Abstract
New glass compositions containing high concentrations of Tb3+ ions were developed aiming at the production of magneto-optical (MO) fibers. This work reports on the structural and MO properties of a new glass composition based on (100 − x)(41GeO2–25B2O3–4Al2O3–10Na2O–20BaO) − xTb4O7. Morphological analysis (HR-TEM) of the sample with the highest concentration of Tb3+ ions confirmed the homogeneous distribution of Tb3+ ions and the absence of nanoclusters. All the samples presented excellent thermal stability against crystallization (ΔT > 100 °C). An optical fiber was manufactured by a fiber drawing process. The UV–Vis spectra of the glasses showed Tb3+ electronic transitions and optical windows varying from 0.4 to 1.6 μm. The magneto-optical properties and the paramagnetic behaviors of the glasses were investigated using Faraday rotation experiments. The Verdet constant (VB) values were calculated at 500, 650, 880, 1050, 1330, and 1550 nm. The maximum VB values obtained at 650 and 1550 nm for the glass with x = 18 mol% were -128 and − 17.6 rad T−1 m−1, respectively. The VB values at 500 and 1550 nm for the optical fiber containing 8 mol% of Tb4O7 were − 110.2 and − 9.5 rad T−1 m−1, respectively, while the optical loss at around 880 nm was 6.4 dB m−1.
Similar content being viewed by others
Introduction
Magneto-optical (MO) materials based on the Faraday effect have been increasingly studied for use in new technologies1,2. MO materials have been applied as modulators, as optical isolators, and as magneto-optical fiber sensors3,4,5. A wide variety of transparent MO glasses, crystals, and transparent glass–ceramics containing rare earth (RE) ions such as Tb3+, Dy3+, Pr3+, and Gd3+ have been investigated and are considered promising materials for photonics and spintronics6,7,8.
The Faraday effect is defined by the rotation angle (θ) of a linearly polarized light beam, when the light travels through an optical path of known length (l), under the application of a longitudinal magnetic field (B) along the light propagation direction9. The MO performance of a material is evaluated and quantified by the magnitude of the Verdet constant (VB) value, and it may be maximized by the incorporation of paramagnetic species10.
Single crystals have larger VB values than magneto-optical glasses with similar composition. However, MO materials produced from glasses are more attractive than crystals, due to their lower cost, isotropy, and simple preparation procedures, in addition to great flexibility in obtaining materials with different shapes and lengths, such as fibers for applications in integrated devices11. Among the MO materials, especially attractive are paramagnetic glasses containing high concentrations of Tb3+ ions and with large VB values in UV–Vis-NIR regions12,13.
The development of MO glass compositions able to support high RE ions contents and with high thermal stability represents an important step towards achieving successful fiber drawing processes without crystallization. Studies have reported the production of MO fibers based on Pr3+-doped aluminosilicate14, Eu-doped silica glass15, Gd2O3 NPs-doped aluminosilicate glass16, and Tb3+-doped silicate glasses17.
The main prerequisites in selection of a good candidate glass material for MO applications are large VB values and transparency in the visible and near-infrared (NIR) regions. In particular, for the NIR region (at 1550 nm), MO materials are good options for applications in telecommunication systems. Among all the paramagnetic ions, Tb3+ is one of the most attractive1,13,18. It has the electronic configuration 4f8 → 4f75d8,9, providing good paramagnetic behavior and among the highest magnetic moments (μeff = 9.5–9.72) and susceptibilities (J = 6, g = 1.46) of all the rare earth ions1. Additionally, terbium gallium garnet single crystals (Tb3Ga5O12), known as TGG, are available commercially and are considered one of the most important bulk MO materials, with VB of − 134 rad T−1 m−1 at 632 nm19.
Currently, heavy metal oxide (HMO) glasses based on borogermanates and borate glasses containing high Tb3+ ions contents stand out, due to their large VB values in the red region of the visible spectrum13. Gao et al.1 reported the MO properties of heavily Tb3+-doped materials with large Verdet constant values for use in fiber-integrated magneto-optics. In this case, the highest VB (at 632.8 nm) was − 119 rad T−1 m−1 for glass containing 25 mol% of Tb2O3. Franco et al.20 reported MO glasses based on the GeO2–B2O3–Al2O3–Na2O–PbO–Tb4O7 composition, with maximum VB values at 650 and 1550 nm equal to − 83.9 and − 15.5 rad T−1 m−1, respectively, for a sample containing 8 mol% of Tb4O7 (35 wt% of Tb dopant). In addition, the authors showed the production of an MO fiber from the drawing process of a glass containing 4 mol% of Tb4O7. Yin et al.13 reported the structural and MO properties of 20Tb2O3–20Ga2O3–xB2O3–(35 − x)SiO2–20GeO2 (20 ≤ x ≤ 35) glasses. In this case, the authors reported the variation of VB values at 450 nm (from − 210 to − 236 rad T−1 m−1), as a function of B2O3 content. Guo et al.21 showed that the VB values (at 632.8 nm) of GeO2–B2O3–SiO2–Ga2O3–xTb2O3 (15 ≤ x ≤ 30) glasses ranged from − 49.98 to − 120.54 rad T−1 m−1, with increase of the Tb2O3 content. Suzuki et al.12 reported large Faraday effects in bulk borate glasses containing Tb4O7 (45, 55, and 60 mol%). The large Verdet constant values for these Tb3+-borate glasses (at 633 nm) were − 172, − 212, and − 234 rad T−1 m−1, respectively12. Savinkov et al.22 described the MO properties of transparent and colorless Tb2O3–B2O3–GeO2 (TBG) glasses containing up to 33 mol% (or 12.13 × 10–21 ion cm−3) of Tb2O3. The maximum VB value obtained at 632.8 nm was − 0.409 arc min cm−1 Oe−1 (~ -119 rad T−1 m−1). As mentioned above, the literature includes very interesting works concerning MO characterization of bulk HMO glasses containing RE oxides, but very few papers have addressed MO fiber production17,20,23,24.
This work reports the synthesis and characterization of a new set of magneto-optical glasses based on Tb3+-containing borogermanate glass compositions. The thermal, structural, morphological, spectroscopic, and optical properties were investigated using differential scanning calorimetry (DSC), X-ray powder diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), and Raman, UV–Vis–NIR, luminescence, and M-Lines spectroscopy methods. The magneto-optical properties were evaluated by Faraday rotation, with Verdet constant (VB) values measured at different wavelengths in the Vis–NIR range. In addition, a magneto-optical fiber was produced and characterized.
Results and discussion
Thermal, structural, and morphological analysis
Figure 1 shows the color evolution of the BGB-xTb glasses, as a function of the Tb3+ content. The same color change has been reported for calcium aluminosilicate and borogermanate glasses containing high Tb3+ contents, with the effects being attributed to the Tb3+–Tb4+ redox process and the conditions of melting1,22.
Figure 2a shows the DSC curves for all the BGB-xTb glasses. The characteristic temperatures of glasses, including the glass transition (Tg), onset of crystallization (Tx), and maximum crystallization (Tp), together with the thermal stability parameters (ΔT = Tx − Tg), were calculated for all the BGB-xTb samples. Table 1 summarizes the values of Tg, Tx, Tp, and ΔT, together with the density (g cm−3) and Tb3+ ions density (ions cm−3) values. The Tb3+ ions density was calculated using Eq. (1):
where, \(N_{{Tb^{3 + } }} \) is the density of Tb3+ ions, x is the mole fraction of Tb4O7, NA is the Avogadro constant, and M is the average molecular weight of the BGB-xTb composition.
As can be seen in Fig. 2b, the Tg values increased as a function of the Tb3+ content, ranging from 545 °C (BGB-4Tb) to 720 °C (BGB-16Tb), followed by a decrease for the most concentrated sample, suggesting the occurrence of structural changes. It has been shown that in borogermanate glasses, RE ions may act as glass modifiers by breaking Ge–O–Ge bonds and inducing the formation of boroxol rings8,25. However, for the highest RE content, the structural connectivity was lost, resulting in a decrease of Tg. The BGB-xTb glasses presented high ΔT values, with a maximum of 305 °C for the BGB-8Tb sample, so this sample was the one selected for fiber production.
The optical basicity concept is based on the nature of the chemical bonding, according to Lewis acid–base theory and electronic polarization26,27. Duffy26,27 proposed an expression (Eq. 2) based on optical basicity theory to calculate the average donor power of different constituents of a medium such as a multi-component oxide glass, since Λth is related to the physical and chemical properties of glasses, including their structures and Verdet constants13:
where, xi is the mole fraction for one of the glass precursors (i) and Λi is the theoretical optical basicity value of an individual glass precursor. The intrinsic optical basicities of GeO2, B2O3, Al2O3, Na2O, BaO, and Tb2O3 are 0.600, 0.420, 0.600, 1.15, 1.15, and 0.954, respectively27.
Qualitatively, Λth is related to the electron donor power in a glass. In structural terms, the Λth values assist in understanding the increase or decrease of non-bridge oxygen (NBO) (negatively charged) generated by a modifier agent in the glass13. According to Liu et al.28 and Duffy27, a lower value of Λth reflects a lower content of NBO, so lower oxidation numbers of positively charged cations in the glass composition are consequently preferred.
As shown in Fig. 2c, the Λth values increased as a function of the Tb4O7 content. This increase of Λth could be explained by the greater polarizability of the glasses after the addition of Tb4O7, as well as the modifier action of Tb3+ ions in the glass network, which contributed to increasing the NBO bonds from the depolymerization of the germanate chains of the glass. Further evidence of NBO bonds in the BGB-xTb glass will be discussed in the Raman spectroscopy section. The Λth values ranged from 0.720 (0 Tb glass) to 0.763 (18 Tb glass), with increase of Tb4O7. As reported by Yin et al.13 and Sontakke et al.29, high glass optical basicity is one of the factors contributing to the oxidation of Tb3+ to Tb4+ during the melting process, responsible for the red shift of the cutoff wavelength. Furthermore, it has been suggested that the addition of high concentrations of Tb4O7 may influence the modifier action of the glassy network constituted by Ge–O–Ge and B–O–B bridging bonds (BO), due to the fact that the Tb4O7 structure contains voluminous polyhedrons larger than those formed by GeO2 and B2O320.
From Table 1, it can be seen that increasing the content of Tb3+ led to densification of the matrix, as shown by the higher density values. It should be noted that higher density values are reflected in a higher refractive index, resulting in higher Verdet constants, as will be discussed below.
Figure 3 shows the XRD patterns for the BGB-xTb samples. The diffractograms showed the presence of an amorphous halo and the absence of crystallization peaks, even for the highest Tb4O7 content. The halo maximum shifted from 27.5° to 30.5°, while the second halo shifted from 45° to around 50°, corroborating the structural changes induced by the addition of Tb4O7, as discussed above.
Figure 4A shows an HRTEM image of the BGB-18Tb glass, revealing a homogeneous distribution of atoms, with no evidence of clustering at the atomic scale. Fast Fourier transform (FFT) (inset of Fig. 4A) confirmed the absence of crystalline spots and the existence of long-range structural order, corroborating the XRD measurements. Figure 4B shows a high-angle annular dark-field (HAADF) image of the homogeneous structure of the BGB-18Tb glass (analyzed area) and the corresponding elemental EDS mapping (Ba-K, Tb-L, and O-K). Supplementary Figure S1 shows the EDS spectrum of the BGB-18Tb glass.
Raman spectroscopy
Raman spectra of all the BGB-xTb glasses are shown in Supplementary Fig. S2a. The Raman spectrum of the undoped glass (BGB-0Tb) is also plotted to facilitate discussion of the role of Tb3+ ions in the glassy network. Raman spectra of the glass formers GeO2 (α-quartz-like) and B2O3 (vitreous) are shown in Figure S2(b).
The Raman spectra showed broad bands typical of glassy structures, assigned to a large distribution of bonds and angles, as well as several overlapping vibrational modes of the glass components. For these reasons, it was necessary to identify the contributions of the individual vibrational modes by deconvolution, involving the fitting of Gaussian peaks in different frequency regions of the spectra. Such Gaussian deconvolution has been described in previous spectroscopic studies of germanate, borate, and borogermanate glasses8,20,25,30,31,32,33,34,35.
Raman spectra of the BGB-xTb glasses at low (~ 130–650 cm−1), medium (~ 650–1050 cm−1), and high (1050–1800 cm−1) frequencies are shown in Fig. 5a–c. Figure 5d–o shows the deconvolution at low, medium, and high frequencies for the BGB-xTb glasses (x = 0, 4, 8, and 18 mol% of Tb4O7). It can be seen that the addition of Tb4O7 caused structural changes in the BGB glass network. The main vibrational modes assigned from the Raman spectra for the BGB-xTb glasses are summarized in Table 2.
Figure 5d shows the peak fitting for the undoped BGB glass. In this case, six Gaussian peaks were fitted at low frequencies: 220 (peak 1), 305 (peak 2), 350 (peak 3), 470 (peak 4), 518 (peak 5), and 561 cm−1 (peak 6). In the region below 400 cm−1, peaks 1 and 2 could be attributed to bending modes of Ge–O–Ge in the glassy network31,32. Peak 3 could be assigned to the vibration of Ge–O− (Q1 species)32. Peak 4 was assigned to symmetrical stretching vibrations of Ge–O–Ge bonds in 3-membered GeO4 rings35. Between 500 and 600 cm−1, peaks 5 and 6 could be attributed to symmetrical vibrations of Ge–O–Ge bonds in three-membered GeO4 rings34,35 and Ge–O–Ge bending mode25, respectively. The presence of only Ge4+ in borogermanate glasses was recently elucidated using Ge K-edge EXAFS and XANES measurements8,20.
Two main features could be observed after addition of terbium oxide: (I) the intensity of the broad band between 130 and 400 cm−1 increased, and (II) the intensity of the broad band between 500 and 650 cm−1 was strongly attenuated. These behaviors could be explained by the gradual increase of Ge–O− non-bridge bonds (NBO), due to depolymerization of the BGB glass network after addition of Tb3+ ions. In region I, according to Kamitsos et al.32, the appearance of vibrational modes at low frequencies (200–400 cm−1) could be assigned to the bending modes of Q2 and Q1 species derived from GeO4 units, as detailed in Fig. 5d–g. In region II, depolymerization increased the NBO number, consequently decreasing the average distribution of Ge–O–Ge bridges.
The deconvolution of the middle region is shown in Fig. 5h–k. Deconvolution of the broad band centered at around 828 cm−1 resulted in the fitting of five peaks centered at around 752, 778, 815, 828, and 908 cm−1. The first two (peaks 7 and 8) were assigned to the symmetrical stretching vibrations of metaborate chains35,36 and borate rings (di-triborate rings)35, respectively. Previous investigations of germanate and borogermanate glasses using Raman spectroscopy found that the region between 800 and 900 cm−1 was dominated by vibrational modes of Q2 and Q3 units derived from the breakdown of tetrahedral [GeO4] units31,34,35,36. Peaks 9 (815 cm−1) and 10 (828 cm−1) could be attributed to symmetrical stretching vibrations of Ge–O− in Q2 and Q3 species, respectively35,37. In addition, peak 11 (~ 908 cm−1) was assigned to diborate groups35.
As shown in Fig. 5b, the addition of Tb4O7 shifted the broad band centered at 828 cm−1 (BGB-0Tb glass) to 792 cm−1 (BGB-18Tb glass), while the shoulder at 909 cm−1 in the BGB-0Tb spectrum was shifted to 931 cm−1. Koroleva et al.35 used Raman spectroscopy to evaluate the individual contributions of the vibrational modes of B2O3 and GeO2 in borogermanate glasses. Based on the work of Koroleva et al.35 and Kamitsos et al.32, the band observed between 900 and 940 cm−1 (peak 11) in the Raman spectra of the borogermanate glasses could be assigned to the vibration of diborate groups.
At higher frequencies (above 1050 cm−1), there was a predominance of vibrations of borate groups (Fig. 5c). The Raman spectrum for the BGB-0Tb glass presented a band at 1200 cm−1 (peak 12)35, assigned to diborate groups, and a broad band at around 1427 cm−1 with overlapping peaks assigned to asymmetric stretching of B–O bonds of BO3/2 units (peak 13, at 1337 cm−1)35, stretching vibration of BO3/2ring (peak 14, at 1427 cm−1)35, and vibrations related to non-bridging oxygen atoms of B–O− bonds (peak 15, at 1495 cm−1)35.
The Raman analysis revealed an interesting feature of borogermanate glasses that should be considered in the search for glasses presenting extremely high Verdet constants. VB was shown to be dependent on the Tb3+ ions and increased as a function of the rare earth content. In order to introduce high contents of rare earths into glasses, it is necessary to provide a favorable chemical environment, since rare earths need high coordination number of oxygen atoms to be stabilized and to avoid further precipitation. From comparison of the structures of the glasses studied in this work with other compositions presented in the literature, it could be inferred that increase in the number of NBO can assist in the stabilization of rare earths38. For compositions containing lower quantities of NBO, the amounts of rare earths were lower than for those with higher NBO, which was mainly characterized by the intense bands at ~ 300 and 820 cm−1 (Ge–O−) and above 1050 cm−1 (B–O−). Hence, the use of adequate contents of modifiers such as BaO is essential for obtaining higher VB in borogermanate glasses.
Optical analysis
Figure 6a shows the absorption spectra of the BGB-xTb glasses in the region from 250 to 800 nm. The absorption bands at 318, 340, 350, 358, 370, 378, and 485 nm were assigned to the Tb3+ 4f–4f transitions from the 7F6 ground state to the excited states (5D0,1, 5H7), (5G2, 5L6), 5L9, 5G5, 5L10, (5D3, 5G6), and 5D4, respectively39.
As shown in Fig. 6a, the UV cutoff for the undoped glass was at around 300 nm. The red shift to around 600 nm (BGB-18Tb), observed after addition of Tb4O7, was the result of the intense absorption of the Tb3+ transitions. However, the main origin of the red shift could be ascribed to the oxidation of Tb3+ to Tb4+, characterized by the change of color from colorless, passing through pale yellow, and finally to dark brown, as the Tb3+ content increased (see Fig. 1)40.
Figure 6b shows the transmission window for the BGB-xTb glasses, recorded from UV to NIR. In the visible region, there were the absorption bands assigned to the 4f electronic transitions of Tb3+ ions. In the NIR region, at 1.84, 1.93, 2.06, and 2.46 μm, there were the 4f–4f transitions of Tb3+ ions from the 7F6 ground state to the 7F0, 7F1, 7F2, and 7F3 excited states, respectively20,40. It should be highlighted that the BGB-xTb glasses containing up to 8 mol% Tb4O7 presented optical windows from 0.5 μm up to 1.60 μm.
Figure 6c shows the refractive indices (n) for the BGB-xTb samples, in the visible and near-infrared regions, as a function of Tb4O7 content. It can be seen that increase of the Tb4O7 concentration resulted in a higher value of n, mainly due to the high polarizability of the Tb3+ ions. The maximum n obtained was 1.8457 at 532 nm, for the BGB-18Tb glass. Figure 6d shows the refractive indices as a function of wavelength. The curve profiles showed that decrease of n was associated with increase of the wavelength, indicative of chromatic dispersion41.
Luminescence analysis
Figure 7a,b shows the photoexcitation (PLE) and luminescence (PL) spectra of Tb3+ in the BGB-xTb glasses at room temperature. Figure 7a shows the PLE spectrum of the BGB-4Tb glass excited at 545 nm. Overlapping was observed of PLE bands in the UV–Vis region at 483, 375, 370, 357, 350, 339, 325, 316, 301, and 284 nm, corresponding to 4f8 → 4f8 electronic transitions from the 7F6 ground state to the labeled excited states42. The most intense band at 375 nm, assigned to the 7F6 → 5D3 transition, was used as the excitation wavelength for acquisition of the emission spectra shown in Fig. 7b.
Four emission bands at 487, 541, 582, and 620 nm were assigned to the transitions from 5D4 to 7Fj (j = 6,5,4,3) multiplet43. Comparison of all the PL bands of the Tb3+ ions in the BGB glasses showed the same spectral profile for all the samples, although the intensities of the PL bands differed, since strong quenching in BGB glasses is observed with increase of the Tb4O7 concentration. This fluorescence quenching is due to greater interaction between the RE ions present at higher concentrations, with shorter distances between neighboring ions (Tb3+–Tb3+) in the bulk glass44. The inset in Fig. 7b highlights the low intensity emission assigned to the electronic transition at 541 nm for the BGB-xTb glasses (x = 14, 16, and 18 mol% Tb4O7) in the range between 530 and 560 nm.
Supplementary Figure S3 shows the normalized luminescence decay curves for the 5D4 → 5F7 emission of Tb3+ ions for the BGB glasses containing x = 4, 6, 8, and 14 mol% Tb4O7, obtained by monitoring of the green emission at 545 nm, with excitation at 375 nm. The decay times for the the BGB-xTb (x = 16 and 18) glasses are not shown, due to the strong quenching. The decay curves presented a single exponential profile, described by I(t) = I0exp(− t/τ), where τ (in ms) is the lifetime. The graph inserted in Supplementary Fig. S3c shows the fluorescence decay time as a function of Tb4O7 concentration, where τ decreased with increase of the Tb3+ content from 1.38 ms (x = 4 Tb) to 0.175 ms (x = 14 Tb).
Magneto-optical properties and Verdet constant (VB)
In this work, BGB glasses containing high concentrations of Tb3+ ions showed accentuated Faraday rotation effects in the visible and NIR regions. The theory underlying the Faraday effect in glasses is based on the Zeeman effect when the material is submitted to a magnetic field9,11. The magnitude of the Faraday effect in a magneto-optical material is evaluated by calculation of the Verdet constant (VB)9.
Figure 8a shows the set of transparent BGB-xTb bulk glasses and the magnetic attraction of the BGB-18Tb glass using a commercial neodymium-based magnet (N42 grade). This interesting attraction phenomenon is a qualitative way to demonstrate the paramagnetic properties of BGB-xTb bulk glasses. All the glasses studied here could be lifted using the Nd magnet. A video showing the attraction effect is provided with the online version of the manuscript.
Figure 8b,c shows the VB values as a function of the concentration of Tb4O7 (in mol%) and the Tb3+ ion density (in 1021 ions cm−3) for all the glasses, at different wavelengths. The increase of the VB values with the Tb4O7 content is shown in Fig. 8b. The VB values obtained were negative, indicating right-handed rotation of the polarized light, characteristic of paramagnetic materials.
In studies of the MO properties of RE-doped glasses, the VB values are generally expressed as a function of the RE ion density (\(N_{RE ion}\)). In this work, the Tb3+ ion densities \((N_{{Tb^{3 + } }} )\) for all glasses were calculated using Eq. (1) and are shown in Table 1.
Progressive increase of the Tb3+ content led to improvement of the magneto-optical properties of the glasses (Fig. 8c), as confirmed by increase of the VB values. At 500 nm, VB for the BGB-8Tb glass was − 111.5 rad T−1 m−1, while the VB values at 650 nm for the BGB-4 Tb and BGB-18Tb glasses were − 43.0 and − 128.0 rad T−1 m−1, respectively. At 650 nm, the BGB-18Tb glass had the highest VB value among all the glasses studied in this work. The observed values were consistent with those reported in the literature for other borogermanate glasses1,22. In addition, comparison of the VB values for the BGB-18Tb glass and the TGG reference, at 650 nm, showed that the VB of the glass was only 2.3% higher than the value for TGG (VB = − 125 rad T−1 m−1)19.
The MO effect of glasses containing high Tb3+ contents is due to the unfilled 4f electron layer of Tb atoms, since the unpaired 4f electrons generate random magnetic moments, consequently inducing a strong paramagnetic effect. In other words, for Tb3+, the high magnetic moment and the paramagnetic effect are produced from 4f → 4fn−1 5d energy level transitions11,45. In general terms, the Verdet constant for MO glass can be described by the sum of the contributions of paramagnetic and diamagnetic components, according to Eq. (3)13:
where, Vparamag and Vdiamag are the Verdet constants for the paramagnetic and diamagnetic contributions. Therefore, when Vparamag is higher than Vdiamag, the MO glass is predominantly paramagnetic. As discussed before, a useful way to increase the Vparamag component in a MO glass is by adding paramagnetic species such as Tb3+, Dy3+, or Mn2+ ions1,7,46. The proportional inverse relationship between Vparamag and wavelength can be expressed as shown in Eq. (4)13:
where, A, λ, and λt are approximation parameters and are given as the incident light and the effective transition wavelengths, respectively13. Figure 8d shows the inverse relation between VB and wavelength for all the BGB-xTb samples, as described by Eq. (4).
The Fig. 8d inset shows the relationship between 1/VB and \(\lambda^{2} \) for the BGB-18Tb glass data. The value of \(\lambda_{t}\) was obtained as the intersection of the straight line on the x-axis (\(\lambda^{2} )\), obtained from linear fitting of 1/VB vs. \(\lambda^{2}\). In this case, the value of the effective transition wavelength (\(\lambda_{t}\)) for the BGB-18Tb glass was 228 nm, which was close to the 4f8 ↔ 4f7 5d electron energy level transition of Tb3+, specifically the 7F6–7D5 level transition between 220 and 250 nm47. The \(\lambda_{t}\) value of 228 nm for the BGB-18Tb glass was similar to values reported for other glass systems such as fluorophosphate (~ 217 nm)48, borogermanate (225–300 nm)1, sodium borate (~ 220 nm)49, Tb3+-doped phosphate (~ 250 nm)50, aluminoborate (~ 250 nm)51, and borosilicate (~ 259–280 nm)52.
The main wavelengths for applications of MO glasses are in the infrared range, between 1.05 and 1.33 µm53. Figure 8d shows the VB values obtained for all the glasses at 1.03, 1.33, and 1.55 μm. It should be highlighted that the maximum VB value at 1550 nm (in the telecommunications range) was − 17.6 rad T−1 m−1, which was 37-fold higher than the VB of silica glass (~ 0.471 rad T−1 m−1)54.
For practical purposes, the absorption of the glass in the spectral region employed should be minimized. As observed in this work, the optical window diminishes as a function of the terbium content, mainly due to the oxidation of Tb3+ to Tb4+, which occurs at high temperature. However, this problem can be mitigated by the addition of reducing agents such as Ce2O3, as shown in Supplementary Fig. S4, which allow broadening of the optical window in the visible range. As can be seen, the addition of 0.5 mol% Ce2O3 was sufficient to maintain the reduced conditions necessary to avoid oxidation of Tb3+ to Tb4+, without significantly affecting other thermal and structural properties. With this approach, it was possible to shift the absorption band from 0.75 to 0.55 µm and obtain a glass that was light yellow in color, rather than dark brown, as shown in the inset in Supplementary Fig. S4.
Fabrication of magneto-optical glass fiber
Figure 9a,b shows photographs of the polished glass preform and the optical fiber obtained by applying the drawing process to the BGB-8Tb glass. Among all the BGB glasses analyzed, the BGB-8Tb glass presented the highest ΔT, so for this reason it was selected for production of the magneto-optical fiber. The BGB-8Tb fiber was coated with poly(methyl methacrylate) (PMMA) and the length of the fiber obtained was around 50 m (Fig. 9b).
Figure 9c shows an SEM cross-section image of the optical fiber with diameter of around 237 μm. The refractive index of the BGB-8Tb fiber was 1.7514, measured at 532 nm. This value was the same as obtained for the corresponding bulk sample.
Figure 9d,e shows photographs of the preform after the drawing process and the surface of the optical fiber, respectively. In neither case was there any evidence of crystallization on the surface after the drawing process.
Magneto-optical and optical fiber characterizations
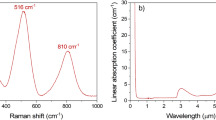
Figure 10a shows the VB values for the BGB-8Tb fiber according to wavelength. The VB values obtained in the visible range at 500 and 650 nm were − 110.2 and 58.8 rad T−1 m−1, respectively. In the NIR range, the VB values were − 32.2, − 22.8, − 15.2, and − 9.5 rad T−1 m−1 at 880, 1050, 1330, and 1550 nm, respectively. Comparison with VB values at 650–660 nm reported in the literature showed that the VB values for the BGB-8Tb fiber were higher than for Eu3+-doped silica11 (− 4.564 rad T−1 m−1), Er3+-doped silica (EDF)11 (− 3.379 rad T−1 m−1), Ho3+-doped silica23 (− 23.6 rad T−1 m−1), and Tb3+-doped borogermanate20 (− 23.6 rad T−1 m−1). However, at 1053 nm, VB obtained here was lower than for silicate fibers containing 56 and 65 wt% Tb3+ ions, for which the values were − 24 and − 32 rad T−1 m−1, respectively17,24. It should be highlighted that the VB values for the BGB-8Tb fiber at 1050 and 1550 nm (− 22.8 and − 9.5 rad T−1 m−1, respectively) were nineteen and sixteen times higher than for a single-mode optical fiber (SMF) (VB ~ 0.589 rad T−1 m−1 at 1550 nm)17,24.
Figure 10b shows the attenuation spectrum of the BGB-8Tb fiber, revealing two main optical losses in the ranges 350–500 nm and 1400–1750 nm. As observed in the transmission spectra for the BGB-xTb bulk glasses (Fig. 6b), there were intrinsic absorptions in UV–Vis–NIR regions, attributed to the Tb3+ ions. Using the cut-back method (from 1.92 m to 0.21 cm fiber length), the minimum attenuation of 6.4 dB m−1 was obtained at around 880 nm (Fig. 10b). The main sources of fiber attenuation include the glass preform preparation process, traces of impurities, water absorption, glass striae, and fiber imperfections.
Among the optical fibers reported in the literature, pure silica fibers are known to provide high performance, due to low attenuation in the NIR region55. On the other hand, silica fibers have very low VB values in the NIR region11. For example, VB of ~ 2.05 rad T−1 m−1 was found for an SMF at 830 nm56. In this work, the BGB-8Tb fiber presented VB of − 32.2 rad T−1 m−1 at 880 nm, which was around 15-fold higher than obtained for the SMF56. In magneto-optical terms, the BGB-8Tb fiber has good potential for application in the so-called “first optical window” at around 820–900 nm, given its high VB value at 880 nm57.
Conclusions
This work reports the synthesis of transparent Tb3+-borogermanate MO glasses using the traditional melt-quenching method. The thermal, structural, morphological, spectroscopic, optical, and magnetic-optical properties of the glasses were investigated. Structural changes in the glass network, following the addition of Tb4O7, were confirmed by Raman spectra of the BGB-xTb glasses, showing the presence of vibrations assigned to non-bridging oxygen bonds, such as Ge–O− in Q2 and Q3 species, and B–O−. The morphological analyses showed that at high Tb3+ content, the BGB-xTb system was free of nanocrystals. The absence of crystals and high thermal stability of the glass containing 8 mol% of Tb4O7 (305 °C) allowed the production of an MO fiber. The magnitude of the Faraday effect in the BGB-xTb glasses was evaluated from the VB values in the visible and NIR regions. Here, it should be emphasized that the VB values for the BGB-xTb glasses were investigated in the NIR region, between 880 and 1550 nm. An important finding was that the maximum VB value at 1550 nm was − 17.6 rad T−1 m−1, which was 37 times higher than for silica glasses. The maximum Verdet constant value for the BGB-18Tb glass at 650 nm was − 128 rad T−1 m−1. For the BGB-8Tb optical fiber, VB at 1550 nm (telecommunications range) was − 9.5 rad T−1 m−1, which was 16 times higher than VB for silica glass. The lowest optical loss of 10 dB m−1 and VB of − 32.2 rad T−1 m−1 were measured at 880 nm. In summary, the BGB-xTb system provides a set of MO glasses with potential to produce Faraday rotator fibers.
Methods
Synthesis
Tb3-doped borogermanate glasses were prepared by the conventional melt-quenching method, using chemical-grade germanium oxide GeO2 (Sigma-Aldrich), boric acid (H3BO3, Sigma-Aldrich), aluminum oxide (Al2O3, Sigma-Aldrich), sodium carbonate (Na2CO3, Sigma-Aldrich), barium carbonate (BaCO3, Sigma-Aldrich), and terbium oxide (Tb4O7, Sigma-Aldrich).
The chemicals were stoichiometrically weighed to yield 10 g of a glass with molar composition of (100 − x)(41GeO2–25B2O3–4Al2O3–10Na2O–20BaO) – xTb4O7 (BGB–xTb), where x = 0, 4, 6, 8, 14, 16, and 18 mol%. The samples were labeled as BGB-xTb. All the compositions are summarized in Table 1.
In the first step, vitreous boron oxide was obtained by thermal decomposition of H3BO3 at 500 °C for 30 min in a resistive furnace. The glass components were ground to fine powder and homogenized in an agate mortar. Batches were loaded into a platinum crucible and melted at between 1350 and 1500 °C (depending on the Tb4O7 content), for 2 h, under atmospheric conditions. The melt was cooled in a preheated stainless steel mold at 30 °C, below the glass transition temperature (Tg), and then annealed at the same temperature for 3 h, to minimize its mechanical stress, followed by slowly cooling to room temperature during 12 h. Pieces with thickness of 3 mm were obtained. As a final step, the samples were polished using silicon carbide (SiC) polishing papers, prior to the optical characterizations.
The glass preform based on the composition 92(41GeO2–25B2O3–4Al2O3–10Na2O–20BaO)–8Tb4O7 (BGB-8Tb) was selected for use in the fiber drawing process, because it showed the highest ΔT value (305 °C), among the set of glasses analyzed. A glass rod was prepared by melt-quenching, using a 30 g batch of glass. The batch was melted in a platinum crucible tube, at 1450 °C for 1 h, under an atmosphere of N2 at a flow rate of 30 mL min−1, in an induction furnace.
The cylindrical stainless steel mold was pre-heated at 555 °C (50 °C below Tg) for 2 h before the melt-quenching process, in order to ensure an even temperature. The dimensions of the mold were 10 cm long and 10 mm diameter. The rod preform was annealed for 6 h at 555 °C and cooled at a rate of 0.5 °C min−1, requiring 17.5 h to reach room temperature. To minimize the structural stress of the BGB-8Tb preform, a second annealing process was performed under the same conditions used previously. Glass preforms containing high concentrations of RE oxides generally present high structural stress, requiring a long annealing time and a slow cooling rate. After the cooling process, the preform was polished in several steps using SiC papers (600–1200 grit).
In the final step, the preform was mounted into the drawing tower and the fiber drawing process was started at 720 °C (Tg + 115 °C). During the drawing process, the BGB-8Tb fiber was coated with a low-index UV-cured poly(methyl methacrylate) (PMMA) polymer, in order to protect the magneto-optical fiber and improve its mechanical properties.
Measurements and characterizations
Differential scanning calorimetry (DSC) measurements of the BGB-xTb glasses were performed using a Netzsch DSC Pegasus 404F3 apparatus. For this, the glass sample (10 mg) was placed in a platinum crucible and heated from 25 to 1000 °C, at a rate of 10 °C min−1, under an atmosphere of nitrogen (20 mL min−1). The maximum errors were ± 2 °C for Tg and Tx, and ± 4 °C for ΔT.
Powder X-ray diffraction measurements were carried out with a Panalytical Aries benchtop diffractometer operating with a Cu Kα radiation source. Scanning was performed in the 2θ range from 10° to 80°, with step size of 0.01° and step time of 2 s.
Raman spectra were recorded at room temperature, in the frequency range from 100 to 1800 cm−1, using a Renishaw inVia Micro-Raman spectrometer equipped with a 633 nm laser delivering 17 mW, resolution of ± 1 cm−1 coupled with a Leica DM2700 microscope.
HRTEM images and SAED patterns for the BGB-18Tb glass were obtained using an FEI Tecnai G2 F20 (200 kV) transmission electron microscope equipped with a field emission gun, coupled with an energy dispersive spectroscopy (EDS) microanalysis system. For the analysis, the BGB-18Tb glass was finely powdered, suspended in ethanol, and deposited onto a copper grid.
Density measurements were performed with a Mettler Toledo Excellence XS densimeter. The measurement precision was ± 0.002 g cm−3.
Optical absorption and transmission spectra of the BGB-xTb glasses were obtained using a Varian Cary 500 dual-beam UV–Vis–NIR spectrophotometer, in the ranges from 200 to 800 nm and from 0.25 to 3 μm, respectively. Linear refractive indexes for the BGB samples were determined at 532, 633, 972, 1038, and 1538 nm by the prism coupling technique, using a Metricon 2010M-Lines instrument, with precision of ± 0.0001.
Excitation, emission, and photoluminescence decay curves were obtained using a Fluorolog near-infrared photomultiplier tube system (NIR-PMT) (Horiba Jobin Yvon) equipped with a xenon lamp (200–900 nm). The PL measurements were performed with bulk samples, at room temperature.
Faraday rotation measurements
Faraday rotation values of the BGB-xTb glasses were obtained at room temperature, using a neodymium magnet with a total magnetic field (B) of 0.46 T. For the Faraday rotation angle (θ), a standardized sample length (l) of 1.9 cm was used. The faces of all the samples were polished to obtain flat surfaces. The Faraday rotation angles were measured at 500, 650, 880, 1030, 1308, and 1550 nm, using a SuperK COMPACT supercontinuum laser (NKT Photonics) with spectral range from 450 to 2400 nm, power of 100 mW, and operating temperature range of 15–30 °C. The laser beam was focused on the BGB-xTb sample and the polarized light transmitted through the glass was measured in quadruplicate using a graduated polarizer with a precision of ± 2° (± 0.035 rad).
The output beam was detected at 500, 650, 880, and 1050 nm, using a PM100D Handheld Optical Power and Energy Meter (Thorlabs), and at 1330 and 1550 nm, using a PDA015C InGaAs Fixed Gain Amplified Detector (Thorlabs) connected to a Model 2512 100 MHz 1 GSa/s Handheld Digital Storage Oscilloscope (BK Precision).
Similarly, Faraday rotation angle measurements for the BGB-8Tb fiber were performed at 500, 650, 880, 1050, 1330, and 1550 nm. The input and output fibers were cleaved using a 24X0-RCL cleaving machine and the optical path length (l) was 2 cm. The fiber was inserted in a holder and a 40 × objective lens was used to focus the laser onto the fiber section. The Faraday rotation angles were measured in triplicate, using a polarizer with precision of ± 2°. The Verdet constant values (VB, rad T−1 m−1) were obtained from the Faraday rotation (Eq. 5).
Optical fiber characterization
The cut-back fiber loss method was used to measure the optical attenuation of the BGB-8Tb fiber in the range from 350 to 1750 nm. The attenuation spectra were acquired using an optical spectrum analyzer (OSA) (Model AQ-6315A, Yokogawa) with wavelength resolution of 5 nm. To obtain flat surfaces, the input and output of the BGB-8Tb fiber were cleaved using a 24X0-RCL cleaving machine, after which the fiber was clamped into two SubMiniature version A (SMA) adaptors. For broadband measurement, the input fiber was clamped at the tungsten-halogen lamp housing and the output was connected to an OSA instrument. The cutback measurements were performed from the initial fiber length of 1.92 m to a final length of 21.8 cm. The output fiber was cut into different lengths using the cleaving machine and the output power was measured for each length, in order to obtain more accurate transmission losses data.
Data availability
All data regarding the work presented here are available upon reasonable request to the corresponding authors.
References
Gao, G. et al. Faraday rotation and photoluminescence in heavily Tb3+-doped GeO2–B2O3–Al2O3–Ga2O3 glasses for fiber-integrated magneto-optics. Sci. Rep. 5, 1–6. https://doi.org/10.1038/srep08942 (2015).
Qiu, X., Wu, B., Liu, Y. & Wen, F. Study on mode coupling characteristics of multimode magneto-optical fibers. Opt. Commun. 456, 124707. https://doi.org/10.1016/j.optcom.2019.124707 (2020).
Valeanu, M. et al. The relationship between magnetism and magneto-optical effects in rare earth doped aluminophosphate glasses. J. Phys. D Appl. Phys. 49(7), 075001. https://doi.org/10.1088/0022-3727/49/7/075001 (2016).
Kruk, A. et al. Transparent yttrium oxide ceramics as potential optical isolator materials. Opt. Appl. 45(4), 585–594. https://doi.org/10.5277/oa150413 (2015).
Chen, Q., Wang, H. & Chen, Q. Structural and properties of heavy metal oxide Faraday glass for optical current transducer. J. Non-Cryst. Solids 429, 13–19. https://doi.org/10.1016/j.jnoncrysol.2015.08.031 (2015).
Babkina, A. et al. Terbium concentration effect on magneto-optical properties of ternary phosphate glass. Opt. Mater. 100, 109692. https://doi.org/10.1016/j.optmat.2020.109692 (2020).
Mollaee, M. et al. Magneto-optical properties of highly Dy3+ doped multicomponent glasses. Opt. Express 28(8), 11789. https://doi.org/10.1364/oe.392008 (2020).
Fernandes, R. G. et al. Thermal and structural modification in transparent and magnetic germanoborate glasses induced by Gd2O3. Ceram. Int. 46, 22079–22089. https://doi.org/10.1016/j.ceramint.2020.05.227 (2020).
Yamane, M. & Asahara, Y. Cambridge University Press 1st Ed. 1, 241–263. https://doi.org/https://doi.org/10.1017/S0263034601224148 (2000).
Chen, Q. Optical linear & nonlinearity and Faraday rotation study on V2O5 nanorod doped glass and glass-ceramic: Impact of optical basicity. J. Alloys Compd. 828, 154448. https://doi.org/10.1016/j.jallcom.2020.155490 (2020).
Huang, Y. et al. Fabrication of europium-doped silica optical fiber with high Verdet constant. Opt. Express 24, 18709–18717. https://doi.org/10.1364/oe.24.018709 (2016).
Suzuki, F. et al. Large Faraday effect of borate glasses with high Tb3+ content prepared by containerless processing. Opt. Mater. 76, 174–177. https://doi.org/10.1016/j.optmat.2017.12.031 (2018).
Yin, H. et al. Effect of B2O3 content and microstructure on Verdet constant of Tb2O3-doped GBSG magneto-optical glass. Ceram. Int. 44(9), 10929–10933. https://doi.org/10.1021/acs.jpcc.8b04989 (2018).
Linganna, K., Ju, S., Basavapoornima, C., Venkatramu, V. & Jayasankar, C. K. Development of aluminosilicate glass fiber doped with high Pr3+ concentration for all-optical fiber isolator application. J. Asian Ceram. Soc. 6(1), 82–87. https://doi.org/10.1007/s10854-019-01644-y (2018).
Guo, Q. et al. Magneto-optical properties and measurement of the novel doping silica optical fibers (2018). IMEKO 127, 63–67. https://doi.org/10.1016/j.measurement.2018.05.032 (2018).
Ju, S. et al. Temperature and vibration dependence of the faraday effect of Gd2O3 NPs-doped alumino-silicate glass optical fiber. Sensors 18(4), 1–13. https://doi.org/10.3390/s18040988 (2018).
Sun, L., Jiang, S., Zuegel, J. D. & Marciante, J. R. All-fiber optical isolator based on Faraday rotation in highly terbium-doped fiber. Opt. Lett. 35, 706–708. https://doi.org/10.1364/OL.35.000706 (2010).
Bellanger, B., Ledemi, Y. & Messaddeq, Y. Fluorophosphate glasses with high terbium content for magneto-optical applications. J. Phys. Chem. C 124(9), 5353–5362. https://doi.org/10.1021/acs.jpcc.9b11696 (2020).
Jin, W. et al. Growth and performance research of Tb3Ga5O12 magneto-optical crystal. J. Cryst. Growth 484, 17–20. https://doi.org/10.1016/j.jcrysgro.2017.12.024 (2018).
Franco, D. F. et al. Fundamental studies of magneto-optical borogermanate glasses and derived optical fibers containing Tb3+. J. Mater. Res. Technol 11, 312–327. https://doi.org/10.1016/j.jmrt.2021.01.010 (2021).
Guo, H. et al. Optical band gap and photoluminescence in heavily Tb3+ doped GeO2–B2O3–SiO2–Ga2O3 magneto-optical glasses. J. Alloys Compd. 686, 635–640. https://doi.org/10.1016/j.jallcom.2016.06.074 (2016).
Savinkov, V. I. et al. Borogermanate glasses with a high terbium oxide content. J. Non-Cryst. Solids 356(33–34), 1655–1659. https://doi.org/10.1016/j.jnoncrysol.2010.06.011 (2010).
Liu, Z. et al. Study of the Verdet constant of the holmium-doped silica fiber. OSA Continuum 3, 1096–1104. https://doi.org/10.1364/OSAC.390111 (2020).
Sun, L., Jiang, S. & Marciante, J. R. Compact all-fiber optical Faraday components using 65-wt%-terbium–doped fiber with a record Verdet constant of −32 rad/(T.m). Opt. Express 18, 12191–12196. https://doi.org/10.1364/oe.18.012191 (2010).
Franco, D. F. et al. Structural study of the germanium–aluminum–borate glasses by solid state NMR and Raman spectroscopies. J. Phys. Chem. C 124(24460–24469), 2020. https://doi.org/10.1021/acs.jpcc.0c07810 (2020).
Duffy, J. A. A review of optical basicity and its applications to oxidic systems. Geochim. Cosmochim. Acta 57(16), 3961–3970. https://doi.org/10.1016/0016-7037(93)90346-X (1993).
Duffy, J. A. Optical basicity: A practical acid-base theory for oxides and oxyanions. J. Chem. Educ. 73(12), 1138–1142. https://doi.org/10.1021/ed073p1138 (1996).
Liu, S. et al. Reduction of Eu3+ to Eu2+ in aluminoborosilicate glasses prepared in air. J. Am. Ceram. 91(8), 2740–2742. https://doi.org/10.1111/j.1551-2916.2008.02496.x (2008).
Sontakke, A. D. & Annapurna, K. Study on Tb3+-containing high silica and low silica calcium aluminate glasses: Impact of optical basicity. Spectrochim. Acta A 94, 180–185. https://doi.org/10.1016/j.saa.2012.03.052 (2012).
Henderson, G. S., Neuville, D. R., Cochain, B. & Cormier, L. The structure of GeO2–SiO2 glasses and melts: A Raman spectroscopy study. J. Non-Cryst. Solids 355, 468–474. https://doi.org/10.1016/j.jnoncrysol.2009.01.024 (2009).
Micoulaut, M., Cormier, L. & Henderson, G. S. The structure of amorphous, crystalline and liquid GeO2. J. Phys. Condens. Matter 18, R753. https://doi.org/10.1088/0953-8984/18/45/R01 (2006).
Kamitsos, E. I., Yiannopoulos, Y. D., Karakassides, M. A., Chryssikos, G. D. & Jain, H. Raman and infrared structural investigation of xRb2O·(1–x)GeO2 glasses. J. Phys. Chem. 100, 11755–11765. https://doi.org/10.1021/jp960434 (1996).
Koroleva, O. N., Shtenberg, M. V. & Ivanova, T. N. The structure of potassium germanate glasses as revealed by Raman and IR spectroscopy. J. Non-Cryst. Solids 510, 143–150. https://doi.org/10.1016/j.jnoncrysol.2019.01.017 (2019).
Koroleva, O. N. & Osipov, A. A. In situ Raman spectroscopy of K2O–GeO2 melts. J. Non-Cryst. Solids 531, 1–6. https://doi.org/10.1016/j.jnoncrysol.2019.119850 (2020).
Koroleva, O. N., Shtenberg, M. V., Zainullina, R. T. & Lebedeva, S. M. Vibrational spectroscopy and density of K2O–B2O3–GeO2 glasses with variable B/Ge ratio. Phys. Chem. Chem. Phys. 21, 12676–12684. https://doi.org/10.1039/c9cp01374a (2019).
Yano, T., Kunimine, N., Shibata, S. & Yamane, M. Structural investigation of sodium borate glasses and melts by Raman spectroscopy. I. Quantitative evaluation of structural units. J. Non-Cryst. Solids 321(3), 137–146. https://doi.org/10.1016/S0022-3093(03)00158-3 (2003).
Verweij, H. & Buster, J. H. J. M. The structure of lithium, sodium and potassium germanate glasses, studied by Raman scattering. J. Non-Cryst. Solids 34, 81–99. https://doi.org/10.1016/0022-3093(79)90008-5 (1979).
Jaba, N., Mermet, A., Duval, E., Champagnon, B. (2005). Raman spectroscopy studies of Er3+-doped zinc tellurite glasses. J. Non-Cryst. Solids. 351(10–11), 833–837. https://doi.org/https://doi.org/10.1016/j.jnoncrysol.2005.02.003 (2005).
Wan, X., Zhong, Q., Tie, S. L. & Shen, J. Y. Synthesis and luminescence properties of Tb3+activated CaO–Al2O3–B2O3 glass. Optoelectron. Adv. Mater. 5(5), 538–544 (2011).
Sontakke, A. D., Biswas, K. & Annapurna, K. Concentration-dependent luminescence of Tb3+ ions in high calcium aluminosilicate glasses. J. Lumin. 129(11), 1347–1355. https://doi.org/10.1016/j.jlumin.2009.06.027 (2009).
Ghosh, G. Sellmeier coefficients and dispersion of thermo-optic coefficients for some optical glasses. Appl. Opt. 36, 1540–1546. https://doi.org/10.1364/AO.36.001540 (1997).
Juárez-Batalla, J., Meza-Rocha, A. N., Muñoz, G. H., Camarillo, I. & Caldiño, U. Luminescence properties of Tb3+ -doped zinc phosphate glasses for green laser application. Opt. Mater. 58, 406–411. https://doi.org/10.1016/j.optmat.2016.06.022 (2016).
Sharma, S., De, M. & Jana, S. Terbium doped sodium phosphate glass: A strong green emitting glass. Optik 154, 576–580. https://doi.org/10.1016/j.ijleo.2017.10.068 (2018).
Zur, L., Sołtys, M., Pisarska, J. & Pisarski, W. A. Absorption and luminescence properties of terbium ions in heavy metal glasses. J. Alloys Compd. 578, 512–516. https://doi.org/10.1016/j.jallcom.2013.07.021 (2013).
Chen, Q., Chen, Q., Wang, H., Wang, G. & Yin, S. Magneto optical properties of rare earth Tb2O3 doped PbO–Bi2O3–B2O3 glass. J. Non-Cryst. Solids 470, 99–107. https://doi.org/10.1016/j.bsecv.2016.07.002 (2017).
Panmand, R. P. et al. Characterisation of spectroscopic and magneto-optical faraday rotation in Mn2+-doped CdS quantum dots in a silicate glass. J. Alloys Compd. 817, 152696. https://doi.org/10.1016/j.jallcom.2019.152696 (2020).
Dongbing, H., Chunlei, Y., Jimeng, C., Shunguang, L. & Lili, H. Energy transfer between Gd3+ and Tb3+ in phosphate glass. J. Rare Earth 29, 48–51. https://doi.org/10.1016/S1002-0721(10)60392-4 (2011).
Letellier, V., Seignac, A., Le Floch, A. & Matecki, M. Magneto-optical properties of heavily rare-earth doped non-crystalline fluorophosphates. J. Non-Cryst. Solids 111, 55–62. https://doi.org/10.1016/0022-3093(89)90423-7 (1989).
Qiu, J., Tanaka, K., Sugimoto, N. & Hirao, K. Faraday effect in Tb3+-containing borate, fluoride and fluorophosphate glasses. J. Non-Cryst. Solids 213–214, 193–198. https://doi.org/10.1016/S0022-3093(97)00101-4 (1997).
Berger, S. B., Rubinstein, C. B., Kurkjian, C. R. & Treptow, A. W. Faraday rotation of rare-earth (III) phosphate glasses. Phys. Rev. 133, A723–A727. https://doi.org/10.1103/PhysRev.133.A723 (1964).
Petrovskii, G. T. et al. Faraday effect and spectral properties of high-concentrated rare earth oxide glasses in visible and near UV region. J. Non-Cryst. Solids 130, 35–40. https://doi.org/10.1016/0022-3093(91)90153-W (1991).
Yuan, S. H. & Shu, X. Z. A new Faraday rotation glass with a large Verdet constant. J. Appl. Phys. 75, 6375–6377. https://doi.org/10.1063/1.355354 (1994).
Kumari, S. & Chakraborty, S. Study of different magneto-optic materials for current sensing applications. J. Sens. Sens. Syst. 7, 421–431. https://doi.org/10.5194/jsss-7-421-2018 (2018).
Ruan, Y., Jarvis, R. A., Rode, A. V., Madden, S. & Luther-Davies, B. Wavelength dispersion of Verdet constants in chalcogenide glasses for magneto-optical waveguide devices. Opt. Commun. 252, 39–45. https://doi.org/10.1016/j.optcom.2005.03.037 (2005).
Kanamori, H. et al. Transmission characteristics and reliability of pure- silica-core single-mode fibers. S. J. Light. Technol 4(8), 1144–1150. https://doi.org/10.1109/JLT.1986.1074837 (1986).
Smith, A. M. Faraday effect in single-mode optical fiber using an injection laser light source. Electron. Lett. 16, 206–208. https://doi.org/10.1049/el:19800148 (1980).
Harrison, M. T., Kershaw, S. V. & Weller, H. Colloidal nanocrystals for telecommunications. Complete coverage of the low-loss fiber windows by mercury telluride quantum dots. Pure Appl. Chem. 72, 295–307. https://doi.org/10.1351/pac200072010295 (2014).
Acknowledgements
The authors are grateful to the São Paulo State Research Foundation (FAPESP, grants 2016/16900-9, 2018/19272-4, and 2013/07793-6) for financial support, and to the Centre d’optique, Photonique et laser (COPL) at Université Laval (Canada).
Author information
Authors and Affiliations
Contributions
D.F.F. wrote the paper and synthesized the glass samples and the optical fiber and carried out the DSC, XRD, Raman, UV–Vis, M-Lines, Photoluminescence, Attenuation and Magneto-optical measurements. The photographs in Figs. 1, 7b, 8a, 9 and Supplementary Fig. S4 were taken by D.F.F. Y.L. participated in the synthesis of the glass samples and the PL measurements. W.C. participated in the SEM and magneto-optical measurements. S.M. was responsible for the fiber drawing process. C.R.M.A. has carried out the morphological characterization (HRTEM). S.M. participated in the review of the manuscript and contributed to the discussion of data. Y.M. supervised the preparation and characterization of the glass samples and optical fiber and contributed to the discussion of data. M.N. is the supervisor of the project, participated in the writing of the manuscript and contributed to the discussion of data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franco, D.F., Ledemi, Y., Correr, W. et al. Magneto-optical borogermanate glasses and fibers containing Tb3+. Sci Rep 11, 9906 (2021). https://doi.org/10.1038/s41598-021-89375-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89375-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.