Abstract
Lysergic acid diethylamide (LSD) is a classic psychedelic substance that is used recreationally and investigated in psychiatric research. There are no pharmacogenetic studies on LSD. In vitro metabolic studies indicate that several cytochrome P450 (CYP) isoforms (e.g., CYP2D6, CYP1A2, and CYP2C9) are involved in LSD metabolism, but in vivo data are scarce. The present study examined the influence of genetic polymorphisms of CYP genes on the pharmacokinetics and acute effects of LSD in healthy subjects. We identified common genetic variants of CYPs (CYP2D6, CYP1A2, CYP2C9, CYP2C19, and CYP2B6) in 81 healthy subjects who were pooled from four randomized, placebo-controlled, double-blind Phase 1 studies. We found that genetically determined CYP2D6 functionality significantly influenced the pharmacokinetics of LSD. Individuals with no functional CYP2D6 (i.e., poor metabolizers) had longer LSD half-lives and approximately 75% higher parent drug and main metabolite 2-oxo-3-hydroxy LSD area-under-the-curve blood plasma concentrations compared with carriers of functional CYP2D6. Non-functional CYP2D6 metabolizers also exhibited greater alterations of mind and longer subjective effect durations in response to LSD compared with functional CYP2D6 metabolizers. No effect on the pharmacokinetics or acute effects of LSD were observed with other CYPs. These findings indicate that genetic polymorphisms of CYP2D6 significantly influence the pharmacokinetic and subjective effects of LSD. Given the potential therapeutic use of psychedelics, including LSD, the role of pharmacogenetic tests prior to LSD-assisted psychotherapy needs to be further investigated.
Similar content being viewed by others
Introduction
Lysergic acid diethylamide (LSD) is a classic psychedelic. After early psychiatric research, recreational use, and prohibition, LSD was rediscovered by modern psychiatric research and may be useful for LSD-assisted psychotherapy1,2,3,4,5,6. However, despite its increasing use, the metabolism of LSD is not fully understood. Two recent in vitro studies reported the involvement of cytochrome P450 (CYP) enzymes in the metabolism of LSD7,8. One study of human liver microsomes showed that CYP2D6, CYP3A4, and CYP2E1 contribute to the N-demethylation of LSD to 6-nor-LSD (nor-LSD), whereas CYP2C9, CYP1A2, CYP2E1, and CYP3A4 participate in the formation of LSD’s main metabolite 2-oxo-3-hydroxy-LSD (O-H-LSD)8. Another study of human liver S9 fractions reported that CYP2C19 and CYP3A4 were involved in the formation of nor-LSD, and CYP1A2 and CYP3A4 contributed to the hydroxylation of LSD7. Some CYP enzymes (e.g., CYP2D6, CYP1A2, CYP2C9, and CYP2C19) have common functional genetic polymorphisms that result in different phenotypes9,10,11,12,13,14. CYP2D6 is associated with several phenotypes, ranging from poor metabolizers (PMs; 5–10% in Caucasians) to ultra-rapid metabolizers (UMs; 3–5% in Caucasians), with different underlying genotypes11. CYP2D6 genotype has previously been shown to influence the pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA)15,16, a substance that is also used for substance-assisted psychotherapy6. Genetic variants of LSD-metabolizing CYPs, especially CYP2D68, may also influence the pharmacokinetics of LSD. Additionally, it has been shown that the acute effects of LSD are dose-dependent and closely linked to the plasma concentration–time curve of LSD within an individual. Thus, the acute pharmacodynamic effects of LSD may also be critically influenced by CYP pharmacogenetics17,18,19.
The present study investigated the influence of prominent genetic polymorphisms of several CYPs involved in the metabolism of LSD (i.e., CYP2D6, CYP1A2, CYP2C9, CYP2C19, and CYP2B6) on pharmacokinetic parameters of LSD and its acute subjective effects. Based on in vitro studies with LSD, we hypothesized that CYP2D6 PMs would exhibit higher LSD concentrations and acute effects compared with individuals with functional CYP2D6. The quality and extent of subjective effects of psychedelics are particularly interesting because more intense and more positive acute psychedelic effects are thought to predict long-term therapeutic outcome in patients who receive psychedelic-assisted therapy20,21,22 and also positive long-term effects in healthy subjects23,24.
Methods
Study design
This was a pooled secondary analysis of four Phase 1 studies that each used a randomized, double-blind, placebo-controlled, crossover design and were conducted in the same laboratory17,25,26,27. The studies were all registered at ClinicalTrials.gov (Study 1: NCT01878942; Study 2: NCT02308969; Study 3: NCT03019822; Study 4: NCT03321136). The studies included a total of 84 healthy subjects. Study 125 and Study 417 each included 16 subjects. Study 2 included 24 subjects26. Study 3 included 29 subjects27. In Study 1, each subject received a single dose of 200 µg LSD or placebo. In Studies 2 and 3, each subject received a single dose of 100 µg LSD or placebo. In Study 4, each subject received 25, 50, 100, and 200, and 200 µg LSD + 40 mg ketanserin (a serotonin [5-hydroxytryptamine, 5-HT] 2A receptor antagonist). For this pooled analysis, we used mean data of the four LSD doses that were used within the same subject in Study 4. The 200 µg LSD + 40 mg ketanserin condition was used for the pharmacokinetic analysis but not for the analysis of the effect of LSD. All of the studies were approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz) and were conducted in accordance with the Declaration of Helsinki. The use of LSD was authorized by the Swiss Federal Office for Public Health (Bundesamt für Gesundheit), Bern, Switzerland. Written informed consent was obtained from all of the subjects. All of the subjects were paid for their participation. The washout periods between doses were 7 days for Studies 1 and 2 and 10 days for Studies 3 and 4. Test sessions were conducted in a quiet hospital research ward with no more than one research subject present per session. The subjects were under constant supervision while they experienced acute drug effects. The subjects comfortably reclined in hospital beds and were mostly listening to music and not engaging in physical activities. LSD was given after a standardized small breakfast in the morning. A detailed overview of the four studies is shown in Supplementary Table S1.
Subjects
A total of 85 healthy subjects of European descent, 25–60 years old (mean ± SD = 30 ± 8 years), were recruited from the University of Basel campus or word-of-mouth advertising and participated in the study. Two participants withdrew from the study, one before the first test session and one before the final LSD session. Two participants did not give consent for genotyping. These four participants were excluded from the final dataset, resulting in a final total of 81 subjects (41 women). The subjects’ mean ± SD body weight was 70 ± 12 kg (range: 50–98 kg). Participants who were younger than 25 years old were excluded from participating in the study because of the higher incidence of psychotic disorders in this age group and because younger ages have been associated with more anxious reactions to hallucinogens28. The exclusion criteria of all the analyzed parent studies included a history of psychiatric disorders, physical illness, tobacco smoking (> 10 cigarettes/day), use of any medication that may interfere with the effects of the study medications, a lifetime history of illicit drug use more than 10 times (with the exception of past cannabis use), illicit drug use within the past 2 months, and illicit drug use during the study, determined by urine tests that were conducted before the test sessions. Twenty-two subjects had prior hallucinogenic drug experiences, of which 16 subjects had previously used LSD (1–3 times), five subjects had previously used psilocybin (1–3 times), and one subject had previously used dimethyltryptamine (4 times), mescaline (1 time), and salvia divinorum (3 times).
Study drug
LSD base (Lipomed AG, Arlesheim, Switzerland) was prepared to be taken orally as gelatin capsules25,26 in Studies 1 and 2 and as a drinking solution in 96% ethanol in Studies 3 and 417,27. The doses that were used in each study are shown in Supplementary Table S1. Content uniformity and long-term stability data were available for the doses that were used in Studies 3 and 417,19,27. The exact actual mean doses of LSD base administered are shown in Supplementary Table S1. The planned mean doses that were used in Studies 1 and 2 were later detected to be lower, and the actual doses that were used were estimated based on comparisons of area-under-the-curve (AUC) values from Studies 1 and 2 with AUC values from Studies 3 and 419. The LSD doses of the analyzed studies were within the range usually used for therapeutic sessions and were not adjusted for body weight or sex6,29.
Pharmacokinetic analyses
Pharmacokinetic parameters were calculated using non-compartmental analysis in Phoenix WinNonlin 6.4 (Certara, Princeton, NJ, USA). Peak effect (Emax) values were obtained directly from the observed data. AUC and area under the time-effect curve AUEC values were calculated using the linear-log trapezoidal method. AUC values were calculated up to the last measured concentration in all studies (AUC10) and extrapolated to infinity (AUC∞). Additionally, a one-compartment model with first-order input, first-order elimination, and no lag time was used in Phoenix WinNonlin 6.4. to compare the pharmacokinetics of LSD among functional and non-functional CYP2D6 groups and to illustrate the LSD concentrations over time (Fig. 1) after a dose of 100 µg LSD base. This analysis included the data from all 81 subjects. For Study 4, only the 100 µg dose was included.
Modeled plasma LSD concentration–time curves over 24 h after LSD administration in subjects with genetically determined non-functional (red) or functional (blue) CYP2D6 enzymes. The shaded area marks the standard error of the mean. Non-functional (n = 7) and functional (n = 74) CYP2D6 subjects received a dose of 100 ± 30 µg LSD and 98 ± 35 µg LSD (mean ± SD), respectively. Both the half-life and AUC values significantly increased in subjects with non-functional CYP2D6 compared with functional CYP2D6.
Physiological effects
Blood pressure, heart rate, and body temperature were assessed before and repeatedly after LSD or placebo administration17,25,26,27. Mean arterial pressure was calculated as diastolic blood pressure + (systolic blood pressure – diastolic blood pressure)/3. The rate pressure product was calculated as systolic blood pressure × heart rate. Core (tympanic) temperature was measured using a Genius 2 ear thermometer (Tyco Healthcare Group LP, Watertown, NY, USA).
Subjective effects
Visual Analog Scales (VASs) were presented as 100 mm horizontal lines (0–100%), marked from “not at all” on the left to “extremely” on the right. The VASs were applied before and repeatedly after LSD or placebo administration17,25,26,27. The onset, offset, and duration of the subjective response were determined using the VAS “any drug effect”-time curve, with 10% of the individual maximal response as the threshold, in Phoenix WinNonlin.
The 5 Dimensions of Altered States of Consciousness (5D-ASC) scale30,31 was administered at the end of the acute drug effects to retrospectively rate peak drug responses. The main subscales that describe alterations of consciousness are Oceanic Boundlessness (OB), Anxious Ego Dissolution (AED), and Visionary Restructuralization (VR).
Genotyping
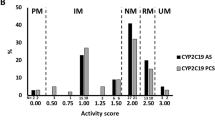
Genomic DNA was extracted from whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Hombrechtikon, Switzerland) and an automated QIAcube system. Single-nucleotide polymorphism (SNP) genotyping was performed using commercial TaqMan SNP genotyping assays (LuBio Science, Lucerne, Switzerland). We were looking at the most common SNPs and haplotypes for the selected CYPs within the population of European ancestry. Nevertheless, we also covered SNPs with a minor allele frequency (MAF) < 1% to catch or rule out mutations with substantial impact (i.e. CYP2D6*3 or CYP2C19*4). We did run uncertain assays twice or more if needed, with duplicates and controls with known genotypes for confirmation. In addition, two experts reviewed the data independently. We assayed the following SNPs and respective alleles: CYP1A2*1F (rs762551, assay: C___8881221_40), CYP2B6 (rs3745274, assay: C___7817765_60), CYP2C9*2 (rs1799853, assay: C__25625805_10), CYP2C9*3 (rs1057910, assay: C__27104892_10), CYP2C19*2 (rs4244285, assay: C__25986767_70), CYP2C19*4 (rs28399504, assay: C__30634136_10), CYP2C19*17 (rs12248560, assay: C____469857_10), CYP2D6*3 (rs35742686, assay: C_32407232_50), CYP2D6*4 (rs3892097, assay: C_27102431_D0; rs1065852, assay: C_11484460_40), CYP2D6*6 (rs5030655, assay: C_32407243_20), CYP2D6*9 (rs5030656, assay: C__32407229_60), CYP2D6*10 (rs1065852, assay: C_11484460_40), CYP2D6*17 (rs28371706, assay: C_2222771_A0; rs16947, assay: C_27102425_10), CYP2D6*29 (rs59421388, assay: C_3486113_20), and CYP2D6*41 (rs28371725, assay: C_34816116_20; rs16947, assay: C_27102425_10). CYP2D6 gene deletion (allele *5) and duplication/multiplication (allele *xN) were determined using a TaqMan Copy Number Assay (Hs04502391_cn). Activity scores for CYP2D6 were assigned according to established guidelines10,12,32,33,34. To see a distinct effect of CYP2D6 functionality on the pharmacokinetic and pharmacodynamic effects of LSD, we classified the subjects as non-functional CYP2D6 (PMs; activity score = 0) and functional CYP2D6 (activity score > 0). The activity score for CYP2C9 was generated using the relative metabolic activity of warfarin35,36. Genetically determined CYP1A2 activity inducibility was combined with the smoking status of the subject (> 5 cigarettes per day = smoker; rs762551 AA = inducible)9,15. Predicted CYP2C19 intermediate metabolizers (IMs) included CYP2C19*1/*2 and CYP2C19*2/*17, extensive metabolizers (EMs) included CYP2C19*1/*1, and UMs included both CYP2C19*17/*17 and CYP2C19*1/*1710. No CYP2C19 PM was identified within the sample. For CYP2B6, we determined the reduced-activity rs3745274 SNP (516G>T, CYP2B6*6 or CYP2B6*9, assay: C_7817765_60). Allele frequencies for the classification of CYP2D6 and CYP2C9 are shown in Supplementary Tables S2 and S3, respectively. All of the tested SNP frequencies were comparable to the Allele Frequency Aggregator Project databank and are listed in Supplementary Table S437.
Statistical analysis
All of the data were analyzed using the R language and environment for statistical computing38. To test for genotype effects, the pharmacokinetic parameters or effects of LSD (Δ LSD-placebo) were compared using one-way analysis of variance (ANOVA), with genotype as the between-group factor. The data are presented as actual nominal values and z-scores per study because the nominal values may be biased by a possible unequal distribution of genotypes across studies using different doses. The statistics were not corrected for sex or body weight because we found no correlations between sex/bodyweight and exposure to the drug (LSD AUC∞; Supplementary Fig. S1). As shown in Supplementary Fig. S1, an outlying individual was identified as non-functional CYP2D6. To minimize the effect of outliers and associated non-normal data distributions on the parametric statistics, we confirmed the results for the influence of CYP2D6 functionality on the pharmacokinetics and effects of LSD with nonparametric statistics (Wilcoxon signed-rank test and Kruskal–Wallis test). The level of significance was set at p < 0.05. Values of p in the pharmacokinetic analysis were not corrected for multiple testing because hypotheses for the influence of certain enzyme activities (i.e., CYP2D6) were made a priori.
Results
LSD produced significant acute subjective effects on all scales and moderately increased blood pressure, heart rate, and body temperature compared with placebo (Supplementary Table S5). Sex and body weight did not significantly alter the pharmacokinetics or acute effects of LSD (Supplementary Fig. S1).
Effects of CYP genotype on LSD pharmacokinetics and acute effects
CYP2D6 function significantly influenced the pharmacokinetics and acute effects of LSD (Table 1, Fig. 1). Specifically, subjects who were genetically classified as CYP2D6 PMs (non-functional) exhibited higher plasma LSD exposure (Fig. 1), reflected by significantly larger AUC∞ and AUC10 values compared with functional CYP2D6 carriers (Table 1). CYP2D6 PMs also had longer T1/2 values, consistent with slower metabolism compared with functional CYP2D6 subjects (Table 1), whereas the Cmax of LSD was not significantly affected. Furthermore, O-H-LSD AUC∞ values were larger in CYP2D6 PMs compared with functional CYP2D6 subjects (Table 1), in parallel with the effects on LSD concentrations and indicating that the conversion to O-H-LSD occurred independently of CYP2D6. Compartmental modeling for 100 µg LSD administration showed LSD AUC∞ and Cmax values for CYP2D6 PMs vs. functional CYP2D6 subjects of (mean ± SD) 24,169 ± 13,112 vs. 13,819 ± 6281 pg/ml*h (F1,79 = 13.8, p < 0.001) and 2369 ± 891 vs. 2061 ± 999 pg/ml (F1,79 = 0.62, p = 0.43), respectively (Fig. 1). Lower CYP2D6 activity was also associated with significantly higher exposure to LSD when analyzed across all CYP2D6 genotype activity score groups (Supplementary Table S6).
Consistent with the pharmacokinetic effect of LSD (Fig. 1), CYP2D6 PMs exhibited a substantially longer duration of the acute subjective response to LSD (Table 1) and significantly greater alterations of mind compared with functional CYP2D6 subjects (Table 1). Specifically, ratings on the 5D-ASC total, AED subscale (including disembodiment, impaired control and cognition, and anxiety), and VR subscale (including complex and elementary imagery and changed meaning of percepts) significantly increased in PMs compared with functional CYP2D6 subjects (Table 1). CYP2D6 genotype had no relevant effect on the autonomic response to LSD (Table 1).
In contrast to CYP2D6, genetic polymorphisms of other CYPs, including CYP1A2, CYP2B6, CYP2C19, and CYP2C9, had no relevant effect on the pharmacokinetics or subjective or autonomic effects of LSD (Supplementary Tables S7 and S8).
Discussion
The present study examined the influence of genetic polymorphisms on the pharmacokinetic and acute subjective effects of LSD in humans. The main finding was that genetic polymorphisms of CYP2D6 significantly influenced the pharmacokinetic and in part also the subjective effects of LSD.
LSD is metabolized almost completely in the human body. Only small amounts of the parent drug (~ 1%) are excreted in urine39. In vitro studies of human liver microsomes and human liver S9 fractions indicated a role for CYP enzymes in the metabolism of LSD7,8. CYP2D6 is involved in the N-demethylation of LSD to nor-LSD8. The present study provided additional in vivo evidence that CYP2D6 is involved in the metabolism of LSD in humans and that polymorphisms of the CYP2D6 gene influence both the metabolism of LSD and acute response to LSD in humans. Plasma nor-LSD concentrations in humans are mostly too low to be measured, even with highly sensitive methods40. However, we found an increase in both plasma LSD and O-H-LSD concentrations in individuals with a non-functional CYP2D6 genotype, consistent with the role of CYP2D6 in the formation of nor-LSD or other metabolites but not O-H-LSD. Thus, CYP2D6 is likely a crucial player in the degradation of LSD but not in the formation of its main metabolite O-H-LSD. The role of CYP2D6 could further be investigated in drug-drug interaction studies using LSD with and without selective CYP2D6 inhibition. This is also interesting because LSD may be therapeutically used in patients with psychiatric disorders and using a serotonin reuptake inhibitor (SSRI) treatment, which may also act as CYP2D6 inhibitors (e.g., fluoxetine and paroxetine)41. Consideration should also be given to discontinuing CYP2D6 inhibitors and allowing sufficient time for the enzyme to regenerate (up to 2 weeks) before LSD is used. Alternatively, in the presence of CYP2D6 inhibitors, the dose of LSD should be reduced, based on the present findings. On the other side, this might not particularly be the case for SSRIs. Chronic administration of antidepressants has been shown to decrease the number of 5-HT2 receptors in various brain regions due to receptor downregulation42. The slowly onset of 5-HT2A receptor downregulation together with the immediate inhibitory property of many SSRIs toward CYP2D6, could lead to an acute increase in LSD effects shortly after initiation of SSRI treatment but eventually to a decrease in effects as the primary target of LSD, 5-HT2A receptors, diminishe43.
With regard to other CYP enzymes, CYP2C19 was found to be involved in the formation of nor-LSD in vitro7. However, we found no influence of its genotype on the pharmacokinetics of LSD. Furthermore, CYP2C9 and CYP1A2 were reported to contribute to the hydroxylation of LSD to O-H-LSD7,8. CYP2C9 also catalyzes the N-deethylation to lysergic acid monoethylamide7. However, no effects of CYP2C9 genotype on the pharmacokinetics of LSD were observed in the present study in humans. For CYP1A2, no common loss-of-function polymorphisms have been identified to date. However, CYP1A2 is inducible by tobacco smoking in subjects with the common A/A genotype of the rs762551 SNP compared with the C/A and C/C genotypes9. Accordingly, we combined CYP1A2 activity inducibility with smoking status of the subjects (> 5 cigarettes per day = smoker). In a similar pharmacogenetic study with MDMA, we found higher 3,4-methylenedioxyamphetamine (MDA) levels (the minor metabolite of MDMA) in subjects who smoked 6–10 cigarettes daily and possessed the inducible genotype of CYP1A2 compared with subjects who smoked less and/or had the non-inducible polymorphism15. We did not find an influence of CYP1A2 genotype/smoking status on the pharmacokinetic of LSD in the present study. However, only five subjects were enrolled in the present study who met both requirements of being a smoker and possessing an inducible CYP1A2 genotype.
The pharmacogenetic influence of metabolizing enzymes on LSD appears quite similar to MDMA. For both psychoactive substances, LSD and MDMA, only polymorphisms of CYP2D6 appear to substantially impact pharmacokinetics and subjective effects15. However, because MDMA inhibits CYP2D6 and its own metabolism (i.e., autoinhibition), the effect of CYP2D6 genotype variations is limited and evident only during the onset of MDMA’s effects during the first 2 h after administration16. For LSD, moderation by CYP2D6 genotype appears to become more relevant later during the elimination phase, increasing the AUC and half-life of LSD and its duration of effect rather than its absorption and early effect peak. CYP2D6 PMs exhibited approximately 75% more total drug exposure than individuals with a functional CYP2D6 enzyme. We observed only a nonsignificant approximately 15% higher mean peak concentration. Therefore, total drug exposure, reflected by the AUC∞, was mainly determined by the lower elimination after the peak. This pattern was also present with the subjective effects of LSD. The VAS peak effects were not different between the different CYP genotypes, and the 5D-ASC ratings that reflected subjective alterations of mind over the entire day showed distinct differences that depended on CYP2D6 functionality. The non-functional CYP2D6 group reported an overall greater altered state of consciousness, with particularly higher ratings on the AED subscale, including Disembodiment, Impaired Control and Cognition, and Anxiety, and VR subscale, including Complex Imagery, Elementary Imagery, and Changed Meaning of Percepts.
Genetic effects on the acute subjective response to LSD is clinically relevant. Several studies in healthy subjects and patients found associations between the extent and quality of the acute subjective experience and long-term effects of psychedelics, including LSD20,21,22,23,24. Typically, greater substance-induced OB and more mystical-type effects could be associated with more favorable long-term effects. Specifically with regard to the 5D-ASC rating scale that was used in the present analysis, greater acutely psilocybin-induced OB and lower AED scores predicted better therapeutic outcomes at 5 weeks in patients with depression, whereas VR scores had no significant effects20. CYP2D6 PMs mainly had greater LSD-induced ratings of AED and VR but not OB, and these subjects may have an overall more challenging acute experience, with higher acute anxiety and possibly even lower therapeutic effects. This possible scenario needs further investigation. Geno- or phenotyping may be useful in patients who undergo LSD-assisted therapy. Based on the present findings, CYP2D6 PMs may benefit from approximately 50% lower doses than those that are used in functional CYP2D6 individuals. This possibility is also consistent with the observation that the higher LSD dose of 200 µg compared with 100 µg doubled plasma LSD exposure but did not result in higher ratings of OB but increased AED and anxiety on the 5D-ASC17.
The present study has limitations. Although this analysis was performed using the largest available sample of healthy human subjects who received LSD in placebo-controlled studies, the sample size is still relatively small. Although the sample size was sufficient to detect an effect of functionally very different genotypes (i.e., CYP2D6), it may have been too small to detect smaller changes with other CYPs. Additionally, CYP3A4 may play a role in the metabolism of LSD, but polymorphisms are rare44. Moreover, type I errors cannot be completely ruled out even if the hypothesis has been rationalized a priori. Drug-drug interaction studies with different selective CYP inducers/inhibitors are needed to confirm and expand the present findings.
The present study has several strengths, including the placebo-controlled design and use of validated psychometric tools. It also used statistical methods to address possible confounders. For example, complementary non-parametric analyses were used to confirm findings from parametric tests45. Additionally, the main analyses in this pooled study used z-transformed values to account for any between-study differences in genotype distribution and the doses used. Although this analysis is reliable for documenting changes between the tested genotypes, the measured values may only approximate the true size of the effect.
In conclusion, the present study revealed the influence of genetic polymorphisms of CYP2D6 on the pharmacokinetics and acute subjective effects of LSD in humans. Genetic polymorphisms of CYP2D6 significantly influenced the pharmacokinetic and subsequently subjective effects of LSD. No effect on the pharmacokinetics of LSD or response to LSD was observed with other CYPs. Given the potential therapeutic use of psychedelics, including LSD, the role of pharmacogenetic tests prior to LSD-assisted psychotherapy needs to be further investigated.
References
Gasser, P., Kirchner, K. & Passie, T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. J. Psychopharmacol. 29, 57–68 (2015).
Liechti, M. E. Modern clinical research on LSD. Neuropsychopharmacology 42, 2114–2127 (2017).
Nichols, D. E. Dark classics in chemical neuroscience: Lysergic acid diethylamide (LSD). ACS Chem. Neurosci. 9, 2331–2343 (2018).
Vollenweider, F. X. & Preller, K. H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 21, 611–624 (2020).
Bogenschutz, M. P. & Johnson, M. W. Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 64, 250–258 (2016).
Schmid, Y., Gasser, P., Oehen, P. & Liechti, M. E. Acute subjective effects in LSD- and MDMA-assisted psychotherapy. J. Psychopharmacol. https://doi.org/10.1177/0269881120959604 (2020).
Wagmann, L. et al. In vitro metabolic fate of nine LSD-based new psychoactive substances and their analytical detectability in different urinary screening procedures. Anal. Bioanal. Chem. 411, 4751–4763 (2019).
Luethi, D., Hoener, M. C., Krahenbuhl, S., Liechti, M. E. & Duthaler, U. Cytochrome P450 enzymes contribute to the metabolism of LSD to nor-LSD and 2-oxo-3-hydroxy-LSD: Implications for clinical LSD use. Biochem. Pharmacol. 164, 129–138 (2019).
Sachse, C., Brockmoller, J., Bauer, S. & Roots, I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br. J. Clin. Pharmacol. 47, 445–449 (1999).
Hicks, J. K. et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 93, 402–408 (2013).
Sachse, C., Brockmoller, J., Bauer, S. & Roots, I. Cytochrome P450 2D6 variants in a Caucasian population: Allele frequencies and phenotypic consequences. Am. J. Hum. Genet. 60, 284–295 (1997).
Hicks, J. K. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98, 127–134 (2015).
Gaedigk, A. Complexities of CYP2D6 gene analysis and interpretation. Int. Rev. Psychiatry. 25, 534–553 (2013).
Preissner, S. C. et al. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 8, e82562 (2013).
Vizeli, P., Schmid, Y., Prestin, K., Meyer zu Schwabedissen, H. E. & Liechti, M. E. Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. Eur. Neuropsychopharmacol. 27, 232–238 (2017).
Schmid, Y. et al. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet. Genom. 26, 397–401 (2016).
Holze, F. et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 46, 537–544 (2021).
Holze, F. et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide microdoses in healthy participants. Clin. Pharmacol. Ther. 109, 658–666 (2021).
Holze, F. et al. Pharmacokinetics and subjective effects of a novel oral LSD formulation in healthy subjects. Br. J. Clin. Pharmacol. 85, 1474–1483 (2019).
Roseman, L., Nutt, D. J. & Carhart-Harris, R. L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 8, 974 (2017).
Griffiths, R. R. et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30, 1181–1197 (2016).
Ross, S. et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 30, 1165–1180 (2016).
Griffiths, R., Richards, W., Johnson, M., McCann, U. & Jesse, R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol. 22, 621–632 (2008).
Schmid, Y. & Liechti, M. E. Long-lasting subjective effects of LSD in normal subjects. Psychopharmacology 235, 535–545 (2018).
Schmid, Y. et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol. Psychiatry. 78, 544–553 (2015).
Dolder, P. C. et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin. Pharmacokinetics. 56, 1219–1230 (2017).
Holze, F. et al. Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45, 462–471 (2020).
Studerus, E., Gamma, A., Kometer, M. & Vollenweider, F. X. Prediction of psilocybin response in healthy volunteers. PLoS ONE 7, e30800 (2012).
Gasser, P. et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 202, 513–520 (2014).
Dittrich, A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31(Suppl 2), 80–84 (1998).
Studerus, E., Gamma, A. & Vollenweider, F. X. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS ONE 5, e12412 (2010).
Gaedigk, A. et al. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 83, 234–242 (2008).
Crews, K. R. et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 91, 321–326 (2012).
Caudle, K. E. et al. Standardizing CYP2D6 genotype to phenotype translation: Consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 13, 116–124 (2020).
Hashimoto, Y. et al. Effect of CYP2C polymorphisms on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Biol. Pharm. Bull. 19, 1103–1105 (1996).
Gage, B. F. et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 84, 326–331 (2008).
Phan, L. et al. ALFA: Allele Frequency Aggregator. National Center for Biotechnology Information, U. S. National Library of Medicine. www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (2020).
R Core Team. R: A Language and Environment for Statistical Computing (2019).
Dolder, P. C., Schmid, Y., Haschke, M., Rentsch, K. M. & Liechti, M. E. Pharmacokinetics and concentration-effect relationship of oral LSD in humans. Int. J. Neuropsychopharmacol. 19, 072 (2015).
Steuer, A. E. et al. Development and validation of an ultra-fast and sensitive microflow liquid chromatography-tandem mass spectrometry (MFLC-MS/MS) method for quantification of LSD and its metabolites in plasma and application to a controlled LSD administration study in humans. Drug Test Anal. 9, 788–797 (2017).
Alfaro, C. L., Lam, Y. W., Simpson, J. & Ereshefsky, L. CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: Intraindividual variability and plasma concentration correlations. J. Clin. Pharmacol. 40, 58–66 (2000).
Meyer, J. H. et al. The effect of paroxetine on 5-HT(2A) receptors in depression: An [(18)F]setoperone PET imaging study. Am. J. Psychiatry. 158, 78–85 (2001).
Bonson, K. R., Buckholtz, J. W. & Murphy, D. L. Chronic administration of serotnergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology 14, 425–436 (1996).
Werk, A. N. & Cascorbi, I. Functional gene variants of CYP3A4. Clin. Pharmacol. Ther. 96, 340–348 (2014).
Zimmerman, D. W. A note on the influence of outliers on parametric and nonparametric tests. J. Gen. Psychol. 121, 391–401 (1994).
Acknowledgements
The authors acknowledge the assistance of F. Müller, L. Ley, and S. Harder in conducting the clinical studies, H. Meyer zu Schwabedissen and J. Hussner for assistance with genotyping, and M. Arends for text editing. This study was supported by the Swiss National Science Foundation (Grant No. 320030_170249 and 32003B_185111).
Author information
Authors and Affiliations
Contributions
P.V. and I.S. analyzed the data and wrote the manuscript. P.V., F.H., P.C.D., and Y.S. performed the research. M.E.L. conceived the study, obtained funding, and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.E.L. is a consultant for Mind Medicine, Inc. The other authors declare no conflicts of interests. Knowhow and data that are associated with this work and owned by the University Hospital Basel were licensed by Mind Medicine, Inc., after study completion. Mind Medicine, Inc., had no role in financing, planning, or conducting the present study or the present publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vizeli, P., Straumann, I., Holze, F. et al. Genetic influence of CYP2D6 on pharmacokinetics and acute subjective effects of LSD in a pooled analysis. Sci Rep 11, 10851 (2021). https://doi.org/10.1038/s41598-021-90343-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90343-y
This article is cited by
-
Acute effects of MDMA and LSD co-administration in a double-blind placebo-controlled study in healthy participants
Neuropsychopharmacology (2023)
-
Dose-response relationships of LSD-induced subjective experiences in humans
Neuropsychopharmacology (2023)
-
Lysergic Acid Diethylamide (LSD) for the Treatment of Anxiety Disorders: Preclinical and Clinical Evidence
CNS Drugs (2023)
-
Safety pharmacology of acute LSD administration in healthy subjects
Psychopharmacology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.