Abstract
Survival of breast cancer patients has improved, and treatment-related changes regarding metabolic profile deterioration after neoadjuvant systemic treatment (NST) become important issues in cancer survivors. We sought to compare metabolic profile changes and the neutrophil-to-lymphocyte ratio (NLR) between patients undergoing neoadjuvant chemotherapy (NCT) and neoadjuvant endocrine therapy (NET) 3 years after the treatment. In a prospective, randomized, phase III trial which compared 24 weeks of NCT with adriamycin and cyclophosphamide followed by docetaxel and NET with goserelin and tamoxifen (NEST), 123 patients in the Asan Medical Center were retrospectively reviewed to evaluate metabolic changes, such as body mass index (BMI), blood pressure (BP), total cholesterol (TC), fasting glucose, and the NLR. The mean age of patients was 42 years. The changes in BMI, serum glucose, and TC during NST and after 3 years were significantly different between NCT and NET. The proportion of overweight + obese group and the mean BMI were significantly increased during NCT (26.6% to 37.5%, 22.84 kg/m2 to 23.87 kg/m2, p < 0.05), and these attributes found to have normalized at the 3-year follow-up. In the NET group, BMI changes were not observed (p > 0.05, all). There were no differences in changes over time among in the Hypertension group during NCT and NET (p = 0.96). The mean value of serum TC and fasting glucose significantly increased (< 0.05, both) during NCT and decreased 3 years after NCT (p < 0.05); however, no significant changes were observed in the NET group. The NLR was increased from 1.83 to 3.18 after NCT (p < 0.05) and decreased from 1.98 to 1.43 (p < 0.05) after NET. Compared with minimal metabolic effect of NET, NCT worsens metabolic profiles, which were recovered over 3 years. The NLR was increased after NCT but decreased after NET.
Similar content being viewed by others
Introduction
According to WHO cancer statistics, breast cancer is the most common cause of cancer-related deaths in women worldwide. With advanced treatments and the consequently improved survival, the long-term and late effects of cancer treatment are of increasing importance1,2. Accumulating evidence suggests that certain cancer therapies may place cancer survivors at an increased risk of worsening metabolic profiles3,4,5. Metabolic complications, such as altered glucose metabolism and increased body weight, may worsen breast cancer prognosis6. Plasma cholesterol levels may also influence the risk of developing breast cancer because cholesterol is a precursor of steroid hormones7,8.
There have been several studies on metabolic changes resulting from the treatment of breast cancer, particularly metabolic syndrome following adjuvant or NCT. Many studies have shown that metabolic biomarkers, such as waist circumference, blood pressure, and fasting levels of blood glucose, triglycerides, and high-density lipoprotein cholesterol worsen after chemotherapy4. Neoadjuvant chemotherapy (NCT) has been established as a standard treatment for locally advanced breast cancer, with anthracyclines and taxanes being among the most frequently used agents9.
Several studies have been conducted on the effects of endocrine therapy on metabolic profiles; however, they have shown conflicting results10. Some studies reported that tamoxifen increases insulin resistance and raises triglyceride (TG) levels11,12. Another study reported that tamoxifen treatment had a favorable effect on lipid metabolism by decreasing TC and LDL levels in postmenopausal women9,13.
However, to our knowledge, no study has compared metabolic changes after NCT and neoadjuvant endocrine therapy (NET) in young breast cancer patients who had no prior underlying condition.
Recently, there is growing evidence that cancer-related inflammatory reactions play an important role in the progression of several malignancies. For example, damage to the adaptive immune response during chronic inflammation may favor cancer cell survival and promotion of tumor growth14,15,16,17,18. The existence of an elevated peripheral neutrophil-to-lymphocyte ratio (NLR), an indicator of systemic inflammation, has been recognized as a poor prognostic factor in various cancers19. Neutrophilia resulting from inflammation inhibits the immune system and promote cancer progression by suppressing the cytolytic activity of immune cells, such as lymphocytes, T cells, and natural killer cells20. Further, some studies suggest that the NLR also functions as a metabolic marker because high NLR values may be a reliable predictive marker of insulin resistance21.
Prospective, randomized, phase III trial which compared 24 weeks of response of NCT and NET (NEST study), 123 patients in Asan Medical Center were retrospectively reviewed to evaluate metabolic changes such as BMI, total cholesterol(TC), fasting glucose, and NLR22.
The purpose of our study was to compare treatment induced changes and long term follow up in patients’ metabolic profiles and NLR ratios between NCT and NET in a series of 123 patients with locally advanced luminal-type breast cancer in young women.
Methods/design
Study design
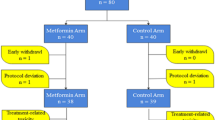
The NEST study is a prospective, multicenter, randomized, parallel group, comparative phase III clinical trial. Seven centers belonging to the Korean Breast Cancer Society Group (KBCSG-012) participated in the study22. Our study is a substudy of the NEST study and was conducted with 123 participants evaluated and treated at the Asan Medical Center to compare the metabolic and immunologic changes between the NCT and NET arms22. Eligible patients included pre-menopausal women with histologically confirmed ER-positive, human epidermal growth factor receptor 2 (HER2)-negative, biopsy-proven lymph node-positive primary breast cancer22. Lymph node positivity was required to be histologically proven with core needle biopsy or fine needle aspiration before starting the treatment. All patients were aged between 20 and 50 years. The goal of this study was to compare the changes in metabolic profiles and NLRs following cytotoxic chemotherapy vs. GnRH agonist and tamoxifen therapy for ER-positive, HER2-negative, lymph node-positive pre-menopausal breast cancer patients.
Procedures
Patients were randomly assigned (1:1) to receive either 3.6 mg goserelin acetate every 4 weeks with 20 mg tamoxifen daily or adriamycin and cyclophosphamide (60 mg/m2 adriamycin + 600 mg/m2 cyclophosphamide intravenously) every 3 weeks for four cycles, followed by taxol (75-mg/m2 docetaxel intravenously) every 3 weeks for four cycles. All patients were premedicated with oral corticosteroids dexamethasone 16 mg per day for 3 days starting 1 day prior to docetaxel administration. Treatment continued for 24 weeks, and all surgeries were performed between the 24th and 26th weeks.
The height, weight, and blood pressure (BP) of the patients were measured at the initial clinical visit. After the neoadjuvant systemic therapy (NST) method was determined, the patients underwent routine lab tests so that baseline data could be obtained, and we considered the results of the complete blood count (CBC) and chemical profile as the initial values. The patients also underwent a routine lab test as a preoperative work-up after 24 weeks of treatment. These values were designated as the post-treatment values. Post-treatment body mass index (BMI in kg/m2) was determined based on the measurements of height and weight when patients were hospitalized for surgery. Patients continued to visit the outpatient center after surgery, and we analyzed the blood test results, height, and body weight for 3 years after the initial clinical visit. BP was measured under resting conditions (participants were seated for 5 min) using an automated BP device (FT-500, Jawon Medical Co., South Korea). A 12-h fasting blood sample was obtained for CBC with differential counts as well as a routine chemical profile including fasting glucose and total cholesterol (TC) levels. On the basis of, patients were categorized as underweight, normal weight, overweight, and obese according to the WHO classification. Initial BP was also divided into normal, elevated, and hypertension (HTN) stages 1 and 2 according to the American Heart Association (AHA) classification.
Statistical analysis
Data were represented as the frequency and percentage for categorical variables and as the mean and standard deviation for continuous variables. The Mann–Whitney U test was used to compare continuous variables, and the chi-square or Fisher’s exact test was used to compare categorical variables. We used the linear mixed model for continuous variables and generalized estimating equations (GEE) for categorical variables to analyze the longitudinal data. The rationale behind the linear mixed model is that it can accommodate an unbalanced study design with unequally spaced repeated measures over time. Fasting glucose and NLR were analyzed after natural logarithmic transformation. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R software version 3.6.0 (R Foundation for Statistical Computing). A two-sided p value < 0.05 was considered statistically significant.
Ethical approval and consent to participate
This study protocol has been approved by the Korea Food and Drug Adminisration (KFDA) as well as institutional review board of erert trial centre, and was conducted in accordance with the Declaration Helsinki, Good Clinical Practice, and the applicable local regulatory requirements on bioethics. Written informed consent was obtained from all participants.
Consent for publication
Authors have agreed to submit it in its current form for consideration for publication in the Journal.
Results
Patient and tumor characteristics
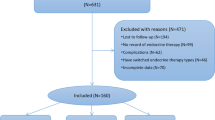
We included 64 patients in the NCT group and 59 patients in the NET group. The mean age was 41.8 ± 5.3 years in the NCT group and 42.0 ± 6.2 years in the NET group. The mean initial tumor size in the NCT group was 3.27 cm (± 1.81 cm) on ultrasonography (USG) and 3.46 cm (± 1.79 cm) on magnetic resonance imaging (MRI). In the NET group, the mean initial tumor size was 3.57 cm (± 1.80 cm) on USG and 4.16 cm (± 1.85 cm) on MRI. Other tumor characteristics, such as clinical T stage, nodal status, and estrogen and progesterone receptor (ER/PR) status, were well balanced between the two groups (Table 1) In addition, body weight groups were not statistically significantly different between both NST groups. The proportion of patients with hypertension was higher in the NET group than in the NCT group.
Metabolic profiles
BMI
There was a significant difference in BMI changes between NCT and NET groups over 3 years (p < 0.05). The change in the NCT group over time was statistically significant (p < 0.05). The mean BMI before NCT was 22.84 kg/m2 [95% confidence interval (CI) 21.94–23.74]. After neoadjuvant treatment, BMI increased to 23.87 kg/m2 (95% CI 23.06–24.68) and decreased to 22.82 kg/m2 (95% CI 22.04–23.61) after 3 years. There was no significant difference in the NET group over this period (Table 2, Fig. 1A).
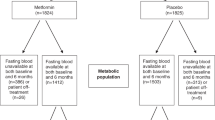
In the obese group, wherein the individuals had a BMI > 25 kg/m2 at diagnosis, the change in the NCT group over time was statistically significant (p < 0.05). There were 17 (26.6%) patients in the obese group before NCT. After neoadjuvant treatment, the number of patients in this group increased to 24 (37.5%) and then decreased to 12 (21.8%) after 3 years. There was no significant difference in the NET group in this regard. The number of obese patients before NET treatment was 14 (23.7%). Thirteen (22.0%) patients were obese after NET and 16 (32%) after 3 years (Tables 3, 4, Fig. 2).
TC
There were significant differences in the TC levels between NCT and NET groups over 3 years (p < 0.05). The change in the NCT group over this period was statistically significant (p < 0.05). The mean TC before NCT was 181.44 mg/dL (95% CI 172.96–189.91). After neoadjuvant treatment, this value increased to 215.23 mg/dL (95% CI 206.75–223.70) and later decreased to 176.15 mg/dL (95% CI 167.58–184.72) after 3 years. There was no significant difference in the NET group over the time (Table 2, Fig. 1B).
Fasting glucose
Fasting glucose was also significantly different between NCT and NET groups after 3 years (p < 0.05). The change in the NCT group over time was statistically significant (p < 0.05). The mean fasting glucose before NCT was 95.36 mg/dL (95% CI 92.55–98.26). After neoadjuvant treatment, the value increased to 111.36 mg/dL (95% CI 106.02–116.98) and then decreased to 99.02 mg/dL (95% CI 95.71–102.45) after 3 years. The metabolic profiles showed significant differences between all time points in the NCT group (Pre-NST vs. Post-NST, Post-NST vs. 3 years p < 0.01) (Table 2, Fig. 1C). There was no significant difference in the NET group in this regard over time.
Blood pressure
The proportion of HTN stage 1 and 2 patients increased from 21.3% (13) to 31.3% (20) in the NCT group and from 38.6% (22) to 50.9% (30) in the NET group (Table 5, Fig. 3). Initially, the proportion of patients with hypertension was significantly higher in the NET group than in the NCT group (Tables 1, 3). However, there were no significant differences in the changes over time between NCT and NET groups (p = 0.96) (Table 3).
NLR
There were significant differences in the NLR between NCT and NET groups over 3 years (p < 0.01). The change in the NCT group over time was statistically significant (p < 0.01). The mean NLR before NCT was 1.83 (95% CI 1.65–2.02). This value increased to 3.18 (95% CI 2.68–3.78) after neoadjuvant treatment and then decreased to 1.42 (95% CI 1.04–1.93) after 3 years. NLR worsening in the NCT group was caused by both neutrophilia and relative lymphopenia (Addendum 1). There was also a significant difference in the NLR in the NET group (p < 0.01). The mean NLR was 1.98 (95% CI 1.78–2.21) before NET treatment, 1.43 (95% CI 1.20–1.72) after NET treatment, and 1.61 (95% CI 1.16–2.22) after 3 years. In the NET group, the NLR changes were significantly different for all time points (Pre-NST vs. Post-NST, Post NST vs. 3 years p < 0.01), with the exception of Post-NST vs. 3-year values (Table 6, Fig. 1D).
Discussion
To our knowledge, this is the first study to demonstrate a relationship between metabolic profiles and NLR variation during neoadjuvant treatment in locally advanced luminal-type breast cancer in young women. This study has shown that NCT worsens metabolic profiles with unfavorable changes in BMI, total cholesterol, and fasting glucose, which recover over 3 years. In the NET group, there were no significant changes in BMI, TC, and fasting glucose. Conversely, NCT increased the NLR, whereas NET decreased the NLR, which recovered over 3 years.
Weight gain during chemotherapy for breast cancer has been well studied, but the reason for this weight gain is not fully understood23. Low physical activity, reduced resting metabolic rate, reduced thermogenesis, chemotherapy induced edema and unreduced dietary intake during chemotherapy24 are all possible reasons for weight gain during chemotherapy; however, no conclusive data exist to support any of these mechanisms25. Many studies have shown that metabolic biomarkers worsen after chemotherapy4. Explanations of potential mechanisms exist to explain the metabolic deterioration after chemotherapy-associated weight gain itself. Some hypotheses suggest that chemotherapy causes endothelial damage which leads to some cytokine alterations, which have an effect on the development of metabolic profile deterioration26,27. As there was a significant increase in BMI after NST in the NCT group, we reclassified the patients based on their BMI to determine the characteristics of those who were initially in the normal BMI group but became overweight after treatment. Eight patients met these criteria, but the sample size was too small to use statistical techniques and showed no special features. Also changes in self-perceived health and mental attitudes with weight gain during NCT, as well as metabolic profiles, could be considered one of the interesting topics. And this is carried out as our upcoming research.
Many studies have been conducted on the effects of endocrine therapy on metabolic profiles, and results are inconsistent. Some studies reported that tamoxifen had an adverse effect on metabolic profiles11,12; conversely, another study reported that tamoxifen had a favorable effect on lipid metabolism9,13 The mechanisms by which tamoxifen affects lipids and lipoproteins are unknown. However, some researchers have suggested that tamoxifen’s estrogenic effects on the hepatic lipoprotein metabolism, which lower LDL levels and increase the synthesis of apolipoprotein A-I, result in high concentrations of high-density lipoprotein (HDL)13. Our study also showed a decrease in TC during NET; however, the changes were not statistically significant.
Elevated NLR after NCT was due to relative neutrophilia, which occurs as a part of the systemic inflammatory response triggered by cancer28,29,30,31. Recent studies have indicated that metabolic profiles are associated with many chronic inflammation risk factors and that the NLR could be a predictive marker for developing metabolic syndrome32,33. It has also been suggested that increased NLR is associated with type 2 diabetes mellitus and increased risk for cardiovascular events34. In our study, the NLR was increased after NCT and decreased after NET, which were well corelated with other metabolic profiles.
High NLR is also known to be associated with an adverse overall survival (OS) and disease free survival (DFS) in patients with breast cancer35,36,37,38. . A few studies analyzing the NLR changes after cancer treatment have been conducted; these studies showed that the NLR after cancer treatment has its own significancy as well as pretreatment NLR35. There are many studies on pretreatment NLR and its significance, but there is also accumulating evidence suggesting that the NLR after treatment is associated with breast cancer-free survival with late recurrence39. In our study, the NLR was worsened by NCT and improved by NET. These NLR changes should be further investigated with survival analysis.
This study has a few limitations. The number of patients was small. Not all patients received the same tests; thus, there were intermediate blank data for the NLR or BMI. Although this gap in data was overcome by statistical techniques, with more patients, it would have been possible to draw more meaningful and reliable conclusions. Another limitation is that certain tests may be included in the metabolic profile, such as HDL and LDL cholesterol and TG, which were not included in the study. Further investigations should be performed to overcome these limitations.
Despite these limitations, the strength of this study is that it was performed using data collected during neoadjuvant treatment for breast cancer. Many studies have been conducted in the adjuvant setting, but few have compared NCT and NET among locally advanced breast cancer patients. Moreover, this study evaluated metabolic changes for young women who had fewer comorbidities than older women; thus, any changes observed in their metabolic profiles are more likely to be a result of the treatment itself. Finally, we herein evaluated metabolic changes not only during NST but also chronologically at 3 years after treatment. To our knowledge, no study has compared the effects of the two neoadjuvant treatments directly in detail or analyzed the long-term data of up to 3 years after the start of the treatment.
Conclusion
NCT worsens metabolic profile parameters, such as BMI, TG, and fasting glucose, which are then recovered over 3 years. In the NET group, however, there were no significant changes in BMI, fasting glucose, and TC. BP was unaffected in both groups, whereas NLR was increased after NCT but decreased after NET.
Data availability
The data supporting the conclusions of this article are included within the article. Any queries regarding these data may be directed to the corresponding author.
References
Kelly, C. M. & Hortobagyi, G. N. J. S. O. C. Adjuvant chemotherapy in early-stage breast cancer: what, when, and for whom?. Surg. Oncol. 19(3), 649–668 (2010).
Gajria, D. & Seidman, A. Adjuvant taxanes: More to the story. Adjuv. Taxanes. 10, S41–S49 (2010).
Demark-Wahnefried, W. et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. Am. J. Clin. Nutr. 65(5), 1495–1501 (1997).
Dieli-Conwright, C. M. et al. An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer 122(17), 2646–2653 (2016).
Gadea, E., Thivat, E., Planchat, E., Morio, B. & Durando, X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: A review of potential mechanisms. Obes. Rev. 13(4), 368–380 (2012).
Goodwin, P. J. et al. Insulin-and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. Obes. Rev. 30(2), 164–171 (2011).
Vatten, L. J. & Foss, O. P. J. Total serum cholesterol and triglycerides and risk of breast cancer: A prospective study of 24,329 Norwegian women. Cancer Res. 50(8), 2341–2346 (1990).
Smethurst, M., Basu, T. & Williams, D. J. Levels of cholesterol, 11-hydroxycorticosteroids and progesterone in plasma from postmenopausal women with breast cancer. Eur. J. Cancer 11(10), 751–755 (1975).
Gupta, S. et al. Effects of tamoxifen therapy on plasma lipid profile in patients of breast cancer. JAPI 54, 183–186 (2006).
Amir, E., Seruga, B., Niraula, S., Carlsson, L. & Ocaña, A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 103(17), 1299–1309 (2011).
Xu, B., Lovre, D. & Mauvais-Jarvis, F. Effect of selective estrogen receptor modulators on metabolic homeostasis. Biochimie 124, 92–97 (2016).
Hozumi, Y., Kawano, M., Saito, T. & Miyata, M. Effect of tamoxifen on serum lipid metabolism. J. Clin. Endocrinol. Metab. 83(5), 1633–1635 (1998).
Love, R. R. et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. J. Natl. Cancer Inst. 115(11), 860–864 (1991).
Elinav, E. et al. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. J. Natl. Res. Cancer 13(11), 759 (2013).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. J. Cancer 140(6), 883–899 (2010).
O’Callaghan, D. S., O’Donnell, D. & O’Connell, F. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thoar. Cancer 5(12), 2024–2036 (2010).
Galizia, G. et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin. Immunol. 102(2), 169–178 (2002).
Mei, Z. et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: Systematic review and meta-analysis. Br. J. Cancer 110(6), 1595 (2014).
Guthrie, G. J. et al. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crir. Rev. Oncol. 88(1), 218–230 (2013).
Chen, J. et al. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. Crir. Rev. Oncol. 5(1), 502–507 (2015).
Lou, M. et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC 15(1), 9 (2015).
Kim, H. J. et al. A phase III, open label, prospective, randomized, multicenter, neoadjuvant study of chemotherapy versus endocrine therapy in premenopausal patient with hormone responsive, HER2 negative, breast cancer (KBCSG 012). J. Clin. Oncol. 35(15 suppl), 517–517 (2017).
Demark-Wahnefried, W. et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J. Clin. Oncol. 19(9), 2381–2389 (2001).
Harvie, M. N., Campbell, I., Baildam, A. & Howell, A. J. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Br. Cancer 83(3), 201–210 (2004).
Demark-Wahnefried, W., Winer, E. P. & Rimer, B. K. J. Why women gain weight with adjuvant chemotherapy for breast cancer. J. Cancer Oncol. 11(7), 1418–1429 (1993).
Nuver, J. et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J. Clin. Oncol. 23(36), 9130–9137 (2005).
Kim, J.-A., Montagnani, M., Koh, K. K. & Quon, M. J. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113(15), 1888–1904 (2006).
Gomez, D. et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. J. Surg. 32(8), 1757–1762 (2008).
Hung, H.-Y. et al. Effect of preoperative neutrophil–lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int. J. Colorect. Dis 26(8), 1059–1065 (2011).
Mallappa, S., Sinha, A., Gupta, S. & Chadwick, S. Preoperative neutrophil to lymphocyte ratio> 5 is a prognostic factor for recurrent colorectal cancer. J. Cancer Dis. 15(3), 323–328 (2013).
Shimada, H. et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 13(3), 170–176 (2010).
Welty, F. K., Alfaddagh, A. & Elajami, T. K. Targeting inflammation in metabolic syndrome. Transl. Res. 167(1), 257–280 (2016).
Liu, C.-C. et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine 98, 43 (2019).
Sefil, F. et al. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J. Int. Res. Med. 42(2), 581–588 (2014).
Iwase, T. et al. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol. Cancer Res. 6(2), 266–270 (2017).
Ethier, J.-L., Desautels, D., Templeton, A., Shah, P. S. & Amir, E. J. B. C. R. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Mol. Cancer Res. 19(1), 2 (2017).
Orditura, M. et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open 1(2), e000038 (2016).
Nakano, K., Hosoda, M., Yamamoto, M. & Yamashita, H. Prognostic significance of pre-treatment neutrophil: Lymphocyte ratio in Japanese patients with breast cancer. Anticancer Res. 34(7), 3819–3824 (2014).
Moon, G., Noh, H., Cho, I.-J., Lee, J.-I. & Han, A. J. Prediction of late recurrence in patients with breast cancer: elevated neutrophil to lymphocyte ratio (NLR) at 5 years after diagnosis and late recurrence. Breast Cancer 12, 1–8 (2019).
Funding
This work was supported by the ASTRAZENEKA Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryu, H.H., Ahn, S.H., Kim, S.O. et al. Comparison of metabolic changes after neoadjuvant endocrine and chemotherapy in ER-positive, HER2-negative breast cancer. Sci Rep 11, 10510 (2021). https://doi.org/10.1038/s41598-021-89651-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89651-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.