Abstract
The role of manganese dioxide (MnO2) in the process of water treatment using metallic iron (Fe0/H2O) was investigated in quiescent batch experiments for t ≤ 60 d. MnO2 was used as an agent to control the availability of solid iron corrosion products (FeCPs) while methylene blue (MB) was an indicator of reactivity. The investigated systems were: (1) Fe0, (2) MnO2, (3) sand, (4) Fe0/sand, (5) Fe0/MnO2, and (6) Fe0/sand/MnO2. The experiments were performed in test tubes each containing 22.0 mL of MB (10 mg L−1) and the solid aggregates. The initial pH value was 8.2. Each system was characterized for the final concentration of H+, Fe, and MB. Results show no detectable level of dissolved iron after 47 days. Final pH values varied from 7.4 to 9.8. The MB discoloration efficiency varies from 40 to 80% as the MnO2 loading increases from 2.3 to 45 g L−1. MB discoloration is only quantitative when the operational fixation capacity of MnO2 for Fe2+ was exhausted. This corresponds to the event where adsorption and co-precipitation with FeCPs is intensive. Adsorption and co-precipitation are thus the fundamental mechanisms of decontamination in Fe0/H2O systems. Hybrid Fe0/MnO2 systems are potential candidates for the design of more sustainable Fe0 filters.
Similar content being viewed by others
Introduction
Water pollution has a significant negative impacts on ecological and human health. These impacts are expected to substantially increase in the coming decades due to: (1) increased urbanization, (2) increased industrialization, and (3) climate change1,2,3. The low-income communities who are spatially scattered, and economically disadvantaged are most impacted by water pollution or lack of safe drinking water2,3,4. Thus, low-income communities need affordable, efficient and applicable technologies for their drinking water supply. Water filtration using sand filters amended with metallic iron (Fe0 filters) has been demonstrated to be such an affordable and applicable technology5,6,7,8,9,10,11.
The use of Fe0 for environmental remediation and water treatment has boomed over the past 30 years12,13,14,15,16,17,18,19,20,21. Fe0 is mainly regarded as an environmentally friendly reducing agent (E0 = − 0.44 V)12,20,21. It has been successfully applied for the removal of a myriad of environmental pollutants from groundwater and wastewater12,15,20. Fe0 was initially used as micro-sized granular material12. Efforts to alleviate material passivation has prompted to develop granular bimetallics6,7,8, granular composites13,15, granular material mixtures (e.g. Fe0/Fe3O4, Fe0/FeS2, Fe0/MnO2)20,21, and their nano-sized counterparts16,17,18,19. There is increasing interest on using granular Fe0 for the design of decentralized drinking water supply units6,10. In particular, the ternary mixture Fe0/MnO2/sand seems very promising21.
Fe0 filters are a special case of "metal corrosion in porous media"22. This process has two key characteristics23,24: (1) the time-dependent decrease of the reaction kinetics of iron corrosion (“reactivity loss”), and (2) the progressive decrease of the hydraulic conductivity (permeability loss) due to the initial porosity being filled by in-situ generated iron oxides and hydroxides. In other words, the design of sustainable Fe0 filters should account for the long-term corrosion rate of the used Fe0 specimens. A mathematical modelling has enabled a spatial solution of the issue of permeability loss24,25. It is established that only hybrid Fe0 filters containing Fe0 and other aggregates (e.g. Fe0/Fe3O4, Fe0/gravel, Fe0/MnO2, Fe0/sand) are sustainable because non-expansive aggregates are not contributing to porosity/permeability loss24. Yet how fast the porosity decreases as a function of time is an open issue and has received very little attention26,27,28. However, it is known from the broad corrosion literature that the corrosion kinetics of metals, including Fe0 under environmental conditions, is neither constant nor linear22,29. Therefore, it is impossible to predict the service life of a Fe0 filter without more accurate data on the corrosion rate, which is material-dependent29,30. It is thus not surprising that all models presented for the prediction of the operation of Fe0-based permeable reactive barriers were not successful26,27,31. The present work is devoted at qualitatively characterizing the efficiency of Fe0/H2O systems for water treatment as influenced by the presence of MnO2. The methylene blue discoloration (MB method) developed in earlier studies is used21,31,32,33,34,35. In particular, MnO2 is used to control the availability of 'free' iron corrosion products (FeCPs).
Since the seminal work of Bischof5, Fe0, gravel/sand and MnO2 have been used in Fe0 filters. Fe0 is electrochemically oxidized by water to generate Fe2+ and H2 (Eq. 1). The original device of Bischof contained a layer of pyrulosite (a MnO2 mineral) after the Fe0/gravel layer5. Pyrulosite acts as a Fe2+ scavenger (Eq. 2) to lower the iron concentration in filtered water. Equation (2) represents the reductive dissolution of MnO2 (or MnOx) which can be regarded as a stand alone branch of environmental geochemistry36,37,38.
In other words, Fe2+ is oxidized at the surface of MnO2 and is ideally not transported out of the filter. It is obvious that the Fe2+ scavenging efficiency of the pyrulosite layer depends on the amount used and its intrinsic reactivity.
During the past two decades, MnO2 and Fe0 have been mixed to enhance the efficiency of Fe0/H2O systems for the removal of various contaminants, including uranium and radon39, diclofenac40,41, heavy metals42, methylene blue21,32,33,34,35,43, chromium44,45, arsenic46, and tetracycline47. In these efforts, Fe0/MnO2 composites were also used6,46,48,49 and enhanced contaminant removal explained by electrochemical cells between Fe0 and MnO2 like in some Mn-rich Fe0 specimens21,50,51. On the other hand, Dong et al.47 tested the sequence MnO2–Fe0 (MnO2 layer before Fe0) and also reported on enhanced tetracycline removal compared to the single-aggregate-systems (e.g., Fe0 and MnO2). Clearly, information rationalizing the positive impact of MnO2 on the efficiency of Fe0/H2O systems is confusing. The methylene blue method32,33,52 can help in elucidating the mechanisms of contaminant removal in Fe0/H2O systems.
Methylene blue (MB, a cationic dye) has been demonstrated to be an indicator of the reactivity of the Fe0/H2O system21,32,33,34,35,52. Its suitability is based on its cationic nature and its differential affinity to positively charged iron oxide surfaces and negatively charged surface of sand21,53. Using MB as a reactivity indicator has improved our knowledge on the Fe0/H2O system during the past decade35,43,52,54,55,56,57,58. In particular, Miyajima and Noubactep35 utilized the systems used in this study and reported on lowered MB discoloration in MnO2 amended Fe0/H2O systems for 14 d.
The objective of this study is to investigate the impact of various amounts of three different MnO2 on the efficiency of Fe0/H2O systems for MB discoloration. The specific objective is to confirm the suitability of ‘MB discoloration’ as powerful tool for the characterization of processes in Fe0/H2O systems while using MnO2 and sand to control the availability of ‘free’ FeCPs. The extent of MB discoloration is characterized using five different systems: (1) Fe0 alone, (2) sand alone, (3) Fe0/sand, (4) Fe0/MnO2, and (5) Fe0/MnO2/sand for up to 60 days. A comparison of the results from the five systems will provide critical information on the contaminant removal mechanisms and the role of MnO2.
Materials and methods
Solutions
The used methylene blue (MB – Basic Blue 9 from Merck) was of analytical grade. The working solution was 10.0 mg L−1 prepared by diluting a 1000 mg L−1 stock solution. The stock solution was prepared by dissolving accurately weighted MB in tap water. The use of tap water rather than deionised water was motivated by the fact that tap water is closer to natural water in its chemical composition. The MB molecular formula is C16H18N3SCl corresponding to a molecular weight of 319.85 g. MB was chosen in this study because of its well-known strong adsorption onto solids32,53.
Solid materials
Metallic iron (Fe0)
The used Fe0 material was purchased from iPutech (Rheinfelden, Germany). The material is available as filings with a particle size between 0.3 and 2.0 mm. Its elemental composition as specified by the supplier was: C: 3.52%; Si: 2.12%; Mn: 0.93%; Cr: 0.66% (balanced by Fe). The material was used without any further pre-treatment. Fe0 was proven as a powerful discoloration agent for MB given that discoloration agents in the form of FeCPs are progressively generated in-situ54,55.
Manganese dioxide (MnO2)
Three natural MnO2-bearing minerals were tested: (1) Manganit (Ilfeld/Harz; Thüringen/Germany), (2) x-MnO2 (mineral of unknown origin), and (3) Psilomelan (Minas Gerais – Brazil). The three samples were used to characterize the impact of differences in MnO2 intrinsic reactivity. Manganit was the quantitatively more abundant mineral available and was used in all experiments, while x-MnO2 and Psilomelan were used only in parallel comparative experiments. The natural minerals were crushed and fractionated by sieving. The fraction 0.5–1.0 mm was used without any further pre-treatment. No chemical, mineralogical nor structural characterizations were performed. MnO2 is a reactive mineral33,59 and is used to delay the availability of ‘free’ iron corrosion products (FeCPs) in the system. This results in a delay of quantitative MB discoloration28,35. Using three different natural MnO2 minerals intended to validate the premise that each material has it own intrinsic reactivity33.
Sand
The used sand was a commercial material for aviculture (“Papagaiensand” from RUT – Lehrte/Germany). The sand was used as received without any further pre-treatment. The particle size was between 2.0 and 4.0 mm. Sand was used as an adsorbent because of its worldwide availability and its use as admixing agent in Fe0 barriers60,61. The adsorption capacity of sand for MB has been systematically documented as early as in 1955 by Mitchell et al.53.
MB discoloration
Quiescent batch experiments (non-shaken) were conducted in assay tubes for experimental durations of up to 60 d. The batches consisted of 0.0 to 1.0 g of sand, 0.0 to 1.0 g of Fe0, 0.0 to 1.0 g of MnO2 and mixtures thereof in 22.0 mL of a 10.0 mg L–1 MB solution. The investigated systems were: (1) Fe0 alone, (2) sand alone, (3) MnO2 alone, (4) Fe0/sand, (5) Fe0/MnO2 and (6) Fe0/sand/MnO2. The efficiency of individual systems at discolouring MB was characterized at laboratory temperature (about 22° C). Initial pH was about 8.2. After equilibration, up to 3.0 mL of the supernatant solutions were carefully retrieved (no filtration) for MB measurements (no dilution). Each experiment was performed in triplicates, and averaged values are presented.
Three different MB discoloration experiments were conducted: (1) in Fe0/sand systems for 3 to 60 d; (2) in Fe0i/sand/MnO2 for 47 d (varying Fe0 loading); and (3) in Fe0/sand/MnO2i for 47 d (varying MnO2 loading). The subscripts ‘i’ refers to the aggregate which mass loading is varying (Fe0 or MnO2). Table 1 summarizes the aggregate content of the 7 Fe0/MnO2/sand systems investigated herein. The operational reference (blank experiment) is also added. Note that the pure Fe0 system (Fe0 alone) is regarded as a ‘Fe0/MnO2/sand system’ without MnO2 nor sand.
Analytical methods
Iron and MB aqueous concentrations were determined by a Cary 50 UV–Vis spectrophotometer (Varian). The working wavelengths for MB and iron were 664.5 and 510.0 nm, respectively. Cuvettes with 1.0 cm light path were used. The spectrophotometer was calibrated for Fe and MB concentrations ≤ 10.0 mg L−1. The pH value was measured by combined glass electrodes (WTW Co., Germany).
Expression of MB discoloration results (E value)
In order to characterize the magnitude of the tested systems for MB discoloration, the discoloration efficiency (E) was calculated (Eq. 3). After the determination of the residual MB concentration (C), the corresponding percent MB discoloration (E value) was calculated as:
where C0 is the initial aqueous MB concentration (ideally 10.0 mg L−1), while C gives the MB concentration after the experiment. The operational initial concentration (C0) for each case was acquired from a triplicate control experiment without additive material (so-called blank). This procedure was to account for experimental errors during dilution of the stock solution, MB adsorption onto the walls of the reaction vessels, and all other possible side reactions during the experiments.
Results and discussion
Evidence for chemical reactions
Triphasic MB discoloration in the Fe0/sand system
Figure 1a shows a triphasic pattern in the process of MB discoloration in the Fe0/sand system. The initial discoloration (up to day 15) is very rapid (phase A), followed by slower discoloration between days 16 and 35 (phase B), and a plateau for the rest of the experimental duration (d > 35) (phase C). It can be considered that after 35 days, a pseudo-equilibrium stage is achieved. This stage is characterized by the complete coverage of sand by FeCPs such that further MB discoloration solely results from adsorption and co-precipitation with free FeCPs (Table 2). Herein, “free” operationally characterizes (not quantifies) the fraction of FeCPs which precipitates after the sand surface is completely coated. This means that, if the impact of MnO2 on the Fe0/H2O should be characterized under the named operational conditions, experiments should last for more than 35 days. Based on this observation, the impact of MnO2 was investigated for an equilibration time of 47 days. Note that the absolute E values from the data in Fig. 1 and the rest of the work are not necessarily directly comparable because the experiments were not performed in parallel. Although the Fe0 material was from the same supplier, the reactivity of a Fe0 specimen also depends on it surface state, which depends on the storing conditions and the duration of storage21,56,57,62. The experiments yielding the results in Fig. 1a were performed more than 12 months18 after the other experiments35. The objective was to understand why results of MB discoloration experiments for 14 days35 and 47 days28 in ternary systems (Fe0/MnO2/sand) vary widely.
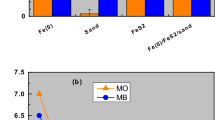
Comparison of the efficiency of tested materials for methylene blue (MB) discoloration: (a) for 0 to 60 days in Fe0/sand system, and (b) by the tested systems for 47 days. Experimental conditions: [Fe0] = 2.3 to 45 g L−1 in (a), 4.5 g L−1 in (b); [sand] = 45 g L−1; and [MnO2] = 2.3 g L−1 in (a) and 4.5 g L−1 in (b). The lines are not fitting functions, they simply connect points to facilitate visualization.
Figure 1b summarizes the extent of MB discoloration in the seven possible systems (Table 1): (1) three single-aggregates (Fe0, MnO2, sand), (2) three binary-aggregates (Fe0/sand, Fe0/MnO2, sand/MnO2), and (3) the ternary Fe0/sand/MnO2 system. The uniqueness of the single-sand MnO2 (100% MnO2) relative to the other systems is that it contains no in-situ generated FeCPs (Table 2) and is not a good adsorbent for MB according to its low points of zero charge63. It is seen that Fe0-based systems performed better than single and binary systems with sand and MnO2 for the selected equilibration time (47 d). For the Fe0-based systems, those containing MnO2 performed the best. While this observation contradicts the report of Miyajima and Noubactep35, it is consistent with literature reports40,41,44,45. Because MnO2 alone had the least E value (only 4%), the contribution of this mineral for MB discoloration is indirect and coupled to the presence of Fe0.
Dissolved iron in the investigated systems
The results of iron release are not shown herein. In Fe0/sand and Fe0/sand/MnO2 systems, the iron level was constantly lower than 0.2 mg L–1 as the contact time varies from 3 to 60 d. This observation is rationalized by: (1) the progressive in-situ coating of sand in the Fe0/sand, (2) sand coating and Fe2+ consumption in the Fe0/sand/MnO2 system. Note that, measured dissolved iron is the balance between Fe0 oxidative dissolution (Fe source) and (3) all processes consuming Fe (Fe sinks). Fe sinks include: (1) Fe adsorption onto minerals (FeCPs, MnO2 and sand), (2) iron precipitation as hydroxides, and (3) Fe2+ utilization for the reductive dissolution of MnO221,35,43,64. Thus, the ternary system (Fe0/sand/MnO2) has one Fe sink more than the Fe0/sand system. This is the major reason of the observed absence of Fe release. Previous works testing the pure Fe0 system in parallel reported on [Fe] > 0.2 mg L−1 for contact time lower than 20 days35. Similar low Fe levels were reported by Gatcha-Bandjun et al.64 in quiescent batch experiments for up to 60 days. This observation recalls that, at pH > 4.5, the dissolved Fe level can only support the interpretation of achieved results65. This observation also reiterates the complex nature of the Fe0/H2O system and the crucial importance of long-term experiments under various operational conditions21,28,65,66 for a better understanding. Remember that the addition of sand and MnO2 is a tool to better characterize the Fe0/H2O system as a whole under natural-near conditions.
MB discoloration in Fe0/sand/MnO2 systems
Figure 2a shows a biphasic pattern in the process of MB discoloration in the three systems with the Fe0/MnO2 being the most interesting. In the sand-based systems, MB discoloration at [Fe0] = 0 g L−1 (> 50%) is explained by the strong adsorption affinity of sand for MB35,53. This means that the observed enhanced MB discoloration due to Fe0 and MnO2 is explained by the continuous corrosion beyond sand coating, as free precipitation of FeCPs becomes possible. MB is adsorbed onto sand and/or precipitated with FeCPs, but note that is a slow process occurring on a “passivated Fe0” (“reactivity loss”). In this study, the investigation of “residual” reactivity is rendered possible by in-situ coating of sand and Fe2+ oxidation by MnO2 (Eq. 2). Both processes delay the “passivation” of Fe0.
Changes in the Fe0, Fe0/sand, and Fe0/sand/MnO2 systems as impacted by the addition of various Fe0 loading for 47 days: (a) Methylene blue discoloration, and (b) pH value. Experimental conditions: [Fe0] = 2.3 to 45 g L−1; [sand] = 22.5 g L−1; and [MnO2] = 2.3 g L−1. The lines are not fitting functions, they simply connect points to facilitate visualization.
In the binary Fe0/MnO2 system, MB discoloration occur solely due to Fe0 oxidation and the subsequent precipitation of FeCPs which eliminate MB from the aqueous solution. Under the test conditions, 47 d were long enough to enable in-situ generation of enough FeCPs for MB discoloration to an extent larger that in the binary Fe0/sand and the ternary Fe0/sand/MnO2 systems (Fig. 2a). It can be considered that sand is a confounding factor in the kinetics of MB discoloration. However, without sand, Fe0 particles are cemented to each other and become less or non-reactive67. Note that sand is a central component for the MB method35,56,57,58. As seen in Fig. 1b, MnO2-based systems are the most efficient systems in the long-term. Accordingly, Fe0/sand/MnO2 systems are more efficient than Fe0 and Fe0/sand because of the sustained Fe0 reactivity. The discussion herein suggests that binary Fe0/MnO2 systems are more efficient than Fe0/sand because besides being non-expansive, MnO2 is also reactive (Eq. 2).
Figure 2b shows changes of the pH value in the three systems as the Fe0 loadings increase from 0 to 45 g L−1. The initial pH value for all systems was 8.2. It is evident that there is a general increase in pH value with increasing Fe0 loading. The fact that there is a slight pH decrease for lower Fe0 loading ([Fe0] < 7.5 g L−1) is reproducible and has been observed by other researchers67. This shows that for these low Fe0 loadings, iron corrosion according to Eq. (1) has not dominated concurrent processes like SiO2 dissolution (acidifying). For [Fe0] < 7.5 g L−1, sustained iron corrosion is clearly reflected even though: (1) no clear difference is observed in the extent of MB discoloration, and (2) no Fe was detected in the aqueous phase (data not shown). Again, the MnO2-bearing systems depicted the highest pH increase, corroborating the fact that MnO2 enhances the efficiency of Fe0/H2O systems by sustaining Fe0 corrosion (Eq. 2)32,39,45. It is expected that a different Fe0 specimen, or a different loading of the same specimen will reproduce the trend observed herein. For example, while using two different mass loadings of the same Fe0 specimen, Touomo-Wouafo et al.68 reported on different pics in the concentration of aqueous Fe2+. In fact, the corrosion rate of Fe0 materials varies largely under environmental conditions29,30,62,69. Unfortunately, despite 30 years of intensive research on the application of Fe0 materials for environmental remediation, no single standardized/unified protocol for the characterization of the material intrinsic reactivity has been presented69,70,71,72. The next section compares the behavior of the same Fe0/sand system as the mass loading of three different natural MnO2 specimens vary from 0 to 45 g L−1.
The impact of the different MnO2
Figure 3a shows changes of the extent of MB discoloration by the Fe0/sand system as the loading of three different natural MnO2 varies from 0 to 45 g L−1. The E value for [MnO2] of 0 g L−1 corresponds to MB discoloration by the Fe0/sand system which is about 67%. This value decreases to about 55% for all three MnO2 specimens for the lowest mass loading ([MnO2] = 2.3 g L−1) and subsequently increase with increasing MnO2 loading. The increase of the E value is not linear but monotonous, revealing the complexity of processes in the ternary-aggregate systems. It is seen that Manganit performed better than the two other MnO2 specimens. The two most important features from Fig. 3a are: (1) MnO2 enhances the efficiency of Fe0/H2O systems, and (2) each MnO2 specimen has its own dissolution kinetics (intrinsic reactivity). Considering that the redox reactivity of contaminants is not considered in the presentation until now, it becomes clear that the semi-conductive nature of MnO2 and its redox reactivity for selected contaminants need to be properly addressed in the remediation context.
Changes in the Fe0/sand/MnO2 systems as impacted by the addition of various MnO2 loading for 47 days: (a) Methylene blue discoloration, and (b) pH value. Three different natural minerals were tested. Experimental conditions: [Fe0] = 4.5 g L−1; [sand] = 22.5 g L−1; and [MnO2] = 2.3 to 45.0 g L−1. The lines are not fitting functions, they simply connect points to facilitate visualization.
Figure 3b shows changes of the pH value in the three systems as the MnO2 loadings increase from 0 to 45 g L−1. The pH of the Fe0/sand system ([MnO2] = 0 g L−1) increased from an initial value of 8.2 to about 8.5 after 47 days of equilibration. For the lowest tested MnO2 loading, the pH value decreased from 8.2 to about 7.8, then subsequently increased to reach a maximum of 8.9 around [MnO2] = 25 g L−1. Thereafter, the pH value decreased to about 7.9 for [MnO2] = 45 g L−1. The largest pH variation was observed with Psilomelan while the behavior of the two other minerals were very close to each other. In the absence of the mineralogical composition of the MnO2 specimens, these observations cannot be further interpreted. It is however certain that: (1) for higher MnO2 loading, the final pH value was not determined by iron corrosion, and (2) each MnO2 mineral is a stand-alone operation parameter. Clearly the results presented herein are highly qualitative. Michel et al.33 recently advocated for the introduction of procedure standardizing the reactivity of manganese oxides (Mnx) for water treatment.
Discussion
Promoting iron corrosion under natural conditions
The main result of this work is that by scavenging Fe2+ from Fe0 oxidative dissolution, MnO2 minerals intensify the process of decontamination. In other words, the reductive dissolution of MnO2 (Eq. 2) sustains iron corrosion (Eq. 1) according to Le Chatelier principle. Sand is used to sustain the discussion in the framework of the used MB method but has the practical significance of being the most available aggregate to build Fe0-based reactive zones24,73. This result leads to several avenues for addressing both the reactivity loss and the permeability loss of Fe0 filters. As for addressing the “reactivity loss” the experiments presented herein, quiescent batch experiments for 47 days, were performed under conditions corresponding to a “passivated” state for Fe0. Nevertheless significant changes within the systems (e.g., pH value) could be documented. It shall be recalled that MB is an indicator of reactivity and that using a species with strong affinities to FeCPs (e.g., Orange II or methylorange) would have enabled more differentiation between the systems64,74,75,76. In essence, by combining the most suitable Fe0 and MnO2 a large array of appropriate remediation systems for site-specific applications can be designed. As far as filtration systems are concerned, beside the nature and the extent of pollution and the required quality of treated water, relevant operation parameters include: (1) the Fe0 nature (e.g., form, reactivity, size), (2) the MnO2 nature and reactivity, (3) the Fe0/MnO2 mixing ratio (including in composites), (4) the exact amount of each material (e.g. mass or volume), (5) the size of the filter, (6) the flow velocity (e.g. residence time), and (7) depth of the filter. More research is needed to enable the realization of the huge potential of the Fe0/MnO2 system for water treatment.
Significance of the results in the design and operation of Fe0/H2O systems
The common underlying mechanism for water decontamination using Fe0 is its electrochemical oxidative dissolution by protons and by protons alone66,77. This century old knowledge has been distorted while introducing the Fe0 remediation technology in the 1990s78. Since then, researchers are desperately seeking for ways to establish reliable non-site-specific criteria for the design of Fe0-based systems58,79. If such design criteria are established, then, site-specific treatability studies may only be required to fine-tune design criteria for the optimal performance of Fe0 systems79. Unfortunately, all data provided in the framework of these efforts have not really helped and a clear circular reasoning is established within the Fe0 remediation research community58,80. Clearly, numerous laboratory, pilot, and field-scale studies for water treatment by Fe0 have just demonstrated the feasibility of the technology, but the science behind is yet to be established. The results of the study are regarded as a decisive contribution on the path to establish the science of the Fe0/H2O system.
Since the seminal work of Schreier and Reinhard81 describing a lag time between Fe0 immersion and the start of contaminant “reductive transformation”, many arguments have been advanced to rationalize this observation, the most prominent being that is corresponds to the time to reduce atmospheric corrosion products, FeIII oxides and hydroxides82,83,84. However, these earlier arguments should be collectively regarded as wrong because if contaminant reduction was occurring at the Fe0 surface, it should be quantitative when this surface is free, that is immediately after immersion45,64,68,85,86,87. Interested readers are referred to the cited literature, in particular Touomo-Wouafo et al.68,86 using polarography to follow changes in Fe2+ and metallic ions (including Zn2+) of polluted waters. Touomo-Wouafo et al.68 described an induction phase following Fe0 immersion, followed by a reactive phase during which Fe2+ appears in solution and Zn2+ is removed. The reactive phase was followed by a passivation phase characterized by no detection of Fe2+ in solution and insignificant Zn2+ removal. The experiments of Touomo-Wouafo et al.68 lasted for up to 16 days under buffered conditions. The first merit of the present work is to have extended the reactive phase beyond 16 days, while demonstrating that despite the absence of Fe2+ in the bulk solution, there is no passivation. In other words, the system is still reactive and will maintain reactivity as long as Fe0 is not completely depleted. The research question is thus: How to sustain the residual Fe0 corrosion to the extent that satisfactorily water treatment is achieved in the long-term? A qualitative answer from the results achieved herein is “add MnO2”. The next step is to pilot test this idea while keeping in mind that each Fe0 and MnO2 is a different reactive material that require prior characterization before use38,69. Only systematic investigations with well-characterized reactive (e.g. Fe0 and MnO2) and non-reactive aggregates (e.g., gravel, pumice, sand) will enable the design of more efficient and sustainable MnO2-amended Fe0/H2O systems.
The role MnO2 in the context of the inconsistencies in Fe0 literature
Decentralized wastewater treatment and safe drinking provision is increasingly using hybrid Fe0-based filtration systems. Commonly used mineral materials are anthracite, diatomite. gravel, magnetite (Fe3O4), manganese ores (MnOx), pumine, quartz, sand and zeolite38,88. From these minerals quartz and sand are most commonly used and are considered "neutral" filter media61,67,73,89. In this context, neutral means non-reactive or inert. In the framework of Fe0 filters, the non-expansive nature of sand is already very important as Fe0/sand filters are more sustainable than pure Fe0 filters24,25,43. The operating mode of sand in sustaining Fe0/sand filters relies on the evidence that sand is in-situ coated with iron oxyhydroxides which spatially precipitate far from the Fe0 surface, thereby delaying its passivation (‘reactivity loss’). The contribution of sand to the sustainability of Fe0 filters arises from it inert nature making it a non-expansive aggregate and retarding clogging compared to a pure Fe0 filter25. Apart from quartz and sand, other minerals are considered to have some adsorptive (and redox) affinities to many dissolved species and are not employed as pure filling material. The two most investigated minerals in the context of Fe0 filters are Fe3O488 and FeS258.
To the best of authors’ knowledge, manganese ores (MnOx) have been introduced as means to investigate the mechanism of uranium removal in Fe0/H2O systems90,91. The achieved results inspired Burghardt and Kassahun39 to test a Fe0/MnO2 reactive zone for the removal of radium and uranium from groundwater. Further investigations of the Fe0/MnO2 were performed around 2010 by two different research groups: Dr. Ghauch in Beirut/Lebanon41,42, and Dr. Noubactep in Göttingen/Germany32,42,51,55. Their results unequivocally confirmed enhanced contaminant removal by Fe0/MnO2 relative to pure Fe0 systems. This observation was justified by the ‘reinforcement’ of the corrosion process by the reductive dissolution of MnO2 or better MnOx. From more recent reports available in the scientific literature, only the research group of Dr. Gheju in Timisoara/Romania has designed his investigations based on this past knowledge. All other researchers have not considered this decades-old knowledge while others even distort it. The most representative example is perhaps Dong et al.47 who investigated the MnO2/Fe0 sequence (Fe0 after MnO2) and discussed their results as if the aggegates were mixed in the same layer. In a effort to build a common knowledge database for Fe0-based remediation systems, it is very disappointing that new experiments are not designed based on available knowledge and their interpretation ignored them as well. In the past two decades, the importance of the integrity of science has gained the due importance92,93,94,95. There will be no progress on this path until authors consider their personal integrity. This proliferation of scientific misconduct92,94 suggest that, beyond the current metrics (e.g., h-index, number of citations) individual researchers have to be evaluated based on their personal integrity. It is at least certain that while cheating the editors and reviewers with wrong novelties, publications contrary to the state-of-the-art knowledge confuse early-career researchers, including PhD candidates80,96,97.
The present study has reinforced the view that water treatment in Fe0 filters is characterized by the in-situ generation of iron oxyhydroxides (FeCPs) and their retention in the filter bed. FeCPs are excellent scavengers of both biological and chemical contaminants5,85,98,99,100. Because oxyhydroxides are larger in volume than the parent metal (Fe0), Fe0/sand filters are more sustainable than pure Fe0 filters. Yet if sand is partly or completely replaced by MnO2, in addition to ‘creating’ space for generated FeCPs, the corrosion process is ‘reinforced’ or the passivation delayed51. By demonstrating this in the present paper with an indicator of reactivity for the Fe0/H2O system (MB), its universality is proved. The remaining task is to characterize both Fe0 and MnO2 and determine their ratio in site-specific applications. That means seeking for knowledge of surface morphology of Fe0 and MnO2 or their time-dependant changes. The next section outlines a possible research program.
Designing MnO2-amended Fe0 filters
MnO2-amended Fe0 filters are a particular case of Fe0 filters in which the reduced oxidation kinetics of Fe0 (‘reactivity loss’) is reinforced by the addition of MnO2. MnO2 addition ultimately increases the service life of the Fe0 filter39. Despite the knowledge of the operating mode of MnO2 to enhance the efficiency of Fe0 filters, little is understood about the intrinsic reactivity of both materials, and how they behave in the long-term38,69,72. Moreover, limited data exist on the reactivities of the various forms of MnO2, including those invested in the present study38. There are numerous Fe0 and MnO2 suppliers around the world and each individual material is a stand-alone operational variable for a MnO2-amended Fe0 filter. Therefore, the major research question for the next-generation Fe0 filters is: How are existing chemical, physical and structural differences between available and/or newly manufactured Fe0 and MnO2 affecting their efficiency for water treatment? To answer this research question, the major research objectives for the coming filters are as follows:
-
1.
To characterize the available Fe0 and MnO2 in terms of the chemical, compositional and physical properties, and identify the structure of each material;
-
2.
To characterize the available Fe0 for their intrinsic reactivity in aqueous solutions using available tools69,72;
-
3.
To develop and validate tools to characterize the intrinsic reactivity of MnO2 in aqueous solutions;
-
4.
To analyse and compare the performance of various Fe0 and MnO2 (and their mixtures) for MB disccoloration and for the removal of selected model contaminants from aqueous solution using long-lasting fixed-bed columns (> 6 months);
-
5.
To investigate changes in contaminant removal performance when different Fe0/MnO2 ratios are used;
-
6.
Finally, to synthesize serviceable column media comprising of desired Fe0/MnO2 mixtures for any site-specific application.
On a positive note, with regard to frugal technologies, apart from Objective 1 (structural characterization), research to address these objectives does not require sophisticated laboratory analytical equipment, and can be implemented with limited research budgets. Thus, all other objectives can be addressed in low-equipped laboratories including those in the developing world52.
Concluding remarks
The concept that aqueous contaminant removal in the presence of metallic iron (Fe0/H2O system) is caused by the process of Fe precipitation is consistent with many experimental observations. In particular, by delaying Fe precipitation in the bulk solution, MnO2 delays the removal process at local-grain scale. However, because in a Fe0 bed this process occurs thousands of times (filter scale), the presence of MnO2 is favourable for the sustainability of Fe0/H2O systems. In fact, without MnO2, “Fe0 passivation” occurs earlier and the Fe0/H2O system may fail despite abundance of Fe0. The present study has used the MB method to demonstrate this elegantly. Past efforts to rationalize the operating mode of MnO2 amended Fe0/H2O systems were challenging also because biotic and/or abiotic interactions of relevant contaminants with both Fe0 and MnO2 were to be considered.
The merit of this study is to have identified a reaction time (> 35 d) and experimental conditions (quiescent bath with the given material loadings) under which the made demonstration was possible. The presentation was limited at highlighting the key result: Fe0 generates Fe minerals which interact with sand and MnOx to treat water. Exploiting this knowledge to design more efficient and sustainable Fe0/H2O systems goes through systematic investigations. The chemistry, mineralogy, morphology, and structure of both Fe0 and MnOx affect the results of water treatment. Their relative amounts and proportions in filters as well as the water chemistry are other equally important variables. This multitude of inter-dependent factors makes a systematic approach mandatory if comparable and transferable results are sought. In other words, thoroughly planed experiments, designed variously with well-characterized materials and using controlled flow conditions are urgently necessary to make Fe0 filtration a sort of “best available technology” among appropriate technologies for decentralized water treatment.
References
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Kearns, J. P., Bentley, M. J., Mokashi, P., Redmon, J. H. & Levine, K. Underrepresented groups in WaSH—the overlooked role of chemical toxicants in water and health. J. Water Sanit. Hyg. Dev. 9, 786–793 (2019).
Amrose, S. E., Cherukumilli, K. & Wright, N. C. Chemical contamination of drinking water in resource-constrained settings: global prevalence and piloted mitigation strategies. Annu. Rev. Environ. Resour. 45, 195–226 (2020).
Jepson, W. & Vandewalle, E. Household water insecurity in the Global North: a study of rural and periurban settlements on the Texas-Mexico Border. Prof. Geogr. 68, 66–81 (2016).
Bischof, G. The purification of water: Embracing the action of spongy iron on impure water. Bell and Bain, Glasgow (1873).
Hussam, A. & Munir, A. K. M. A simple and effective arsenic filter based on composite iron matrix: development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 42, 1869–1878 (2007).
Ngai, T. K. K., Shrestha, R. R., Dangol, B., Maharjan, M. & Murcott, S. E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A42, 1879–1888 (2007).
Noubactep, C., Schöner, A. & Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean: Soil, Air, Water 37, 930–937 (2009).
Bradley, I., Straub, A., Maraccini, P., Markazi, S. & Nguyen, T. H. Iron oxide amended biosand filters for virus removal. Water Res. 45, 4501–4510 (2011).
George, D. & Ahammed, A. M. Effect of zero-valent iron amendment on the performance of biosand filters. Water Supply 19, 1612–1618 (2019).
Mutemi, S., Hoko, Z. & Makurira, H. Investigating feasibility of use of bio-sand filters for household water treatment in Epworth, Zimbabwe. Phys. Chem. Earth Parts A/B/C 117, 102864 (2020).
Guan, X. et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 75, 224–248 (2015).
Maamoun, I., Eljamal, O., Falyouna, O., Eljamal, R. & Sugihara, Y. Stimulating effect of magnesium hydroxide on aqueous characteristics of iron nanocomposites. Water Sci. Technol. 80, 1996–2002 (2019).
Eljama, O. et al. Investigating the design parameters for a permeable reactive barrier consisting of nanoscale zero-valent iron and bimetallic iron/copper for phosphate removal. J. Mol. Liq. 299, 112144 (2020).
Falyouna, O., Eljamal, O., Maamoun, I., Tahara, A. & Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 571, 66–79 (2020).
He, F., Gong, L., Fan, D., Tratnyek, P. G. & Lowry, G. V. Quantifying the efficiency and selectivity of organohalide dechlorination by zerovalent iron. Environ. Sci. Process. Impacts 22, 528 (2020).
Maamoun, I., Eljamal, O., Falyouna, O., Eljamal, R. & Sugihara, Y. Multi-objective optimization of permeable reactive barrier design for Cr(VI) removal from groundwater. Ecotoxicol. Environ. Saf. 200, 110773 (2020).
Mokete, R., Eljamal, O. & Sugihara, Y. Exploration of the reactivity of nanoscale zero-valent iron (NZVI) associated nanoparticles in diverse experimental conditions. Chem. Eng. Process. Process Intensif. 150, 107879 (2020).
Pasinszki, T. & Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 10, 917 (2020).
Calabrò, P. S., Bilardi, S. & Moraci, N. Advancements in the use of filtration materials for the removal of heavy metals from multicontaminated solutions. Curr. Opin. Environ. Sci. Health 20, 100241 (2021).
Cao, V., Alyoussef, G., Gatcha-Bandjun, N., Gwenzi, W. & Noubactep, C. The suitability of methylene blue discoloration (MB method) to investigate the Fe0/MnO2 system. Processes 9, 548 (2021).
Stefanoni, M., Angst, U. M. & Elsener, B. Kinetics of electrochemical dissolution of metals in porous media. Nat. Mater. 18, 942–947 (2019).
Henderson, A. D. & Demond, A. H. Long-term performance of zero-valent iron permeable reactive barriers: a critical review. Environ. Eng. Sci. 24, 401–423 (2007).
Domga, R., Togue-Kamga, F., Noubactep, C. & Tchatchueng, J. B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 263, 127–134 (2015).
Caré, S. et al. Modeling the permeability loss of metallic iron water filtration systems. Clean: Soil, Air, Water 41, 275–282 (2013).
Moraci, N., Lelo, D., Bilardi, S. & Calabrò, P. S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 53, 946–961 (2016).
Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: modeling gone astray. Water 8, 162 (2016).
Alyoussef, G. Characterizing the impact of contact time in investigating processes in Fe0/H2O Systems. Freiberg Online Geosci. 44, 1–60 (2021).
Romanoff, M. Underground Corrosion. United States Department of Commerce, National Bureau of Standards. Circular 579 (1957).
Melchers, R. E. & Petersen, R. B. A reinterpretation of the Romanoff NBS data for corrosion of steels in soils. Corros. Eng. Sci. Technol. 53, 131–140 (2018).
Santisukkasaem, U. & Das, D. B. A non-dimensional analysis of permeability loss in zero-valent iron permeable reactive barrier (PRB). Transp. Porous Media 126, 139–159 (2019).
Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freiberg Online Geosci. 32, 1–60 (2012).
Miyajima, K. & Noubactep, C. Effects of mixing granular iron with sand on the efficiency of methylene blue discoloration. Chem. Eng. J. 200–202, 433–438 (2012).
Miyajima, K. & Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 217, 310–319 (2013).
Miyajima, K. & Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 262, 891–896 (2015).
Appelo, C. A. J. & Postma, D. Variable dispersivity in a column experiment containing MnO2 and FeOOH-coated sand. J. Cont. Hydrol. 40, 95–106 (1999).
Appelo, C. A. J. & Postma, D. A consistent model for surface complexation on birnessite (δ MnO2) and its application to a column experiment. Geochim. Cosmochim. Acta 63, 3039–3048 (1999).
Michel, M. M. et al. Mineral materials coated with and consisting of MnOx—characteristics and application of filter media for groundwater treatment: a review. Materials 13, 2232 (2020).
Burghardt, D. & Kassahun, A. Development of a reactive zone technology for simultaneous in situ immobilisation of radium and uranium. Environ. Geol. 49, 314–320 (2005).
Ghauch, A., Abou Assi, H. & Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: bimetallic systems. J. Hazard. Mater. 182, 64–74 (2010).
Ghauch, A., Abou Assi, H., Baydoun, H., Tuqan, A. M. & Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: mechanism and kinetics. Chem. Eng. J. 172, 1033–1044 (2011).
Noubactep, C., Btatkeu-K, B. D. & Tchatchueng, J. B. Impact of MnO2 on the efficiency of metallic iron for the removal of dissolved metal. Chem. Eng. J. 178, 78–84 (2011).
Btatkeu-K, B. D., Olvera-Vargas, H., Tchatchueng, J. B., Noubactep, C. & Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 250, 416–422 (2014).
Gheju, M., Balcu, I. & Vancea, C. An investigation of Cr(VI) removal with metallic iron in the co-presence of sand and/or MnO2. J. Environ. Manag. 170, 145–151 (2016).
Gheju, M. & Balcu, I. Sustaining the efficiency of the Fe(0)/H2O system for Cr(VI) removal by MnO2 amendment. Chemosphere 214, 389–398 (2019).
Bui, T. H., Kim, C., Hong, S. P. & Yoon, J. Effective adsorbent for arsenic removal: core/shell structural nano zero-valent iron/manganese oxide. Environ. Sci. Pollut. Res. 24, 24235–24242 (2017).
Dong, G. et al. Effect and mechanism analysis of MnO2 on permeable reactive barrier (PRB) system for the removal of tetracycline. Chemosphere 193, 702–710 (2018).
Liang, Y. et al. Stabilization of arsenic sludge with mechanochemically modified zero valent iron. Chemosphere 168, 1142–1151 (2017).
Qiu, Z., Tian, Q., Zhang, T., Yang, D. & Qiu, F. Fabrication of dynamic zero-valent iron/MnO2 nanowire membrane for efficient and recyclable selenium separation. Sep. Purif. Technol. 230, 115847 (2020).
Su, C. & Puls, R. W. Arsenate and arsenite removal by zerovalent iron: kinetics, redox transformation, and implications for in situ groundwater remediation. Environ. Sci. Technol. 35(7), 1487–1492 (2001).
Noubactep, C., Caré, S., Btatkeu-K, B. D. & Nanseu-Njiki, C. P. Enhancing the sustainability of household Fe0/sand filters by using bimetallics and MnO2. Clean: Soil, Air, Water 40, 100–109 (2012).
Btatkeu-K, B. D., Tchatchueng, J. B., Noubactep, C. & Caré, S. Designing metallic iron based water filters: light from methylene blue discoloration. J. Environ. Manag. 166, 567–573 (2016).
Mitchell, G., Poole, P. & Segrove, H. D. Adsorption of methylene blue by high-silica sands. Nature 176, 1025–1026 (1955).
Noubactep, C. Characterizing the discoloration of methylene blue in Fe0/H2O systems. J. Hazard. Mater. 166, 79–87 (2009).
Noubactep, C. Characterizing the reactivity of metallic iron upon methylene blue discoloration in Fe0/MnO2/H2O systems. J. Hazard. Mater. 168, 1613–1616 (2009).
Xiao, M., Hu, R., Cui, X., Gwenzi, W. & Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 8, 409 (2020).
Xiao, M., Cui, X., Hu, R., Gwenzi, W. & Noubactep, C. Validating the efficiency of the FeS2 method for elucidating the mechanisms of contaminant removal using Fe0/H2O systems. Processes 8, 1162 (2020).
Hu, R., Cui, X., Xiao, M., Gwenzi, W. & Noubactep, C. Characterizing the impact of pyrite addition on the efficiency of Fe0/H2O systems. Sci. Rep. 11, 2326 (2021).
Zhang, H. et al. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: behavior and mechanism. Sci. Rep. 10, 6067 (2020).
Mackenzie, P. D., Horney, D. P. & Sivavec, T. M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 68, 1–17 (1999).
Varlikli, C. et al. Adsorption of dyes on Sahara desert sand. J. Hazard. Mater. 170, 27–34 (2009).
Hu, R. et al. The impact of selected pre-treatment procedures on iron dissolution from metallic iron specimens used in water treatment. Sustainability 11, 671 (2019).
Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 238, 1–61 (2016).
Gatcha-Bandjun, N., Noubactep, C. & Loura Mbenguela, B. Mitigation of contamination in effluents by metallic iron: the role of iron corrosion products. Environ. Technol. Innov. 8, 71–83 (2017).
Lavine, B. K., Auslander, G. & Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 70, 69–83 (2001).
Cao, V. et al. Tracing the scientific history of Fe0-based environmental remediation prior to the advent of permeable reactive barriers. Processes 8, 977 (2020).
Kaplan, D. I. & Gilmore, T. J. Zero-valent iron removal rates of aqueous Cr(VI) measured under flow conditions. Water Air Soil Pollut. 155, 21–33 (2004).
Touomo-Wouafo, M. et al. Electrochemical monitoring of heavy metals removal from aqueous solutions by aged metallic iron. Competitive effects of cations Zn2+, Pb2+ and Cd2+. Monatsh. Chem. 151, 1511–1523 (2020).
Lufingo, M., Ndé-Tchoupé, A. I., Hu, R., Njau, K. N. & Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 11, 2465 (2019).
Kim, H., Yang, H. & Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Health A 49, 514–523 (2014).
Li, S., Ding, Y., Wang, W. & Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 8, 1239–1248 (2016).
Li, J. et al. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 148, 70–85 (2019).
Ndé-Tchoupé, A. I., Makota, S., Nassi, A., Hu, R. & Noubactep, C. The suitability of pozzolan as admixing aggregate for Fe0-based filters. Water 10, 417 (2018).
Phukan, M., Noubactep, C. & Licha, T. Characterizing the ion-selective nature of Fe0-based filters using three azo dyes in batch systems. J. Environ. Chem. Eng. 4, 65–72 (2016).
Heimann, S. Testing granular iron for fluoride removal. Freiberg Online Geosci. 52, 1–80 (2018).
Heimann, S., Ndé-Tchoupé, A. I., Hu, R., Licha, T. & Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 209, 578–587 (2018).
Whitney, W. R. The corrosion of iron. J. Am. Chem. Soc. 25(4), 394–406 (1903).
Matheson, L. J. & Tratnyek, P. G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 28, 2045–2053 (1994).
McGeough, K. L., Kalin, R. M. & Myles, P. Carbon disulfide removal by zero valent iron. Environ. Sci. Technol. 41, 4607–4612 (2007).
Makota, S. et al. Metallic iron for water treatment: leaving the valley of confusion. Appl. Water Sci. 7, 4177–4196 (2017).
Schreier, C. G. & Reinhard, M. Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 29, 1743–1753 (1994).
Ritter, K., Odziemkowski, M. S. & Gillham, R. W. An in situ study of the role of surface films on granular iron in the permeable iron wall technology. J. Cont. Hydrol. 55, 87–111 (2002).
Odziemkowski, M. S. & Simpraga, R. P. Distribution of oxides on iron materials used for remediation of organic groundwater contaminants—implications for hydrogen evolution reactions. Can. J. Chem./Rev. Can. Chim. 82, 1495–1506 (2004).
Hao, Z., Xu, X. & Wang, D. Reductive denitrification of nitrate by scrap iron filings. J. Zhejiang Univ. Sci. 6B, 182–187 (2005).
Ghauch, A. Iron-based metallic systems: an excellent choice for sustainable water treatment. Freiberg Online Geosci. 32, 1–80 (2015).
Touomo-Wouafo, M. et al. Role of pre-corrosion of Fe0 on its efficiency in remediation systems: an electrochemical study. Chemosphere 209, 617–622 (2018).
Antia, D. D. J. Water treatment and desalination using the eco-materials n-Fe0 (ZVI), n-Fe3O4, n-FexOyHz[mH2O], and n-Fex[Cation]nOyHz[Anion]m [rH2O]. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications (eds Kharissova, O. V. et al.) (Springer, 2020). https://doi.org/10.1007/978-3-030-11155-7_66-1.
Han, S., Huang, Y. & Liu, Z. Bacterial indicator reduction in dairy manure using hybridzero-valent iron (h-ZVI) system. Environ. Sci. Pollut. Res. 26, 10790–10799 (2019).
Bi, E., Devlin, J. F. & Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Ground Water Monit. Remed. 29, 56–62 (2009).
Noubactep, C., Meinrath, G., Dietrich, P. & Merkel, B. Mitigating uranium in groundwater: prospects and limitations. Environ. Sci. Technol. 37, 4304–4308 (2003).
Noubactep, C., Meinrath, G. & Merkel, J. B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2, 235–242 (2005).
Lewellyn, P. G. & Rodriguez, L. C. Does academic dishonesty relate to fraud theory? A comparative analysis. Am. Int. J. Contemp. Res. 5, 1–6 (2015).
Egan, A., Maguire, R., Christophers, L. & Rooney, B. Developing creativity in higher education for 21st century learners: a protocol for a scoping review. Int. J. Educ. Res. 82, 21–27 (2017).
Egan, A. Improving Academic Integrity through Assessment Design (Dublin City University, 2018).
Burgess-Jackson, K. Why I publish in “predatory” journals—and why you should, too. Philos. Int. J. 3, 000160 (2020).
Noubactep, C., Makota, S. & Bandyopadhyay, A. Rescuing Fe0 remediation research from its systemic flaws. Res. Rev. Insights https://doi.org/10.15761/RRI.1000119 (2017).
Ebelle, T. C., Makota, S., Tepong-Tsindé, R., Nassi, A. & Noubactep, C. Metallic iron and the dialogue of the deaf. Fresenius Environ. Bull. 28, 8331–8340 (2019).
Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 129, 449–461 (1890).
You, Y., Han, J., Chiu, P. C. & Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 39, 9263–9269 (2005).
Chopyk, J. et al. Zero-valent iron sand filtration reduces concentrations of virus-like particles and modifies virome community composition in reclaimed water used for agricultural irrigation. BMC Res Notes 12, 223 (2019).
Acknowledgements
For providing the iron materials investigated in this study the authors would like to express their gratitude to iPutec GmbH (Rheinfelden, Germany). The natural MnO2-minerals were provided by the Department of Geology of the Technical University Bergakademie Freiberg/Germany (Mineralsammlung). The manuscript was improved by the insightful comments of anonymous reviewers from Scientific Reports. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
G.A., V.C., N.G.-B. and C.N. conceived the presented idea and developed the theory. G.A. carried out the experiment. C.N. supervised this work. W.G. supervised the redaction of the first draft by V.C. and N.G.-B. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, V., Alyoussef, G., Gatcha-Bandjun, N. et al. Characterizing the impact of MnO2 addition on the efficiency of Fe0/H2O systems. Sci Rep 11, 9814 (2021). https://doi.org/10.1038/s41598-021-89318-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89318-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.