately increase the effect, but produces significant differences for samples with heavy metal concentrations at the safety limits. Interestingly, the hindered Paramecia’s swimming speed becomes stable with time, reaching the same constant slow swimming speed in the first 24 h for all the samples within permissible concentration levels. This may be due to the adjustment/adaptation of the microorganism to the surrounding presence of the chemical pollutants. Those concentrations beyond safe levels result in cell death in the first 24 h for all the samples, except for twice the threshold concentration of copper sulfate, when Paramecia death occurs between 24 to 48 h in the contaminated media.
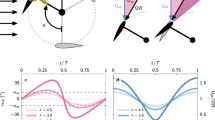
Paramecia in the presence of heavy metals. (A) Swimming speed of paramecia in different concentrations of zinc chloride (left). Red circular markers represent the speed immediately after the Paramecia are mixed in the Zinc chloride contaminated sample. Purple triangles and blue squares represent the average swimming speed of Paramecia after 24 and 48 h, respectively. Concentrations are relative to the PL (5 mg/l). The green and orange areas are the average and range of Paramecia speeds in unpolluted (green) and polluted (orange) media. The image on the right side is two characteristic paths followed by the Paramecia for 3 min at PL (light blue) and at five times PL (dark blue). Both are taken immediately after the Paramecia were mixed in the contaminated samples, and it represents the whole field of view. (B) Same representation than (A) but for copper sulfate. Markers use the same color code. PL, in that case, is 2 mg/l. The paths at the right side are for PL (light green) and five times PL (dark green). Error bars represent standard deviation of the average swimming speeds. Statistical significances are analyzed in Supplementary Fig. 2 and cumulative distances in Supplementary Fig. 4.
Among all the pollutants tested, heavy metals showed the highest impact on the microorganisms’ swimming speed, resulting in death at concentrations immediately beyond those considered safe for drinking. This result could be interpreted as higher toxicity of heavy metals for Paramecia or a less strict regulation for heavy metal pollution with respect to that for organic pollutants tested in this study.
Similar patterns of swimming speed to those for heavy metals were observed from the impact of erythromycin, a common pollution indicator for an urbanizing global water cycle28 (Figs. 3A, Supplementary video 4). While erythromycin’s primary use is the selective treatment of bacterial infections, Paramecia also gets strongly affected by the antibiotic, which impacts its mitochondrial DNA29. However, in this case, cell death was only observed when erythromycin concentration reached five times the currently regulated limit. In the case of the VOCs tested (i.e., tetrachloroethylene and trichloroethylene, Supplementary video 5), none of them resulted in cell death even at the extreme concentrations tested, well beyond the safe limits (Figs. 3B and C).
Measurement of the movement of Paramecium in the presence of organic pollutants. (A) Movement of Paramecium in Erythromycin solutions. The color code of the graph is similar to that in Fig. 2. The PL of erythromycin is 0.1 µg/l. Paths at the right side are for PL (light purple) five times PL (dark purple). (B) Swimming speed of Paramecia in samples contaminated with tetrachloroethylene (PL = 40 µg/l). Paths are for PL (blue) and five times PL (yellow). (C) Same representation but for trichloroethylene at (PL = 20 µg/l). Error bars represent standard deviation of the average speeds, and the paths are represented over the whole area of the chamber. Statistical significances are analyzed in Supplementary Fig. 2 and cumulative distances in Supplementary Fig. 4.
There is a common pattern in all the pollutants tested (Fig. 4). There was an immediate and significant reduction in the swimming speed of Paramecia in all the pollutants at the concentrations considered an environmental/health hazard (Supplementary Fig. 2). Paramecia in all those samples where they were alive after 48 h achieved the same swimming speed (0.71 ± 0.08 mm/s), almost three times lower than the speed in uncontaminated water (1.86 ± 0.16 mm/s). Most of them reached this slow-swimming state in the first 24 h. The rate at which Paramecia reach this state of slow swimming speed depends on the concentration—reaching the state almost immediately in large concentrations, but taking up to two days in low concentrations. In the case of erythromycin and VOCs, this state is reached even at half the PL concentrations, while for heavy metals, the threshold is at the PL concentration.
Swimming speed of Paramecia in all the pollutants tested. (A) Swimming speed immediately after their mixing in the contaminated samples. (B) Speed after 24 h in the contaminated sample. (C) Swimming speed 48 h in the contaminated samples. White is the average swimming speed of the Paramecia in the unpolluted reference environment.
This behavior raises two possible scenarios for using the swimming speed of Paramecia to assess water quality. The immediate and high relative change in Paramecia’s swimming speed suggests a suitable application to quickly evaluate, in a research environment, a specific pollutant’s impact. This type of test could, for example, substitute the standard IC50, based on reproducibility/mortality of the microorganisms, which is laborious, time-consuming and requiring trained personnel, reducing it to a few minutes of video recording, being the result also of a quantitative measurement with zero compromises on the quality.
Additionally, the existence of a stable and differentiable swimming speed in contaminated samples could be used as a rapid qualitative test field. In such situations, the suitability of a water sample could be assessed based on the average speed of the large microorganisms swimming in it and, in particular, those that are ubiquitous such as Paramecia.
Conclusion
The swimming speed of Paramecia in a confined space is strongly correlated to environmental conditions, and it is easily measurable using a simple object tracking algorithm and CCD sensor. Inorganic and organic pollutants have been demonstrated to affect such speed significantly and consistently when they are present in concentrations around drinking water thresholds. The reliability of the parameters was studied using previous reports based on the viscosity of the environment, showing similar speeds and strong correlation in the range of 1–10 cP and also fitted to a power-law model within those boundaries.
The immediate impact of the presence of pollutants on the swimming speed and the persistence of that change for days suggest two modes of utilization of the magnitude: One, as a rapid alternative to traditional IC50 measurements in preestablished samples, dropping the required time from several days to few minutes; and the other, using the ubiquity of Paramecia in water bodies as an immediate and resource-poor field measurement to detect the presence of pollutants at levels that could pose a risk to human health.
Material and methods
Paramecium aurelia was used for all the experiments in this study (Nantah Capital One Pte Ltd, Singapore). The Paramecia were further maintained in the laboratory as a mixed culture containing bacteria in a lettuce infusion, the latter prepared by brewing dried lettuce leaves in boiling water following a standardized protocol30. This media was used as a control/baseline to measure the swimming speeds of Paramecia in an unpolluted environment. Methylcellulose (MC) was purchased in a local store. The water pollutants used in the experiments (i.e., Zinc chloride, Copper sulfate, Erythromycin, Tetrachloroethylene, Trichloroethylene) were all purchased from Sigma-Aldrich (Germany). The polydimethylsiloxane (PDMS) is branded as SYLGARD 184 Silicone Elastomer Kit (Dow Chemical Company, USA) and was purchased from Tatlee Engineering Pte Ltd (Singapore).
Sample preparation for rheology study
Different concentrations (w/v) of MC solutions (0.01%, 0.025%, 0.05%, 0.1%, 0.25% and 0.5%) in the reference media were prepared and filtered. The respective viscosity measurements were taken using the capillary viscometer and tabulated in Supplementary Table 1. Then 1 ml of a concentrated culture of Paramecia (by centrifuging at 300 × g ) was pipetted and incubated with 100 ml of the prepared samples with different viscosities in containers and stored on a bench-top at room temperature.
Sample preparation for water pollutant detection study
The concentrations of the pollutants were chosen based on the concentrations of PL in water issued by PUB Singapore’s National Water Agency. The selected concentrations of 0.5x, 1x, 2.5x, and 5 × times the concentration of PL compared with 0x (water media control). The concentrations used were as follows: (1) Zinc chloride at 2 mg/l, 5 mg/l, 12.5 mg/l & 25 mg/l, (2) for Copper sulfate at 1 mg/l, 2 mg/l, 5 mg/l & 10 mg/l, (3) Erythromycin at 0.05 µg/l, 0.1 µg/l, 0.25 µg/l & 0.5 ug/l, (4) Tetrachloroethylene at 20 µg/l, 40 µg/l, 100 µg/l & 200 µg/l, and (5) Trichloroethylene at 10 µg/l, 20 µg/l, 50 µg/l & 100 µg/l. The stock solutions were prepared by diluting the pollutants in DI water, at the concentrations of 0.5 mg/ml (Zinc chloride), 1 mg/ml (Copper sulfate), 1 mg/ml (Erythromycin), 15 mg/ml (Tetrachloroethylene), and 17 mg/ml (Trichloroethylene). The testing solutions were prepared by adding stock solutions to the control media at the corresponding ratios. The “polluted” samples were then incubated with Paramecia (centrifuging at 300 × g and pipetted) in a process similar to that described above for viscosity measurements. The experiments were conducted at three time points: 0 h, 24 h, and 48 h at room temperature.
Experimental setup
The experiments were carried out in an open well made up of PDMS with dimensions of 1.3 × 1.1 × 1 mm3 (w × l × h). From the samples prepared, Paramecia were pipetted out from the incubation container and transferred to the PDMS well, and their movements were recorded at a rate of 15 frames/sec using an inverted light microscope (Axio Observer D1, Carl Zeiss, Germany) and a custom-built camera at 5x, 0.15 NA during the experiment at room temperature. A customized MATLAB script (Math Work, USA) with its Vision and Image Analysis toolboxes was used to analyse the recorded videos15. All videos were processed frame by frame, and a Kalman filter with a path predictor was used to locate the microorganism and generate the data. A sample size of 30 Paramecia was used for each experimental condition, including the control samples, totaling approximately 2500 Paramecia for the rheology and water pollutant detection studies. Each Paramecium was considered a single object and assigned with a unique number as ID, and they were tracked frame by frame consecutively. Short-lived paths were automatically discarded as noise. The path prediction algorithm was used for more accurate tracking, enabling to track movement even in the events of losing the Paramecium for few frames (e.g., moving too close to the edge of the chamber, crossing with another Paramecium, or impurity in the media). All the tracked information about the generated path for each Paramecium was stored under the same ID in separate files as cartesian coordinates at each frame. With these obtained (x,y,t) values, total distance traveled and average swimming speed (= total path length/time) were calculated for each Paramecium (Supplementary Tables 2 and 3). Error bars in the figures represent the standard deviation for the averaged speed values of all the Paramecia used at each experimental condition (~ 30 cells).
References
Makiola, A. et al. Key questions for next-generation biomonitoring. Front. Environme. Sci. https://doi.org/10.3389/fenvs.2019.00197 (2020).
Bohan, D. A. et al. Next-generation global biomonitoring: large-scale, automated reconstruction of ecological networks. Trends Ecol. Evol. 32, 477–487. https://doi.org/10.1016/j.tree.2017.03.001 (2017).
Derocles, S. A. P. et al. In Advances in Ecological Research Vol. 58 (eds Bohan, D. A. et al.) 1–62 (Academic Press, 2018).
Miyoshi, N. et al. Use of Paramecium Species in Bioassays for Environmental Risk Management: Determination of IC50 Values for Water Pollutants. J. Health Sci. 49, 429–435. https://doi.org/10.1248/jhs.49.429 (2003).
Doherty, F. G., Cherry, D. S. & Cairns, J. Valve closure responses of the Asiatic clam Corbicula fluminea exposed to cadmium and zinc. Hydrobiologia 153, 159–167. https://doi.org/10.1007/BF00006647 (1987).
Duque, G., Gamboa-García, D. E., Molina, A. & Cogua, P. Effect of water quality variation on fish assemblages in an anthropogenically impacted tropical estuary, Colombian Pacific. Environ. Sci. Pollut. Res. 27, 25740–25753. https://doi.org/10.1007/s11356-020-08971-2 (2020).
Grue, C. E. & Shipley, B. K. Interpreting population estimates of birds following pesticide applications-behavior of male starlings exposed to an organophosphate pesticide. Stud. Avian Biol 6, 292–296 (1981).
Kenna, D. et al. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 9, 5637–5650. https://doi.org/10.1002/ece3.5143 (2019).
Zala, S. M. & Penn, D. J. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 68, 649–664. https://doi.org/10.1016/j.anbehav.2004.01.005 (2004).
Fernandez, J. G. & Dritsas, S. The biomaterial age: the transition toward a more sustainable society will be determined by advances in controlling biological processes. Matter 2, 1352–1355. https://doi.org/10.1016/j.matt.2020.04.009 (2020).
Ng, S., Song, B. & Fernandez, J. G. Environmental attributes of fungal-like adhesive materials and future directions for bioinspired manufacturing. J. Clean. Prod. 282, 125335. https://doi.org/10.1016/j.jclepro.2020.125335 (2021).
Prabhu, J. Frugal innovation: doing more with less for more. Philos. Trans. Royal Soc. A Math. Phys. Eng. Sci. 375, 20160372. https://doi.org/10.1098/rsta.2016.0372 (2017).
Sonneborn, T. M. in Handbook of Genetics: Plants, Plant Viruses, and Protists (ed Robert C. King) 469–594 (Springer US, 1974).
Wichterman, R. The biology of Paramecium. (Springer US, 1986).
Shunmugam, A. P. & Fernandez, J. G. Characterization, Manipulation, and Isolation of Paramecium aurelia Using a Micro‐Electromigration Chip and Computer Vision. Adv. Mat. Tech. 5, 2000152. https://doi.org/10.1002/admt.202000152 (2020).
Orth, A., Wilson, E. R., Thompson, J. G. & Gibson, B. C. A dual-mode mobile phone microscope using the onboard camera flash and ambient light. Sci. Rep. 8, 3298. https://doi.org/10.1038/s41598-018-21543-2 (2018).
Vasudevan, J., Lim, C. T. & Fernandez, J. G. Cell migration and breast cancer metastasis in biomimetic extracellular matrices with independently tunable stiffness. Adv. Func. Mater. 30, 2005383. https://doi.org/10.1002/adfm.202005383 (2020).
Larsen, P. S. & Riisgård, H. U. Viscosity and not biological mechanisms often controls the effects of temperature on ciliary activity and swimming velocity of small aquatic organisms. J. Exp. Mar. Biol. Ecol. 381, 67–73. https://doi.org/10.1016/j.jembe.2009.09.021 (2009).
Beveridge, O. S., Petchey, O. L. & Humphries, S. Mechanisms of temperature-dependent swimming: the importance of physics, physiology and body size in determining protist swimming speed. J. Exp. Biol. 213, 4223–4231. https://doi.org/10.1242/jeb.045435 (2010).
Lee, S. A. & Yang, C. A smartphone-based chip-scale microscope using ambient illumination. Lab Chip 14, 3056–3063. https://doi.org/10.1039/C4LC00523F (2014).
Ishikawa, T. & Hota, M. Interaction of two swimming <em>Paramecia</em>. J. Exp. Biol. 209, 4452–4463. https://doi.org/10.1242/jeb.02537 (2006).
Au, L. & Shu, J. The relationship between kinematic viscosity and the swimming speeds of Paramecium aurelia, Tetrahymena spp., and Euglena spp. (University of British Columbia, 2013).
Valles, J. M., Jr., Jung, I., Mickalide, H., Park, H. & Powers, T. Paramecia Swim with a constant propulsion in Solutions of Varying Viscosity. APS Meeting Abstracts, 40.007 (2012).
Gómez, S., Godínez, F. A., Lauga, E. & Zenit, R. Helical propulsion in shear-thinning fluids. Journal of Fluid Mechanics. 812, R3. https://doi.org/10.1017/jfm.2016.807 (2017).
Qiu, T. et al. Swimming by reciprocal motion at low Reynolds number. Nat. Commun. 5, 5119. https://doi.org/10.1038/ncomms6119 (2014).
Plaut, I. Critical swimming speed: its ecological relevance. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 131, 41–50. https://doi.org/10.1016/S1095-6433(01)00462-7 (2001).
Helmer, R., Hespanhol, I. & World Health, O. Water pollution control: a guide to the use of water quality management principles. (E & FN Spon, 1997).
Schafhauser, B. H., Kristofco, L. A., de Oliveira, C. M. R. & Brooks, B. W. Global review and analysis of erythromycin in the environment: occurrence, bioaccumulation and antibiotic resistance hazards. Environ. Pollut. 238, 440–451 (2018).
Adoutte, A., Balmefrézol, M., Beisson, J. & André, J. The effects of erythromycin and chloramphenicol on the ultrastructure of mitochondria in sensitive and resistant strains of Paramecium. J. Cell Biol. 54, 8–19. https://doi.org/10.1083/jcb.54.1.8 (1972).
Van Wagtendonk, W. & Hackett, P. L. The culture of Paramecium aurelia in the absence of other living organisms. Proc. Natl. Acad. Sci. U.S.A. 35, 155 (1949).
Acknowledgements
This work was partially supported by the National Research Foundation, Singapore (Grant Number: CRP20-2017-0004).
Author information
Authors and Affiliations
Contributions
A.P.S. and J.G.F. designed the experiments. A.P.S. performed the experiments and collected the data. A.P.S. and G.S. analysed data. J.G.F. conceived the project and planned the experiments. All authors contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Supplementary Video 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shunmugam, A.P., Subramanian, G. & Fernandez, J.G. Measurements of the swimming speeds of motile microorganisms using object tracking and their correlation with water pollution and rheology levels. Sci Rep 11, 11821 (2021). https://doi.org/10.1038/s41598-021-91134-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91134-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.