Abstract
In this study, we investigated a new application of bubble-eye goldfish (commercially available strain with large bubble-shaped eye sacs) for immunological studies in fishes utilizing the technical advantage of examining immune cells in the eye sac fluid ex vivo without sacrificing animals. As known in many aquatic species, the common goldfish strain showed an increased infection sensitivity at elevated temperature, which we demonstrate may be due to an immune impairment using the bubble-eye goldfish model. Injection of heat-killed bacterial cells into the eye sac resulted in an inflammatory symptom (surface reddening) and increased gene expression of pro-inflammatory cytokines observed in vivo, and elevated rearing temperature suppressed the induction of pro-inflammatory gene expressions. We further conducted ex vivo experiments using the immune cells harvested from the eye sac and found that the induced expression of pro-inflammatory cytokines was suppressed when we increased the temperature of ex vivo culture, suggesting that the temperature response of the eye-sac immune cells is a cell autonomous function. These results indicate that the bubble-eye goldfish is a suitable model for ex vivo investigation of fish immune cells and that the temperature-induced infection susceptibility in the goldfish may be due to functional impairments of immune cells.
Similar content being viewed by others
Introduction
Infection control is key in aquaculture1. Failure to control infection can lead to colony collapse (i.e., death), which can be very damaging to the local industry. Despite significant investments in infection control, including the use of antibiotics, the aquaculture industry is still affected by infectious diseases1,2,3,4. Rapid temperature rises, often caused by heat waves, increase the risk of infection through a combination of accelerated pathogen growth and suppression of host immune defenses1,5,6. Understanding such complex host–pathogen interactions requires efficient models; models that allow in vivo, in vitro, and ex vivo studies will accelerate research in fish immunology and contribute to the fight against infectious diseases.
Temperature alteration impairs fish immunity and develops infections as evidenced by increased infection rates and shortened lifespan in aquatic species5,7,8,9,10,11,12,13. Most of those studies reported that a warm temperature is associated with increased infection resistance, while it is not always true for some contexts. Increased temperature triggers a stress response that suppresses the immune system of fish species1, and such hyperthermia may impact the fishery. To prevent potential damage to the industry, immunostimulants and vaccination may be effective, if not complete14,15. For such immunostimulant screening, it is essential to use both live animals raised in laboratory tanks and ex vivo analysis using freshly harvested immune cells. Ex vivo systems allow high-throughput screening of potent immunostimulant candidates, while in vivo confirmatory experiments are required for extensive testing at the industrial level. Lack of either approach will delay the research. In this study, we propose to use bubble eye goldfish to fulfill this requirement. Goldfish can be kept in a wide temperature range (typically 15–30 °C) 16, which is suitable for studying the effects of acute temperature changes on the immune system of goldfish.
The bubble-eye goldfish is a commercially available strain of goldfish in East Asia. Bubble-eye goldfish have large eye sacs (ocular sacs) under each eye. The eye-sac is surrounded by membranous epithelial cells with blood vessels running through the structure, and lymphatic fluid containing immunocompetent cells flows into the inner part of the sac. The sacs make it possible for experimenters to retrieve the immune cells using a needle without sacrificing the animal. The ocular sacs can be sampled repeatedly, allowing for time-course studies that track the same set of individuals over time. The lymph of the eye sac contains growth-promoting factors that act on fish cell cultures17, and cells in the eye sac fluid may play an immunological role. In this study, we will demonstrate the expression of immune-related genes in eye sac cells in response to immune challenge with heat-killed bacteria to elucidate their immune function. We will also demonstrate how temperature elevation affects immune function in goldfish, both in vivo and ex vivo, using a bubble eye goldfish model.
Results
Temperature rise promotes infection in the common goldfish
As shown in Fig. 1, the common goldfish injected intraperitoneally with P. aeruginosa died earlier when kept at elevated temperature (33 ℃) than when kept at the normal temperature (25 ℃). The two groups were statistically significantly different (p = 0.021, Log-rank test). No death was observed for the saline-injected groups (Fig. 1).
Temperature sensitivity of common goldfish for Pseudomonas infection. After the acclimation period, the common goldfish were divided into four groups as descried in the Materials and Methods section (two infection paradigms and two temperature paradigms). The goldfish were either infected (P. aeruginosa; 3 × 107 CFU) or uninfected (saline) at either 25 \(^\circ \)C or 33 \(^\circ \)C. Survival curve for each group is shown in the figure over 7 days after the infection. Results from two experiments were combined and shown in the figure. Symbols: ● (saline, 25℃, n = 8), ○ (saline, 33℃, n = 9), ■ (P. aeruginosa infection, 25℃, n = 14) and □(P. aeruginosa infection, 33℃, n = 15). There was a statistically significand difference between the survival curves of P. aeruginosa infection (25ºC) and P. aeruginosa infection (33 \(^\circ \)C) groups (P = 0.021 by Log-rank test).
Inflammatory responses to P. aeruginosa in the bubble-eye goldfish eye-sacs
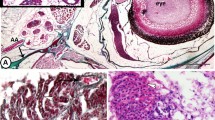
Eye sac cells (Fig. 2) can be harvested and cultured ex vivo. In the response to immune challenge by heat-killed P. aeruginosa, redness (prominent blood vessels) was observed in the ipsilateral eye sac membrane, but not in the contralateral membrane (Fig. 3), which represents an inflammatory response induced in the eye-sac. We further examined the gene expressions of pro-inflammatory cytokines in the eye-sac immune cells (see Materials and Methods for technical details). As shown in Fig. 4, the mRNA level increased by 800 fold for IL1β1 (p = 0.0001), 200 fold for IL1β2 (p = 0.0005), tenfold for TNFα1 (p = 0.0090), and 300 fold for TNFα2 (p = 0.0470) in response to the immune challenge (Fig. 4).
Inflammatory symptom (redness) of the eye sac. Heat-killed P. aeruginosa cells (50 µL of tenfold concentration of full growth) were injected into the right eye sac of the bubble-eye goldfish, while saline (50 µL) was injected into the left eye sac. Redness was observed on the right eye sac as shown in the figure after 20 h.
Pro-inflammatory cytokines are expressed in the eye-sac cells in the response to heat-killed P. aeruginosa cells. Twenty-four hours after the injection of heat-killed P. aeruginosa cells (50 µL of tenfold concentration of full growth; P) or saline (50 µL; S) into eye sac, eye sac cells were collected and analyzed for gene expression (see Materials and Methods section for details). Levels of mRNA for IL1β1, IL1β2, TNFα1 and TNFα2 were shown in the figure (values were normalized by a housekeeping gene (EF1α)). A representative result from three replicates is shown.
Temperature rise suppresses the pro-inflammatory cytokine expressions in the eye-sac immune cells both in vivo and ex vivo
In the eye-sac immune cells in vivo, mRNA levels of induced pro-inflammatory cytokine genes were lower when the fish were kept at elevated temperature (33 ℃) than when kept at the normal rearing temperature (25 ℃) (Fig. 5). We then harvested the eye-sac immune cells from untreated bubble-eye goldfish to obtain an ex vivo culture at 25 ℃ (see Materials and Methods for details), where induction of pro-inflammatory cytokine genes by heat-killed P. aeruginosa was observed in adherent cells but not apparent in non-adherent cells (Fig. 6, and Fig. S4). Using the ex vivo culture, we examined the effect of temperature rise on the pro-inflammatory cytokine expressions in the eye-sac cells induced by heat-killed P. aeruginosa cells. Compared with the normal temperature, increased culture temperatures resulted in reduced cytokine expressions (Fig. 7, and Fig. S1–3).
High temperature suppresses the expression level of pro-inflammatory cytokines in the eye-sac cells. Levels of mRNA for IL1β1, IL1β2, TNFα1 and TNFα2 were measured by qRT-PCR. Results from the increased-temperature (33 \(^\circ \)C, n = 3) condition and the normal temperature condition (25 \(^\circ \)C, n = 2) are shown in the figure (values were normalized to EF1α). P values from Student’s t-test are shown in the figure. The asterisks represent statistically significant differences between temperatures (significance levels were corrected by Benjamini–Hochberg procedure).
Ex vivo stimulation of eye-sac cells using heat-killed P. aeruginosa cells. The eye-sac cells of bubble-eye goldfish were suspended in medium (see Materials and Methods for details) and pre-incubated at 25 ℃ in 24-well plate (8 × 104/0.8 mL/well) for two hours. Then, adherent (Ad) and non-adherent (Non-ad) cells, were separated by gentle pipetting and cultured. The result from unseparated cell population (Ad + Non-ad; Whole) are also shown in the panels. Gene expressions of pro-inflammatory cytokines in response to heat-killed P. aeruginosa (P) or saline (S) (four hours after adding of the Pseudomonas cells or saline into the culture media) are shown in the figure. The values were normalized to EF1α.
Temperature sensitivity of the ex vivo function of the eye-sac cells. Eye-sac cells, collected from three bubble-eye goldfish were cultured either at 25 \(^\circ \)C or 33 \(^\circ \)C. Gene expressions of pro-inflammatory cytokines in the presence of heat-killed P. aeruginosa cells (four hours after adding of the Pseudomonas cells) are shown in the figure. The values were normalized to EF1α. P values obtained from Student’s t-tests are shown in the figure. The asterisks represent statistically significant differences (significance levels were corrected by Benjamini–Hochberg procedure).
Discussion
In the present study, we established a model system to study immune responses using the bubble-eye goldfish. As demonstrated in the present study, this model is suitable for both in vivo and ex vivo analyses of immune cells. For the ex vivo experiments, the eye sacs allow us to collect immune cells without sacrificing the animal, and the harvested immune cells can be cultured on a plastic dish. The ex vivo cultured cells expressed cytokine genes in response to the bacterial challenge, which is consistent with the cytokine gene expressions triggered by an immune challenge observed in vivo. The immune cells showed attenuated expressions of cytokine genes in response to temperature rise as observed in vivo and ex vivo, which sheds light on the underpinning mechanism of the increased infection risks in aquaculture upon temperature rises.
Among the cytokines, IL1β and TNFα play important roles both in innate and acquired immunity, such as the activation of phagocytic cells and the promotion of immune-related gene expressions in a series of immunoreactive cells both in mammals and fishes18, indicating that whether an animal is capable of inducing the expression of IL1β or TNFα in response to immune challenges reflects its infection resistance. In this sense, the suppressed induction of these cytokines at elevated temperatures may explain the enhanced bacterial infection of goldfish in this experimental temperature increase, but the optimal temperature for bacterial growth may also be an important factor in assessing resistance.
The microscopy suggests that most of the eye-sac cells are heterogeneous in multiple histological properties such as cell size and nucleocytoplasmic ratio. It should be noted that the adhesion rate to the plastic dish was approximately 50%, and those cells that did not adhere to the dish may represent distinct population from the adherent cell populations. At least, the adherent population showed apparent expressions of pro-inflammatory cytokines in response to bacterial challenge, suggesting its role in the pro-inflammatory cytokine production. Also, mammalian studies revealed that dish-adhering cell populations are rich in monocyte/macrophage lineage cells19,20, while goldfish macrophages express IL1β and TNFα21. These are consistent with our finding, and the dish-adhering eye-sac immune cells are most likely monocyte/macrophage lineage cells that respond to the immune challenge by expressing the pro-inflammatory cytokines.
Goldfish are eurythermic16 and often kept in a wide range of temperatures (usually 15 to 30 ℃). Goldfish can tolerate temperature rise after enough period of acclimation22. In our experiments, physiological conditions of goldfish were not investigated but no apparent changes were made between at normal (25 ℃) and at increased temperature (33 ℃). Regarding the temperature sensitivity of cytokine expressions, empirical knowledge in fish immune cells is slim. A very recent in vivo study in the crucian carp demonstrated that the host viability and the gene expression of pro-inflammatory cytokines after a bacterial infection was reduced by increased temperature23, supporting the present study in the bubble-eye goldfish. In the bubble-eye goldfish, the eye sac enables immune cell analyses as it provides up to 1 mL of lymphoid fluid from each individual, even without sacrificing the animal. This feature is the major advantage of this model using the bubble-eye goldfish and will accelerate the molecular study of fish immunity and contribute to improved aquaculture productivities.
Methods
Goldfish strains (Carassius auratus)
The common goldfish (‘Wakin’) and the bubble-eye goldfish were obtained from a local supplier Kingyo-Zaka (Tokyo, Japan). Goldfish were fed with a commercial diet for goldfish (Kyorin, Hyougo, Japan). All experiments were done after an acclimation period (more than a week) in the laboratory after each purchase, where we kept the goldfish at 25 ℃ in a fish tank (30 × 45 × 23 cm). The ranges of body weight were 7–10 g for the common goldfish, and 20–28 g for the bubble-eye goldfish upon the start day of each experiment. The research protocol was approved by Animal Welfare Ethics Committee of Genome Pharmaceuticals Institute Co., Ltd. and was conducted in compliance with all relevant guidelines and regulations applicable at the time and place of the experiments, including the ARRIVE guidelines.
Temperature rise paradigms
Temperature rise paradigms were given to the goldfish following the acclimation period at the normal temperature (25 ℃). For the infection experiments and the sterile immune challenge experiments, goldfish were kept at either 25 ℃ or 33 ℃ for 24 h before treatments (i.e., infection or sterile challenge). For ex vivo experiments using harvested eye-sac immune cells, cells were harvested from bubble-eye goldfish reared at 25 ℃, and the harvested cells were cultured either at 25 ℃ and 33 ℃.
Bacteria (Pseudomonas aeruginosa)
P. aeruginosa, strain PAO124 was aerobically cultured overnight at 37 ℃ in LB10 medium. In infection experiments, the live P. aeruginosa cells were washed and suspended in saline (0.9% NaCl aqueous solution). For the heat-killed P. aeruginosa cells used in this study, we washed the live cells with saline, and then autoclaved the cells at 121 ℃ for 20 min.
Infection experiments
To know the effect of temperature on the goldfish immunity, we intraperitoneally injected P. aeruginosa live cells (3 × 107 CFU/fish) to the common goldfish (Wakin). The injected fish were then kept at either 25 ℃ (normal rearing temperature) or 33 ℃ (increased temperature) and monitored for their survival.
Sterile immune challenge to the eye-sac using heat-killed bacterial cells
An overnight culture of P. aeruginosa was spun and the pellet was resuspended in a tenth volume of saline. We autoclaved this suspension at 121 ℃ for 20 min to obtain heat-killed P. aeruginosa cells. We injected 50 µL of the heat-killed P. aeruginosa cells into the eye-sac of one side, and 50 µL of saline into the eye-sac of the other side. The immune challenges were done either at the normal rearing temperature (25 ℃) or at the increased temperature (33 ℃).
Collection of eye-sac cells
Because the common goldfish is difficult to collect their immune cells without sacrificing the animal, we used the bubble-eye goldfish in the following part of this study to investigate the molecular responses of the goldfish immune system to temperature rises. Eye-sac cells floating in the eye-sac fluid can be easily collected from the eye sacs of bubble-eye goldfish (≧2 × 105 cells/mL). Eye-sac cells consisted of cells of various sizes, ranging from 5 to 20 µm, and different nucleocytoplasmic ratios (Fig. 2). Some of the cells showed adhesion to plastic dishes in vitro (Fig. S4). From the bubble eye-goldfish, the eye-sac fluid (containing the eye-sac cells) was collected from the eye sacs using a disposable plastic syringe with a 21-Gauge sterile needle.
Gene expression analysis of eye-sac immune cells
Twenty-four hours after the injection of heat-killed P. aeruginosa into the eye sac, we collected the eye-sac cells as described in the ‘Collection of eye-sac cells’ section. We then isolated the mRNA and performed qRT-PCR analyses (details describe in the following section).
Ex vivo culture of the eye-sac cells
We used a disposable syringe (5 mL) with needle (21 Gauge) to harvest the eye-sac immune cells. The harvested cells (typically 4 mL) were suspended in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated calf serum (SAFC Biosciences, USA), 3% autologous eye-sac fluid, 20 mM HEPES buffer and antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin) and cultured in 24-well plastic plate (catalog# 3820–024, AGC Techno Glass) (8 × 104 cells/0.8 mL/well) for at least 2 h. The medium had been preincubated at 25 ºC. In this condition, approximately 50% of the harvested cells adhered to the plastic dish. To give an immune challenge, 1.5 × 108 cells of heat-killed P. aeruginosa were added in the medium when the cells were suspended in the culture dish.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from eye-sac cells by using TRIzol Reagent (ThermoFisher Scientific), treated with DNase I (Promega), and reverse transcribed to obtain cDNA using the High Capacity RNA-to-cDNA Kit (ThermoFisher Scientific) following the manufacturer’s protocol. Using the cDNA, gene expressions were analyzed by qRT-PCR. The qRT-PCR was performed (relative standard method using a standard curve for each target), using 7500 Fast Real-Time PCR System (ThermoFisher Scientific) and Fast SYBR Green Master Mix (ThermoFisher Scientific). Primers for each target gene were described in the literature21. We chose the elongation factor 1α (EF1α) gene as an internal control as done in the preceding study in the goldfish21. As reported in the literature25, the goldfish has L-type and S-type ohnologs. The target genes we analyzed in this study were either on S-type or L-type chromosome. Further information is summarized in Supplementary Table S1.
Statistical analysis
To test the differences between survival curves, log-rank tests were performed using GraphPad Prism version 8.4.3 (GraphPad Software Inc.). To test the differences between mean values, Student’s t-tests were performed using Microsoft Excel 2013.
References
Lafferty, K. D. et al. Infectious diseases affect marine fisheries and aquaculture economics. Ann. Rev. Mar. Sci. 7, 471–496. https://doi.org/10.1146/annurev-marine-010814-015646 (2015).
Moran, J., Whitaker, D. & Kent, M. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172, 163–196 (1999).
Vendramin, N. et al. Viral Encephalopathy and Retinopathy in groupers (Epinephelus spp) in southern Italy: a threat for wild endangered species?. BMC Vet. Res. 9, 1–7 (2013).
Traxler, G., Roome, J., Lauda, K. & LaPatra, S. Appearance of infectious hematopoietic necrosis virus (IHNV) and neutralizing antibodies in sockeye salmon Onchorynchus nerka during their migration and maturation period. Dis. Aquat. Org. 28, 31–38 (1997).
Jiravanichpaisal, P., Soderhall, K. & Soderhall, I. Effect of water temperature on the immune response and infectivity pattern of white spot syndrome virus (WSSV) in freshwater crayfish. Fish Shellfish Immunol. 17, 265–275. https://doi.org/10.1016/j.fsi.2004.03.010 (2004).
Ndong, D., Chen, Y.-Y., Lin, Y.-H., Vaseeharan, B. & Chen, J.-C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 22, 686–694. https://doi.org/10.1016/j.fsi.2006.08.015 (2007).
Vidal, O. M., Granja, C. B., Aranguren, F., Brock, J. A. & Salazar, M. A profound effect of hyperthermia on survival of Litopenaeus vannamei juveniles infected with white spot syndrome virus. J. World Aquacult. Soc. 32, 364–372 (2001).
Guan, Y., Yu, Z. & Li, C. The effects of temperature on white spot syndrome infections in Marsupenaeus japonicus. J. Invertebr. Pathol. (Print) 83, 257–260 (2003).
Amend, D. F. Control of infectious hematopoietic necrosis virus disease by elevating the water temperature. J. Fisher. Board Canada 27, 265–270 (1970).
Castric, J. & De Kinkelin, P. Experimental study of the susceptibility of two marine fish species, sea bass (Dicentrarchus labrax) and turbot (Scophthalmus maximus), to viral haemorrhagic septicaemia. Aquaculture 41, 203–212 (1984).
Oseko, N., Yoshimizu, M. & Kimura, T. Effect of water temperature on artificial infection of Rhabdovirus olivaceus (hirame rhabdovirus: HRV) to hirame (Japanese flounder, Paralichtys olivaceus). Fish Pathol. 23, 125–132 (1988).
Kobayashi, T., Shiino, T. & Miyazaki, T. The effect of water temperature on rhabdoviral dermatitis in the Japanese eel, Anguilla japonica Temminck and Schlegel. . Aquaculture 170, 7–15 (1999).
Dorson, M. & Touchy, C. The influence of fish age and water temperature on mortalities of rainbow trout, Salmo gairdneri Richardson, caused by a European strain of infectious pancreatic necrosis virus. J. Fish Dis. 4, 213–221 (1981).
Smith, V. J., Brown, J. H. & Hauton, C. Immunostimulation in crustaceans: does it really protect against infection?. Fish Shellfish Immunol. 15, 71–90. https://doi.org/10.1016/s1050-4648(02)00140-7 (2003).
Lillehaug, A., Lunestad, B. T. & Grave, K. Epidemiology of bacterial diseases in Norwegian aquaculture–a description based on antibiotic prescription data for the ten-year period 1991 to 2000. Dis. Aquat. Organ. 53, 115–125. https://doi.org/10.3354/dao053115 (2003).
Ferreira, E. O., Anttila, K. & Farrell, A. P. Thermal optima and tolerance in the eurythermic goldfish (Carassius auratus): relationships between whole-animal aerobic capacity and maximum heart rate. Physiol. Biochem. Zool. 87, 599–611. https://doi.org/10.1086/677317 (2014).
Sawatari, E. et al. Cell growth-promoting activity of fluid from eye sacs of the bubble-eye goldfish (Carassius auraitus). Zoolog. Sci. 26, 254–258 (2009).
Zou, J. & Secombes, C. J. The function of fish cytokines. . Biology 5, 23 (2016).
Mosier, D. E. A requirement for two cell types for antibody formation in vitro. Science 158, 1573–1575 (1967).
Nathan, C. F., Karnovsky, M. L. & David, J. R. Alterations of macrophage functions by mediators from lymphocytes. J. Exp. Med. 133, 1356–1376 (1971).
Grayfer, L., Garcia, E. G. & Belosevic, M. Comparison of macrophage antimicrobial responses induced by type II interferons of the goldfish (Carassius auratus L.). J. Biol. Chem. 285, 23537–23547 (2010).
Brett, J. R. Rate of gain of heat-tolerance in goldfish (Carassius auratus). Univ. Tor. Stud. Biol. Ser. 1, 5–30 (1946).
Jiang, M., Chen, Z.-G., Zheng, J. & Peng, B. Metabolites-enabled survival of crucian carps infected by edwardsiella tarda in high water temperature. Front. Immunol. 10, 1991 (2019).
Miyashita, A., Takahashi, S., Ishii, K., Sekimizu, K. & Kaito, C. Primed immune responses triggered by ingested bacteria lead to systemic infection tolerance in silkworms. PLoS ONE 10, e030486 (2015).
Kon, T. et al. The Genetic Basis of Morphological Diversity in Domesticated Goldfish. Curr. Biol. 30, 2260–2274. https://doi.org/10.1016/j.cub.2020.04.034 (2020).
Acknowledgements
We thank Genome Pharmaceuticals Institute and Kingyozaka (the local goldfish supplier) for providing materials, animals, and technical advice. This work was supported by JSPS KAKENHI for Early-Career Scientists for AM (Grant# 20K16253).
Author information
Authors and Affiliations
Contributions
Study conception and design: K.S.; Acquisition of data: H.N., K.S.; Analysis and interpretation of data: H.N., A.M., H.H., K.S.; Drafting of manuscript: H.N.; Critical revision: H.N., A.M., H.H., K.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakajima, H., Miyashita, A., Hamamoto, H. et al. A novel application of bubble-eye strain of Carassius auratus for ex vivo fish immunological studies. Sci Rep 11, 10757 (2021). https://doi.org/10.1038/s41598-021-89882-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89882-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.