Abstract
Obesity is a known factor increasing the risk of developing breast cancer and reducing disease free survival. In addition to these well-documented effects, recent studies have shown that obesity is also affecting response to chemotherapy. Among the multiple dysregulations associated with obesity, increased level of the apelin adipokine has been recently shown to be directly involved in the association between obesity and increased breast cancer progression. In this study, we analyzed in a retrospective cohort of 62 breast cancer patients the impact of obesity and tumoral apelin expression on response to neoadjuvant chemotherapy. In the multivariate logistic regression, obesity and high tumoral apelin expression were associated with a reduced response to NAC in our cohort. However, obesity and high tumoral apelin expression were not correlated, suggesting that those two parameters could be independently associated with reduced NAC response. These findings should be confirmed in independent cohorts.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer death among women1. It is now recognized that obesity, a condition that has reached pandemic proportions, is a risk factor for BC2. This association is especially well described for postmenopausal luminal BC but has also been observed for other BC subtypes regardless of the menopausal status3,4,5,6. Several potential mechanisms linking obesity and cancer have been identified as for instance higher estrogen exposure7,8, systemic low-grade inflammation9,10, increased insulin and insulin-like growth factor expression11,12 or altered adipokines secretion13,14. Obese patients have increased circulating levels of apelin15,16. Upon binding to the apelin receptor APJ, this adipokine is involved in several physiological functions as angiogenesis, heart contractility, energy metabolism and tumor progression17,18. Moreover, we recently demonstrated in an vivo study that this adipokine is implicated in the relation between obesity and BC19. Reproducing obesity-related levels of apelin is sufficient to promote BC growth and metastatization19. Besides promoting BC, recent data showed that obesity affects response to neoadjuvant or adjuvant chemotherapy for BC patients20,21,22. As tumor apelin expression or its receptor APJ have been associated with poor survival in humans23,24,25,26, we hypothesized that the apelinergic system could also be implicated in the adverse relation between obesity and pathological complete response (pCR) to neoadjuvant chemotherapy (NAC) in BC patients.
Results
Patients’ characteristics
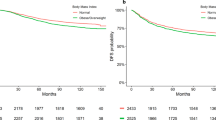
Body mass index (BMI) at diagnosis was used to classify 62 non-metastatic BC patients into three categories: normal weight (BMI < 25 kg/m2), overweight (BMI 25–30 kg/m2) and obese (BMI > 30 kg/m2) (Table 1). Thirty-five percent of patients were of normal weight, 40% were overweight and 25% were obese. The mean age of patients at diagnosis was 52.6 years old with a standard deviation of ± 12.4 (range 33–75). There was a trend towards overrepresentation of older patients in the overweight group (56.8 ± 9.96) as compared to the normal weight group (49.1 ± 13.73). This tendency was not observed in the obese category (50.5 ± 12.86). Altogether, age did not correlate with BMI in this cohort (Fig. 1). Compared to the normal weight and obese patients, overweight patients were more likely postmenopausal (41–40–72% of patients, respectively, p = 0.01). Two obese patients (3%) were diabetic. The majority of BC cases in the normal weight and obese groups were luminal. BC subtype was not significantly different between the subgroups. However, the overweight BC group was numerically enriched in triple negative BC cases, compared to normal weight and obese patients (36–18–7% respectively). Tumor size and cell proliferation were not significantly different among the three BMI categories. The majority of BC were of grade III. Nevertheless, tumor grade significantly diverged between the three subgroups, with a lower proportion of high-grade tumors in the obese patients (60%). Node infiltration did not differ between the subgroups.
The pCR rate was significantly different between the three subgroups, with a trend towards decreased response to chemotherapy with increasing BMI category (p = 0.03).
BMI and tumor apelin are associated with NAC pCR in BC
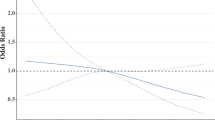
We investigated the link between pCR after NAC and BMI by univariate and multivariate logistic regression, accounting for several clinical relevant parameters [BMI, menopausal status, tumor grade, tumor size, nodal involvement and hormone receptor expression (Table 2)]. As our team recently highlighted in mouse models that obesity promotes tumor apelin expression and that high circulating apelin favors BC aggressiveness19, we also analyzed tumoral expression of the adipokine apelin and its receptor and tested their association with pCR by univariate logistic regression and in the multivariate logistic regression model. Interestingly, only BMI [Odds ratio (OR) of 0.86, 95% confidence interval (CI) 0.74–0.99] and tumor apelin expression (OR of 0.90, 95% CI 0.83–0.97) were significantly associated with pCR in the multivariate analysis. No other factor was significantly associated with pCR. However, tumor apelin did not correlate with BMI (Fig. 2).
Discussion
In this study, we retrospectively assessed the response to anthracycline and taxanes-based NAC in 62 early BC patients. As already highlighted in previous studies20, obesity was in our cohort significantly associated with a poorer response to NAC. We also observed numerical but not statistically significant trends towards higher pCR rate in high-grade tumors, and lower pCR rate in luminal tumors.
To further explore this association between weight status and pCR rate, we measured the tumoral expression of apelin and APJ. Interestingly, we discovered that high tumor apelin expression was associated with a lower rate of pCR in these patients. This finding further suggests that this adipokine plays a detrimental role in cancer. Indeed, several preclinical and clinical studies have shown that apelin correlates with metastatization and poor overall survival23,27. Here, our clinical exploratory study suggests that tumor expression of apelin in BC is also associated with a poor response to NAC, a factor associated with worse disease-free survival28.
In our previous preclinical study, we found that BC tumors developing in obese mice display an increased tumoral apelin expression. In the current cohort, BMI and tumor apelin did not correlate, suggesting these two parameters might independently affect response to NAC. This should be interpreted cautiously, as only 3% of patients were diabetic in our cohort, whereas the mice we used in preclinical studies were diabetic. Indeed, insulin is the main inducer of apelin expression29 and could explain why obese subjects have increased apelin expression levels.
The small sample size and retrospective nature of our study are other limitations. Indeed, we could question the representativeness of our cohort as no factor commonly associated with pCR shows a significant association with this parameter in our study. Therefore, our findings are only hypothesis generating and must be validated in an independent, prospective cohort. In addition, a new study would allow us to refine our model by including other obesity-related parameters such as insulin sensitivity and circulating levels of apelin expression. Indeed, in a retrospective study, circulating levels of apelin were directly correlated with cancer stage in several different forms of tumors, including BC30. Moreover, even if the use of BMI as a parameter for obesity is used in daily clinical practice, the use of waist-to-hip ratio as parameter for central obesity could be more appropriate to study the implication of obesity and apelin in response to NAC in BC patients. Larger studies would also allow to reduce heterogeneity between patients by considering separately the different BC subtypes, but also menopausal status.
In conclusion, in this retrospective exploratory study on 62 early BC patients treated with taxane and anthracycline-based NAC, obesity and high tumor apelin expression were independently associated with poorer pCR rates. This observation supports the notion that besides their role in the development of BC, both obesity and specific adipokines could play a role in the response to chemotherapy. Confirmation of these findings in independent cohorts would allow assessing their clinical relevance.
Patients and methods
Patients
We retrospectively collected a series of 64 patients with early BC, treated with NAC at Cliniques universitaires Saint-Luc (a tertiary care center in Brussels, Belgium) between 2012 and 2020. Patients had received similar chemotherapy regimen and had remaining pre-treatment tumor samples available. Two anorexic patients with a BMI below 18.5 were excluded and the final cohort was 62 patients. Baseline information at diagnosis included anthropometric measurements, menopausal and diabetic status. This study was approved by the Ethics Committee of Cliniques universitaires Saint-Luc (2017/25JUL/376). All research was performed in accordance with relevant guidelines/regulations, and informed consent was obtained from all participants.
Tumors biopsies
Patients underwent biopsy at diagnosis. Hormone receptor status [estrogen receptor (ER) and progesterone receptor (PR)] was evaluated by immunohistochemistry (IHC) and reported with the Allred score. Human epidermal growth factor receptor 2 (HER2) gene amplification status was determined by IHC and considered as positive for a staining superior to 10% (3+). In case of ambiguous IHC score (2+), FISH was performed to determine the HER2 gene amplification. Subtypes are categorized as following: hormone receptor positive (HR + HER2−) for tumor positive for either ER and/or PR and HER2 negative, HR + HER2+ for tumor positive for ER and/or PR and HER2 positive, HER2+ for tumor positive for HER2 and triple negative in case of negativity of ER, PR and HER2. Histological grade was assessed by the Nottingham scoring system. High Ki-67 index was determined for IHC staining above 15%31. Nodal invasion at diagnosis suspected by clinical evaluation or ultrasound had to be confirmed by cytopunction. Pathological complete response (pCR) was achieved if no invasive carcinoma was found in the breast and in the axilla (ypT0/is ypN0).
Neoadjuvant regimen
Patients underwent neoadjuvant regimen including combination of anthracycline and cyclophosphamide followed by taxanes. Depending on the recommendations at the time of diagnosis, patients have received either: 4 cycles of 5-fluorouracile (500 mg/m2), epirubicin (100 mg/m2), cyclophosphamide (500 mg/m2) followed by 4 cycles of docetaxel (100 mg/m2) or 4 cycles of epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) followed by 12 cycles of paclitaxel (80 mg/m2). Trastuzumab (6 mg/m2) was administered if case of HER2 positive status, and in one case, the patient also received Pertuzumab (fixed dose 420 mg). Three patients received carboplatin (AUC 6) in association of taxanes because of non-response to anthracyclines regimen. In some cases, patients did not receive the complete anthracycline regimen because of intolerance, or the complete taxol regimen because of neuropathy (Supplementary Table 1).
Immunohistochemistry
Biopsies were fixed in 4% paraformaldehyde for 24 h at room temperature before processing for paraffin embedding. Sections of 5 µm were submitted to endogenous peroxidases inhibition. Sections were then subjected to antigen retrieval in 10 mM citrate buffer pH 5.7 and to blocking of aspecific antigen binding sites (TBS containing 5% BSA and 0.05% Triton). Anti-apelin and anti-APJ primary antibody (Apelin: Abcam59469, APJ: Abcam214369) were incubated in TBS containing 1% BSA and 0.05% Triton and detected with anti-rabbit horseradish peroxidase-conjugated polymer secondary antibodies (Agilent) overnight at 4 °C. HRP was then visualized by DAB (Agilent). Cell nuclei were counterstained with hematoxylin. Stained slides were then digitalized using a SCN400 slide scanner (Leica Biosystems) at 40 × magnification and tumor area were detected by a Pathologist. Percentage of stained tissue was analyzed using Visiopharm software.
Statistical methods
Statistical analyses were performed using Graphpad Prism 8.0. For descriptive analyses, categorical parameters were presented as distribution of frequencies and continuous parameters as mean ± standard deviation. Descriptive analyses were performed using Chi-square test for categorical parameters and one-way Anova for continuous parameters. Factor associations with pathological complete response were tested by univariate and multivariate logistic regression. A p-value of ≤ 0.05 was considered significant.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638. https://doi.org/10.1056/NEJMoa021423 (2003).
Bao, P. P. et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control 27, 229–236. https://doi.org/10.1007/s10552-015-0700-7 (2016).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397. https://doi.org/10.3322/caac.21405 (2017).
Nagrani, R. et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur. J. Cancer 66, 153–161. https://doi.org/10.1016/j.ejca.2016.07.022 (2016).
Turkoz, F. P. et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J. BUON 18, 335–341 (2013).
Dall, G. V. & Britt, K. L. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Front. Oncol. 7, 110. https://doi.org/10.3389/fonc.2017.00110 (2017).
Simpson, E. R. & Brown, K. A. Obesity and breast cancer: Role of inflammation and aromatase. J. Mol. Endocrinol. 51, T51-59. https://doi.org/10.1530/JME-13-0217 (2013).
Jordan, B. F., Gourgue, F. & Cani, P. D. Adipose tissue metabolism and cancer progression: Novel insights from gut microbiota?. Curr. Pathobiol. Rep. 5, 315–322. https://doi.org/10.1007/s40139-017-0154-6 (2017).
Cani, P. D. & Jordan, B. F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 15, 671–682. https://doi.org/10.1038/s41575-018-0025-6 (2018).
Papa, V. et al. Elevated insulin receptor content in human breast cancer. J. Clin. Investig. 86, 1503–1510. https://doi.org/10.1172/JCI114868 (1990).
Vigneri, R., Sciacca, L. & Vigneri, P. Rethinking the relationship between insulin and cancer. Trends Endocrinol. Metab. 31, 551–560. https://doi.org/10.1016/j.tem.2020.05.004 (2020).
Niu, J. et al. The association between leptin level and breast cancer: A meta-analysis. PLoS ONE 8, e67349. https://doi.org/10.1371/journal.pone.0067349 (2013).
Gu, L. et al. Serum adiponectin in breast cancer: A meta-analysis. Medicine (Baltimore) 97, e11433. https://doi.org/10.1097/MD.0000000000011433 (2018).
Boucher, J. et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771. https://doi.org/10.1210/en.2004-1427 (2005).
Krist, J. et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes. Facts 6, 57–69. https://doi.org/10.1159/000348667 (2013).
Shin, K., Kenward, C. & Rainey, J. K. Apelinergic system structure and function. Compr. Physiol. 8, 407–450. https://doi.org/10.1002/cphy.c170028 (2017).
O’Carroll, A. M., Lolait, S. J., Harris, L. E. & Pope, G. R. Theapelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 219, R13-35. https://doi.org/10.1530/JOE-13-0227 (2013).
Gourgue, F. et al. Obesity and triple-negative-breast-cancer: Is apelin a new key target?. J. Cell Mol. Med. https://doi.org/10.1111/jcmm.15639 (2020).
Desmedt, C. et al. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J. Clin. Oncol. 38, 2883–2891. https://doi.org/10.1200/JCO.19.01771 (2020).
Karatas, F. et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 32, 237–244. https://doi.org/10.1016/j.breast.2016.05.013 (2017).
Wang, H. et al. Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: A meta-analysis. Breast Cancer https://doi.org/10.1007/s12282-020-01194-w (2021).
Feng, M., Yao, G., Yu, H., Qing, Y. & Wang, K. Tumorapelin, not serum apelin, is associated with the clinical features and prognosis of gastric cancer. BMC Cancer 16, 794. https://doi.org/10.1186/s12885-016-2815-y (2016).
Neelakantan, D. et al. Multifunctional APJ pathway promotes ovarian cancer progression and metastasis. Mol. Cancer Res. 17, 1378–1390. https://doi.org/10.1158/1541-7786.MCR-18-0989 (2019).
Zuurbier, L. et al. Apelin: A putative novel predictive biomarker for bevacizumab response in colorectal cancer. Oncotarget 8, 42949–42961. https://doi.org/10.18632/oncotarget.17306 (2017).
Hao, Y. Z., Li, M. L., Ning, F. L. & Wang, X. W. APJ is associated with treatment response in gastric cancer patients receiving concurrent chemoradiotherapy and endostar therapy. Cancer Biother. Radiopharm. 32, 133–138. https://doi.org/10.1089/cbr.2016.2138 (2017).
Uribesalgo, I. et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol. Med. 11, e9266. https://doi.org/10.15252/emmm.201809266 (2019).
Spring, L. M. et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 26, 2838–2848. https://doi.org/10.1158/1078-0432.CCR-19-3492 (2020).
Dray, C. et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 8, 437–445. https://doi.org/10.1016/j.cmet.2008.10.003 (2008).
Lacquaniti, A. et al. Apelin beyond kidney failure and hyponatremia: A useful biomarker for cancer disease progression evaluation. Clin. Exp. Med. 15, 97–105. https://doi.org/10.1007/s10238-014-0272-y (2015).
Gnant, M., Harbeck, N. & Thomssen, C. St. Gallen 2011: Summary of the consensus discussion. Breast Care (Basel) 6, 136–141. https://doi.org/10.1159/000328054 (2011).
Acknowledgements
We thank Michele de Beukelaer from the 2IP imaging platform of UCLouvain for technical assistance.
Funding
BJ is research director and PDC is a senior research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium. This work was supported by the Fonds de la Recherche Scientifique (FNRS FRFS-WELBIO) under the grants WELBIO-CR-2019C-02R. PDC is a recipient of the Funds Baillet Latour (Grant for Medical Research 2015). FPD received a postdoctoral clinical mandate (2017-034) from the not-for-profit organization ‘Foundation Against Cancer’ (Brussels, Belgium). FG is a FRIA grant holder of the FRS-FNSR, Belgium. FD received a doctoral mandate from the Breast Clinic, King Albert II Cancer Institute, Cliniques universitaires Saint-Luc. CVM received a post-doctoral clinician-researcher FRC mandate from the Cliniques universitaires Saint-Luc.
Author information
Authors and Affiliations
Contributions
F.G., F.D., F.P.D., P.D.C., B.F.J. conceived and designed the study. F.G., F.D., C.V.M., E.V., H.D., C.B. developed the methodology. F.G., F.D., C.V.M., L.D. analyzed and interpreted the data. F.G., F.D., C.V.M., F.D.P., P.D.C., B.F.J. wrote, reviewed, and/or revised the manuscript. All authors have read and approved the manuscript. F.G. & F.D. contributed as co-first author. F.P.D., P.D.C. & B.F.J. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gourgue, F., Derouane, F., van Marcke, C. et al. Tumor apelin and obesity are associated with reduced neoadjuvant chemotherapy response in a cohort of breast cancer patients. Sci Rep 11, 9922 (2021). https://doi.org/10.1038/s41598-021-89385-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89385-z
This article is cited by
-
A retrospective study in tumour characteristics and clinical outcomes of overweight and obese women with breast cancer
Breast Cancer Research and Treatment (2023)
-
The pleiotropic roles of adipocyte secretome in remodeling breast cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
Predictors for development of palbociclib-induced neutropenia in breast cancer patients as determined by ordered logistic regression analysis
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.