Abstract
Nicotinic acetylcholine receptors (nAChRs) play a critical role in the neuropharmacology of learning and memory. As such, naturally occurring alkaloids that regulate nAChR activity have gained interest for understanding and potentially improving memory function. In this study, we tested the acute effects of three known nicotinic alkaloids, nicotine, cotinine, and anatabine, in suppressing scopolamine-induced memory deficit in rodents by using two classic memory paradigms, Y-maze and novel object recognition (NOR) in mice and rats, respectively. We found that all compounds were able to suppress scopolamine-induced spatial memory deficit in the Y-maze spontaneous alternation paradigm. However, only nicotine was able to suppress the short-term object memory deficit in NOR, despite the higher doses of cotinine and anatabine used to account for their potential differences in nAChR activity. These results indicate that cotinine and anatabine can uniquely regulate short-term spatial memory, while nicotine seems to have more robust and general role in memory regulation in rodents. Thus, nAChR-activating alkaloids may possess distinct procognitive properties in rodents, depending on the memory types examined.
Similar content being viewed by others
Introduction
The cholinergic system of the brain is a critical regulator of attention, memory, and higher-order cognitive processing, and its deficits are central to the etiology of dementia1. As such, nicotinic acetylcholine receptors (nAChRs) have gained much interest as a target of drug development2. Neuronal nAChRs are pentameric proteins composed of various combinations of α (α2–α9) and β (β2–β4) nAChR subunits, differentially expressed throughout the nervous system. Their homomeric (e.g., α7) or heteromeric (e.g., α4β2, α3β4, and α6β2β3) assembly generates multiple nAChR subtypes, which differ in their pharmacological and biophysical properties, such as sensitivity and rate of desensitization3,4,5,6. High densities of α4β2 and α7 nAChRs, in particular, can be found in brain regions critical for memory, including prefrontal cortex and hippocampus, while α6 containing nAChRs are more commonly found in other structures, such as striatum, substantia nigra, and locus coeruleus5,7,8,9,10. This diversity of nAChR subtypes allows fine orchestration of neural network activities necessary for proper memory formations5,11.
Alkaloids are a class of naturally occurring organic nitrogen-containing bases with various neurological effects in humans and other animals12,13,14. More than 3000 alkaloids have been identified with diverse chemical structures and pharmacological actions13,15,16,17. In particular, pyridine alkaloids that target nAChRs are of great interest due to the critical role they play in neuropharmacology of memory13,18. Although nicotine analogs and other pyridine alkaloids have shown a certain degree of toxicity in clinical studies19,20,21, they have also demonstrated potential modulatory effects in various neurological conditions, including memory deficit, particularly in nonclinical animal models13,22,23,24,25. Among them, nicotine is one of the most well-known natural alkaloid that can be found in many plants of the Solanaceae family with well-established activities on nAChRs18,26. A number of studies have reported efficacy of nicotine in regulating memory, such as working memory and recognition memory, in rodents and humans2,23,24,27,28,29. The rodent studies have primarily focused on memory improvements using, for example, radial-arm maze, passive avoidance, and water maze2,30. Recent studies have demonstrated that α4, β2, and/or α7 subunit-containing nAChRs participate in the cognitive-enhancing effects of nicotine9,24. Although some controversies remain regarding the nootropic effect of nicotine on specific memory functions and on individual differences in such effects, the preponderance of evidence from nonclinical animal and human studies supports memory-enhancing effects as a clinically relevant dimension of nicotine psychopharmacology9. In contrast, the effects of other alkaloids from the same chemical class in Solanaceae plants, such as cotinine and anatabine, are less well known23,31,32,33,34,35,36,37,38,39. The main findings for cotinine (the major metabolite of nicotine) include, for example, fear memory extinction, working memory, and sensory gating in rodent models of memory deficit23,40,41,42,43,44,45. The results of these studies suggest that the neurobehavioral effects of cotinine seem to significantly differ from those of nicotine46,47,48. Similarly, a few studies that investigated the effects of anatabine on memory used rodent models of neurodegeneration, such as Alzheimer’s disease and mild traumatic brain injury, the result of which may be at least partly attributed to the anti-inflammatory property of anatabine instead of its nootropic effect33,49,50.
In this study, we aimed to investigate these three alkaloids from the same chemical family—nicotine, cotinine, and anatabine—in parallel to understand and compare their potential role in memory. Nicotine served as an ideal, well-established, natural memory enhancing nicotinic reference compound with a very similar chemical structure as cotinine and anatabine. We selected Y-maze spontaneous alteration and novel object recognition (NOR) tasks in rodents to understand their efficacy in regulating spatial and recognition memory. The Y-maze test takes advantage of the innate investigative nature of rodents to explore new environment to assess short-term spatial memory. The test has been shown to be sensitive to hippocampal damage, gene manipulations, and amnesic drugs, for example51,52,53. Similarly, NOR relies on the innate investigative nature of rodents to explore new object to assess recognition memory. Recognition memory is a type of episodic memory that is often reported to be degraded with age in humans and in patients with Alzeheimer’s disease2. The rodent spontaneous NOR task has become a particularly popular method for evaluating nicotinics as well as other nootropic drugs2. From a practical point of view, both Y-maze and NOR are quite efficient, requiring no or short training, respectively. Neither of them require aversive conditioning, such as footshocks, food deprivation, or water immersion, which reduces the influence of sensory modalities and stress and thus, may simulate the natural memory challenges experienced by humans better than memory paradigms requiring aversive conditioning during the training sessions.
We induced a memory deficit using the muscarinic receptor antagonist scopolamine, which is a standard reference chemical for inducing memory deficit in rodents and humans to mimic the memory decline observed during natural aging and in Alzheimer’s disease patients54,55. For example, scopolamine-induced memory deficit in NOR has been used to assess the effect of nicotine and other nicotinic ligands on memory function56,57,58. The efficacy of the nicotinic alkaloids in the indicated memory paradigms was then compared.
Materials and methods
Chemicals
(‒)-Nicotine free base (CAS No. 54-11-5), (‒)-cotinine free base (CAS No. 486-56-6), and (‒)-scopolamine hydrobromide (CAS No. 6533–68‒2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). (±)-Anatabine free base (purity > 95% by HPLC) was custom-synthesized by WuXi AppTec (Shanghai, China). The nAChR agonist activities of nicotine, cotinine, and anatabine are indicated in Fig. 1 for reference18.
α4β2 and α7 nAChR EC50 values of nicotine, cotinine, and anatabine. α4β2 and α7 nAChRs EC50 values were determined in Chinese hamster ovarian (CHO) cells overexpressing the respective human nAChRs as reported by Alijevic et al.18. Nicotine and anatabine are potent, while cotinine is a weak α4β2 nAChR agonist. Nicotine is also a weak α7 agonist. NA not available due to no or low activity.
Animals
Adult male Swiss mice (6–7 weeks old; JANVIER, Saint Berthevin, France) were used for the Y-maze spontaneous alternation paradigm test, conducted by Amylgen SAS (Montferrier-sur-Lez, France). The mice were group-housed (4–8 mice per cage) in a temperature (22 ± 2 °C)- and humidity (40–60%)-controlled animal facility with a 12-h light/dark cycle (7:00 a.m. to 7:00 p.m.) and free access to food and water in accordance with the Direction Régionale de l'Alimentation, de l'Agriculture et de la Forêt du Languedoc-Roussillon (agreement #A 34-169-002). During the experiment, animal health was monitored for general appearance, activity, and acute or delayed mortality. All animal procedures were conducted in strict adherence to the European Union Directive of September 22, 2010 (2010/63/UE).
Adult male Wistar rats (3 months old; Envigo, Indianapolis, IN, USA) were used for the NOR paradigm test, conducted by Prime Behavior Testing Laboratories Inc. (PBTLI; Evans, GA, USA). The rats were double-housed in polycarbonate cages with corncob bedding in a vivarium of constant temperature (21–23 °C) and humidity (40–50%) with a 12 h light/dark cycle (7:00 a.m. –7:00 p.m.) and free access to food and water. All animal procedures employed by PBTLI were reviewed and approved by the Institutional Animal Care and Use Committee of Augusta University and conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. Measures were taken to minimize pain or discomfort in accordance with the Guide for the Care and Use of Laboratory Animals59. The animal studies were carried out in compliance with the ARRIVE guidelines.
Y-maze
Adult male Swiss mice (n = 12 per condition) received an intraperitoneal (i.p.) injection of nicotine (0.125, 0.25, and 0.5 mg/kg body weight [b.w.]), cotinine (0.25, 0.5, and 1 mg/kg b.w.), anatabine (0.25, 0.5, and 1 mg/kg b.w.), or vehicle (saline). The highest dose chosen for each of the compounds for Y-maze were the highest tolerated dose assessed in the tolerability test (Supplementary File S1). After 20 min, the mice were subcutaneously (s.c.) injected with scopolamine (0.5 mg/kg b.w. in saline). The injection volume was 5 mL/kg b.w. At 20 min after scopolamine injection, the animals were tested for spontaneous alternation performance in the Y-maze.
The Y-maze test, an index of short-term spatial memory, was designed in accordance with Itoh et al. and Hiramatus & Inoue60,61. In brief, the mice were placed at the end of one arm of a Y-shaped maze and allowed to move freely during an 8-min session. The series of arm entries were visually scored by an experimenter blind to the treatment. An alternation was defined as entries into all three arms on consecutive occasions. The number of maximum alternations equaled the total number of arm entries minus two, and the percentage alternation was calculated as (actual alternations/maximum alternations) × 100. The percentage alternation (memory index) and total number of arm entries (exploration index) were then analyzed62,63,64. For graphical representations of the results, memory index was normalized to Veh/Veh controls. None of the animals exhibited extreme behavior (i.e., alternation percentage < 20% or > 90% or number of arm entries < 10). Thus, all animals were included in the analysis. All test compounds were tolerated well at all doses. Experimenters were blind to the test conditions.
NOR
The NOR task was adapted from Ennaceur & Delacour as previously published by PBTLI44,65,66,67. In brief, adult male Wistar rats were acclimated, weighed, and individually placed in a dimly lit (10 lx) training/testing chamber without any objects for 10 min. Approximately 24 h after the habituation session, the rats (n = 8 per condition) received i.p. injections of scopolamine (0.2 mg/kg b.w.) and nicotine (0.03, 0.1, and 0.3 mg/kg b.w.), cotinine (30 and 100 mg/kg b.w.), anatabine (0.3, 1, and 3 mg/kg b.w.), or vehicle (saline). The doses were selected based on previous publications that showed nootropic effects of the respective compounds in rodents33,44,65,68,69. In particular, we chose higher doses of cotinine based on a previously published NOR study where the most significant pro-cognitive effect of cotinine was observed at 10 mg/kg when combined with donepezil and no effect at 0.3 or 1 mg/kg44. The injection volume was 1.0–2.0 mL/kg b.w. After 30 min, the rats were placed in the chamber with their nose facing the center of a long wall and allowed to explore two identical objects for 10 min and then returned to their home cages. Object recognition memory was assessed 3 h later by placing the animals back in the chamber with one object identical to that in training (familiar) and one new object (novel). The animals were allowed to freely explore the objects for 5 min. Two plastic multicolored Duplo-Lego block configured towers (12 cm in height, 6 cm in width) or two ceramic conical-shaped green Christmas tree salt/pepper shakers (12 cm in height, 5 cm in diameter) were used as the training objects. The object type that was not used during the training was used during the test session as the novel object as described in Terry et al. and Poddar et al.44,70. The primary behavioral measure was the time spent investigating each object, which was defined as the time the animal spent touching the object or directing its nose to the object at a distance of less than or equal to 2 cm or rearing up against the object. However, physically climbing on the object, using the object to support itself, or digging at the base of the object was not considered as an appropriate object exploratory behavior44,70.
A discrimination ratio (d2) was then calculated in each test trial and defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 ratio = (novel – familiar)/(novel + familiar). For data inclusion, a rat had to explore each individual object for at least 4 s and spend a minimum of 12 s on total object exploration. All rats met this criteria, and thus, all rats were included in the analysis. All test compounds were tolerated well at all doses. Experimenters were blind to the test conditions.
Statistical analysis
One-way analysis of variance (ANOVA) with Bonferroni post-hoc multiple comparison test was used for Y-maze statistical analysis (GraphPad Prism v8.2.1, GraphPad Software, San Diego, CA, USA). For the d2 ratio comparisons in NOR, a one-way ANOVA followed by a Student Newman Keuls post-hoc test by using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA) was conducted. The two-way repeated measures ANOVA was applied to the analysis of exploration times for NOR to understand the effects of treatment (or dose), object type (novel vs. familiar), and the treatment by object type interactions. Values with p < 0.05 were considered as statistically significant. Data are expressed as mean ± standard error of the mean (SEM).
Results
In the Y-maze spontaneous alternation test, the ability of mice to remember the previously visited arm was measured as a parameter of functional spatial memory. A single injection of nicotine, cotinine, and anatabine significantly suppressed the scopolamine-induced memory deficit in this spatial memory task in a dose-dependent manner (Fig. 2; treatment effect: p < 0.001). Nicotine and anatabine showed the most potent effect, being able to fully suppress the effect of scopolamine at 0.25 and 0.5 mg/kg b.w. for nicotine and at 0.5 mg/kg and 1 mg/kg b.w. for anatabine (Fig. 2b,d; p < 0.001 compared to scopolamine only treatment for both compounds at both doses). Cotinine also suppressed the effect of scopolamine at 0.5 and 1 mg/kg b.w. (p < 0.001 and p < 0.01, respectively, compared to scopolamine only treatment for both doses) and not at all at 0.25 mg/kg b.w. (Fig. 2c). The lowest dose of nicotine and anatabine and higher two doses of cotinine improved object recognition that was significantly different from Veh/Scopolamine controls, yet still significantly less than a full performance by Veh/Veh controls (bars in Fig. 2 with both asterisks and hash marks). This observation suggests that the animals did not fully recover from scopolamine-induced memory deficit at these particular doses for the respective compounds.
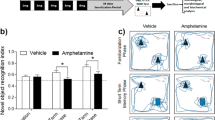
Effects of alkaloids on Y-maze working memory. (a) Schematic diagram of the Y-maze spontaneous alternation is shown. The test duration was 8 min. Memory indices, expressed as a percentage of vehicle response (Veh/Veh), are presented for (b) nicotine (NIC), (c) cotinine (COT), and (d) anatabine (ANAT). All compounds were able to significantly suppress scopolamine (SCOP)-induced memory deficit. Doses are indicated below each graph in mg/kg b.w. The vehicle in all panels are the same due to the fact all compounds were tested at the same time. **p < 0.01 and ***p < 0.001 compared with vehicle control without scopolamine (Veh/Veh; white bars). ###p < 0.001 compared with vehicle control with scopolamine (black bars). Grey bars are the lowest, bars with slashes are the medium, and checkered bars are the highest concentrations tested of the respective compounds. Data are expressed as mean ± SEM.
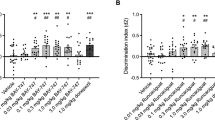
The effects of these chemicals on short-term object memory were also assessed by the NOR test in rats. In contrast to the results obtained in the Y-maze test of spatial memory, only nicotine was effective in suppressing the memory deficit induced by scopolamine (Fig. 3 and Supplementary File S2; treatment effect: p < 0.001 for the d2 ratio and treatment x object type interaction: p < 0.001 for the exploration time). Nicotine was able to fully suppress the effect of scopolamine at 0.1 and 0.3 mg/kg b.w. (Fig. 3b; p < 0.001 and p < 0.01, respectively). Cotinine and anatabine had no significant effect at tested doses (Fig. 3c,d). All rats explored each individual object for at least 4 s and demonstrated a minimum total object exploration time of 12 s, meeting the inclusion criteria for the study. Both right and left objects were explored equally during the training sessions, and the total exploration times were similar in the training and test sessions regardless of the treatment conditions (data not shown). Therefore, the observed treatment effects in the NOR test sessions were related to differences in recognition memory and not due to the confounding non-cognitive behavioral effects of scopolamine or the test compounds.
Effects of alkaloids on NOR short-term memory. (a) Schematic diagram of the NOR test is shown. The familiar objects are indicated as squares with stripes, and the new object is indicated as a checkered round object for presentation purposes. The test was conducted 3 h after training. The discrimination ratios (d2) are presented for (b) nicotine (NIC), (c) cotinine (COT), and (d) anatabine (ANAT). Only nicotine was able to significantly suppress scopolamine (SCOP)-induced memory deficit at 0.1 and 0.3 mg/kg. Doses are indicated below each graph in mg/kg b.w. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with vehicle control without scopolamine (white bars). ##p < 0.001 and ###p < 0.001 compared with vehicle control with scopolamine (black bars). Grey bars are the lowest, bars with slashes are the medium, and checkered bars are the highest concentrations tested of the respective compounds. Data are expressed as mean ± SEM.
Discussion
In this study, we tested the acute effects of three nAChR-activating alkaloids—nicotine, cotinine, and anatabine—on two types of memory: spatial memory and object recognition memory. We report, for the first time, that cotinine and anatabine differentially regulated spatial and object recognition memory in rodents. Nicotine, on the other hand, suppressed both scopolamine-induced memory deficit in the Y-maze memory task and the NOR short-term memory task in rodents, suggesting a more general role of nicotine in nonclinical models of memory function. This is the first time that these three alkaloids have been tested in parallel to observe their differential effects on specific memory classes in rodents. Previous animal behavioral studies have mainly focused on the effect of nicotine only, which have generally shown memory improvements using, for example, radial-arm maze, passive avoidance, and water maze in rodents2,30. The effect of cotinine (the major metabolite of nicotine) on the other hand, is less well-studied and findings are rather specific to, for example, fear memory extinction, working memory, and sensory gating in rodent models of memory deficit23,40,41,42,43,44,45. The results of these studies indirectly suggested that the neurobehavioral effects of cotinine significantly differ from those of nicotine when cotinine is used as a pharmacological tool46,47,48. Similarly, a few studies that investigated the effects of anatabine on memory have shown memory improvement in rodent models of memory deficit, such as Alzheimer’s diseases and mild traumatic brain injury, which may be partially explained by the anti-inflammatory properties of anatabine33,49,50. Thus, no direct comparison has been made among these alkaloids in the past.
The reason for the alkaloid-specific effects on memory is unclear, but it is most probably related to the fact that the alkaloids have differential activities on specific subtypes of nAChRs18. Previous studies have demonstrated distinct roles of nAChR subtypes in memory processes in rodents27,71. For example, α4β2 and α7 nAChRs are the major nAChRs present in the brain, and studies have demonstrated their important regulatory roles in various aspects of memory functions in human and nonclinical studies2,7. Although the expression patterns of α4β2 and α7 nAChRs overlap within the key brain regions for memory-associated processes (e.g., hippocampus and prefrontal cortex), there are crucial differences in substructure, cell type, and cellular localization (e.g., pre- vs post-synaptic) specificity between the two nAChR subtypes reported in rodents5,72,73. In turn, these finer distributions of the receptors are hypothesized to induce distinct effects on memory. For example, α7 nAChRs in the frontal cortex are thought to be involved in spatial working and reference memory in rats, while α4β2 nAChRs are thought to be involved mainly in spatial working memory74. However, in a study that analyzed the ventral hippocampus, the α4β2 nAChR antagonist dihydro-β-erythroidine induced both working and reference memory deficits, while the α7 nAChR antagonist methyllycaconitine only affected the spatial memory in the radial arm maze in rats, indicating a distinct task- and brain region-specific role of these receptors75. More recently, a study showed that, in the medial prefrontal cortex, α7 nAChRs are critical for encoding, while α4β2 nAChRs are required for retrieval of object recognition memory in rats7. However, neither receptor was involved in spatial memory. The observed effects were attributed to α7 nAChR-mediated glutamatergic pyramidal cell activation, resulting in long-term synaptic potentiation, and α4β2 nAChR-mediated GABAergic interneuron activation, resulting in long-term synaptic depression of hippocampal–prefrontal synapses7.
Interestingly, nicotine, cotinine, and anatabine have been reported to differentially bind to and activate α4β2 and α7 nAChRs in vitro18,26. Nicotine can, in fact, potently activate α4β2 nAChRs expressed in CHO cells at concentrations 1/10th of anatabine or 1/100th of cotinine concentrations. In addition, nicotine can also weakly activate α7 nAChRs expressed in CHO cells, while cotinine and anatabine cannot18. An α7 nAChR-positive allosteric modulator-like activity was previously suggested for cotinine44. However, the electrophysiological evidence was rather weak and could not be confirmed by Alijevic et al.18. Thus, these subtle differences in the receptor pharmacology of the three alkaloids may contribute to their unique effects on specific classes of memory. In fact, even slight differences in chemical structures are known to have significant changes in receptor pharmacology as exemplified by intense research effort on structure–activity relationships for drug discovery programs76 in addition to efforts to improve translatability of in vitro findings to animal studies77.
It is worth noting that two different species were used for Y-maze and NOR paradigms. Selections of strains/species of animals used in each test were based on the previous reports, indicating appropriateness and sensitivities of the strains/species to the respective behavioral paradigms44,78,79, which were validated by the respective behavioral test facilities. The fact that we were able to detect the effect of all compounds in Y-maze using Swiss mice suggest that the choice of strain and specie was appropriate for testing nicotinic compounds for this particular behavioral paradigm. We chose to use Wistar rats due to the fact that it was the rodent type used in Terry et al. where cotinine was reported to show procognitive effect when combined with donepezil by using NOR44. In fact, Wistar rats was reported to perform well in NOR compared to other strains or species79 and for testing nicotine2. The differences between pharmacokinetics of nicotine between rats and mice have been previously reported with a half-life of plasma nicotine in mice being shorter (less than 10 min in general) than that of rats (approximately 1 to 2 h) after i.p. or subcutaneous administration due to the specie differences in nicotine metabolism68,80,81,82. However, the fact that similar doses of nicotine suppressed scopolamine-induced memory deficit in both NOR and Y-maze in rats and mice, respectively, in the present study suggests that the specie differences in pharmacokinetics or any other variables introduced by the two testing facilities did not play a significant role in this instance. Instead, it supports the robustness of nicotine findings despite of these variables. In fact, Mohler et al. have demonstrated that effects of a compound can be confirmed across sites despite of varying testing equipment, animal suppliers, and general husbandry parameters used across sites83, which further supports the validity of our methods and robustness of the findings. Nevertheless, the results of our study should be understood with caution that different species were used in two test facilities.
Together, these findings confirm previous reports that nicotine can regulate cognitive functions in nonclinical animal models24,84,85, although it is also not risk free. We hypothesize that spatial and object recognition memory may recruit distinct neural processes that are regulated by specific subtypes of nAChRs in rodents. This specialization of receptor subtypes likely played a part in the memory-type-specific effect of the three alkaloids investigated in the current study in rodent models of memory deficit. The findings presented here is not directly translatable to human findings without additional studies86. Although it was out of scope of this paper, investigations on the effects of these and other alkaloids on long-term memory may provide further insights regarding the memory enhancing potential of these natural compounds that may pose less harm than nicotine.
References
Picciotto, M. R. & Zoli, M. Nicotinic receptors in aging and dementia. J. Neurobiol. 53, 641–655. https://doi.org/10.1002/neu.10102 (2002).
Terry, A. V. & Callahan, P. M. Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tobacco Res. 21, 383–394. https://doi.org/10.1093/ntr/nty166 (2019).
Wonnacott, S., Sidhpura, N. & Balfour, D. J. Nicotine: From molecular mechanisms to behaviour. Curr. Opin. Pharmacol. 5, 53–59. https://doi.org/10.1016/j.coph.2004.12.002 (2005).
Gotti, C. & Clementi, F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 74, 363–396. https://doi.org/10.1016/j.pneurobio.2004.09.006 (2004).
Gotti, C., Zoli, M. & Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 27, 482–491. https://doi.org/10.1016/j.tips.2006.07.004 (2006).
Hogg, R. C., Raggenbass, M. & Bertrand, D. Nicotinic acetylcholine receptors: From structure to brain function. Rev. Physiol. Biochem. Pharmacol. 147, 1–46. https://doi.org/10.1007/s10254-003-0005-1 (2003).
Sabec, M. H., Wonnacott, S., Warburton, E. C. & Bashir, Z. I. Nicotinic acetylcholine receptors control encoding and retrieval of associative recognition memory through plasticity in the medial prefrontal cortex. Cell Rep. 22, 3409–3415. https://doi.org/10.1016/j.celrep.2018.03.016 (2018).
Wallace, T. L. & Bertrand, D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem. Pharmacol. 85, 1713–1720. https://doi.org/10.1016/j.bcp.2013.04.001 (2013).
Valentine, G. & Sofuoglu, M. Cognitive effects of nicotine: Recent progress. Curr. Neuropharmacol. 16, 403–414. https://doi.org/10.2174/1570159X15666171103152136 (2018).
Taly, A., Corringer, P. J., Guedin, D., Lestage, P. & Changeux, J. P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 8, 733–750. https://doi.org/10.1038/nrd2927 (2009).
Swan, G. E. & Lessov-Schlaggar, C. N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 17, 259–273. https://doi.org/10.1007/s11065-007-9035-9 (2007).
Debnath, B. et al. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 9, 56–72. https://doi.org/10.1016/j.mtchem.2018.05.001 (2018).
Hussain, G. et al. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 14, 341–357. https://doi.org/10.7150/ijbs.23247 (2018).
Chaves, S. K., Feitosa, C. M. & da, S. A. L. Alkaloids pharmacological activities: Prospects for the development of phytopharmaceuticals for neurodegenerative diseases. Curr. Pharm. Biotechnol. 17, 629–635. https://doi.org/10.2174/138920101707160503201541 (2016).
Perviz, S., Khan, H. & Pervaiz, A. Plant alkaloids as an emerging therapeutic alternative for the treatment of depression. Front. Pharmacol. 7, 28. https://doi.org/10.3389/fphar.2016.00028 (2016).
Maione, F. et al. Phenols, alkaloids and terpenes from medicinal plants with antihypertensive and vasorelaxant activities. A review of natural products as leads to potential therapeutic agents. Nat. Prod. Commun. 8, 539–544 (2013).
Vina, D., Serra, S., Lamela, M. & Delogu, G. Herbal natural products as a source of monoamine oxidase inhibitors: A review. Curr. Top. Med. Chem. 12, 2131–2144. https://doi.org/10.2174/156802612805219996 (2012).
Alijevic, O. et al. An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human alpha4beta2 and alpha7 nicotinic acetylcholine receptors. Phytochemistry 170, 112187. https://doi.org/10.1016/j.phytochem.2019.112187 (2020).
Kleinsasser, N. H., Wallner, B. C., Harreus, U. A., Zwickenpflug, W. & Richter, E. Genotoxic effects of myosmine in human lymphocytes and upper aerodigestive tract epithelial cells. Toxicology 192, 171–177. https://doi.org/10.1016/s0300-483x(03)00296-8 (2003).
Mishra, A. et al. Harmful effects of nicotine. Indian J. Med. Paediatr. Oncol. 36, 24–31. https://doi.org/10.4103/0971-5851.151771 (2015).
Ji, L., Melkonian, G., Riveles, K. & Talbot, P. Identification of pyridine compounds in cigarette smoke solution that inhibit growth of the chick chorioallantoic membrane. Toxicol. Sci. 69, 217–225. https://doi.org/10.1093/toxsci/69.1.217 (2002).
Echeverria, V., Grizzell, J. A. & Barreto, G. E. Neuroinflammation: A therapeutic target of cotinine for the treatment of psychiatric disorders?. Curr Pharm. Des. 22, 1324–1333 (2016).
Terry, A. V. Jr., Callahan, P. M. & Hernandez, C. M. Nicotinic ligands as multifunctional agents for the treatment of neuropsychiatric disorders. Biochem. Pharmacol. 97, 388–398. https://doi.org/10.1016/j.bcp.2015.07.027 (2015).
Rezvani, A. H. & Levin, E. D. Cognitive effects of nicotine. Biol. Psychiat. 49, 258–267 (2001).
Holtman, J. R. Jr., Crooks, P. A., Johnson-Hardy, J. K. & Wala, E. P. The analgesic and toxic effects of nornicotine enantiomers alone and in interaction with morphine in rodent models of acute and persistent pain. Pharmacol. Biochem. Behav. 94, 352–362. https://doi.org/10.1016/j.pbb.2009.09.017 (2010).
Xing, H., Keshwah, S., Rouchaud, A. & Kem, W. R. A Pharmacological comparison of two isomeric nicotinic receptor agonists: The marine toxin isoanatabine and the tobacco alkaloid anatabine. Marine Drugs 18. https://doi.org/10.3390/md18020106 (2020).
Levin, E. D. Nicotinic receptor subtypes and cognitive function. J. Neurobiol. 53, 633–640. https://doi.org/10.1002/neu.10151 (2002).
Bertrand, D. & Terry, A. V. Jr. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 151, 214–225. https://doi.org/10.1016/j.bcp.2017.12.008 (2018).
Newhouse, P. et al. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology 78, 91–101. https://doi.org/10.1212/WNL.0b013e31823efcbb (2012).
Levin, E. D., McClernon, F. J. & Rezvani, A. H. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184, 523–539. https://doi.org/10.1007/s00213-005-0164-7 (2006).
Andersson, C., Wennström, P. & Gry, J. Nicotine alkaloids in Solanaceous food plants (Ekspressen Tryk & Kopicenter, 2003).
Dwoskin, L. P. et al. Minor alkaloids of tobacco release [3H]dopamine from superfused rat striatal slices. Eur. J. Pharmacol. 276, 195–199 (1995).
Levin, E. D. et al. Effects of tobacco smoke constituents, anabasine and anatabine, on memory and attention in female rats. J. Psychopharmacol. 28, 915–922. https://doi.org/10.1177/0269881114543721 (2014).
Lippiello, P. M. et al. Metanicotine: A nicotinic agonist with central nervous system selectivity-in vitro and in vivo characterization. Drug Dev. Res. 38, 169–176 (1996).
Suemaru, K. et al. Antidepressant-like action of nicotine in forced swimming test and brain serotonin in mice. Physiol. Behav. 88, 545–549. https://doi.org/10.1016/j.physbeh.2006.05.007 (2006).
Anderson, S. M. & Brunzell, D. H. Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at beta2*nicotinic acetylcholine receptors. Br. J. Pharmacol. 172, 2864–2877. https://doi.org/10.1111/bph.13090 (2015).
Andreasen, J. T. & Redrobe, J. P. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: Role of strain, test and sex. Behav. Pharmacol. 20, 286–295. https://doi.org/10.1097/FBP.0b013e32832c713e (2009).
Vazquez-Palacios, G., Bonilla-Jaime, H. & Velazquez-Moctezuma, J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine]. Pharmacol. Biochem. Behav. 78, 165–169. https://doi.org/10.1016/j.pbb.2004.03.002 (2004).
Xia, W., Veljkovic, E., Koshibu, K., Peitsch, M. C. & Hoeng, J. Neurobehavioral effects of selected tobacco constituents in rodents following subchronic administration. Eur. J. Pharmacol. 865, 172809. https://doi.org/10.1016/j.ejphar.2019.172809 (2019).
Buccafusco, J. J. & Terry, A. V. Jr. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: Potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem. Pharmacol. 78, 852–862. https://doi.org/10.1016/j.bcp.2009.06.102 (2009).
Terry, A. V. Jr., Hernandez, C. M., Hohnadel, E. J., Bouchard, K. P. & Buccafusco, J. J. Cotinine, a neuroactive metabolite of nicotine: Potential for treating disorders of impaired cognition. CNS Drug Rev. 11, 229–252 (2005).
Grizzell, J. A., Iarkov, A., Holmes, R., Mori, T. & Echeverria, V. Cotinine reduces depressive-like behavior, working memory deficits, and synaptic loss associated with chronic stress in mice. Behav. Brain Res. 268, 55–65. https://doi.org/10.1016/j.bbr.2014.03.047 (2014).
Terry, A. V. Jr. et al. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem. Pharmacol. 83, 941–951. https://doi.org/10.1016/j.bcp.2011.12.043 (2012).
Terry, A. V. Jr., Callahan, P. M. & Bertrand, D. R-(+) and S-(-) isomers of cotinine augment cholinergic responses in vitro and in vivo. J. Pharmacol. Exp. Ther. 352, 405–418. https://doi.org/10.1124/jpet.114.219881 (2015).
Zeitlin, R. et al. Cotinine enhances the extinction of contextual fear memory and reduces anxiety after fear conditioning. Behav. Brain Res. 228, 284–293. https://doi.org/10.1016/j.bbr.2011.11.023 (2012).
Echeverria, F. & Zeitlin, R. in Neuroscience of Nicotine: Mechanisms and Treatment Ch. 22, 173–180 (Academic Press, 2019).
Echeverria, V. & Zeitlin, R. Cotinine: A potential new therapeutic agent against Alzheimer’s disease. CNS Neurosci. Ther. 18, 517–523. https://doi.org/10.1111/j.1755-5949.2012.00317.x (2012).
Grizzell, J. A. & Echeverria, V. New insights into the mechanisms of action of cotinine and its distinctive effects from nicotine. Neurochem. Res. 40, 2032–2046. https://doi.org/10.1007/s11064-014-1359-2 (2015).
Verma, M. et al. Chronic anatabine treatment reduces Alzheimer’s disease (AD)-like pathology and improves socio-behavioral deficits in a transgenic mouse model of AD. PLoS ONE 10, e0128224. https://doi.org/10.1371/journal.pone.0128224 (2015).
Ferguson, S. et al. Acute or delayed treatment with anatabine improves spatial memory and reduces pathological sequelae at late time-points after repetitive mild traumatic brain injury. J. Neurotrauma 34, 1676–1691. https://doi.org/10.1089/neu.2016.4636 (2017).
Albani, S. H., McHail, D. G. & Dumas, T. C. Developmental studies of the hippocampus and hippocampal-dependent behaviors: Insights from interdisciplinary studies and tips for new investigators. Neurosci. Biobehav. Rev. 43, 183–190. https://doi.org/10.1016/j.neubiorev.2014.04.009 (2014).
Seo, J. Y., Ju, S. H., Oh, J., Lee, S. K. & Kim, J. S. Neuroprotective and cognition-enhancing effects of compound K isolated from red ginseng. J. Agric. Food Chem. 64, 2855–2864. https://doi.org/10.1021/acs.jafc.5b05789 (2016).
Yuede, C. M., Dong, H. & Csernansky, J. G. Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav. Pharmacol. 18, 347–363. https://doi.org/10.1097/FBP.0b013e3282da278d (2007).
Klinkenberg, I. & Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 34, 1307–1350. https://doi.org/10.1016/j.neubiorev.2010.04.001 (2010).
Gilles, C. & Luthringer, R. Pharmacological models in healthy volunteers: Their use in the clinical development of psychotropic drugs. J. Psychopharmacol. 21, 272–282. https://doi.org/10.1177/0269881107077733 (2007).
Ciamei, A., Aversano, M., Cestari, V. & Castellano, C. Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacology 154, 126–130 (2001).
Marcus, M. M. et al. Alpha7 nicotinic acetylcholine receptor agonists and PAMs as adjunctive treatment in schizophrenia. An experimental study. Eur. Neuropsychopharmacol. J. Eur. College Neuropsychopharmacol. 26, 1401–1411. https://doi.org/10.1016/j.euroneuro.2016.07.004 (2016).
Sultan, A. et al. Thujone inhibits the function of alpha7-nicotinic acetylcholine receptors and impairs nicotine-induced memory enhancement in one-trial passive avoidance paradigm. Toxicology 384, 23–32. https://doi.org/10.1016/j.tox.2017.04.005 (2017).
National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. (The National Academies Press, 2011).
Itoh, J., Ukai, M. & Kameyama, T. Dynorphin A-(1–13) markedly improves scopolamine-induced impairment of spontaneous alternation performance in mice. Eur. J. Pharmacol. 236, 341–345. https://doi.org/10.1016/0014-2999(93)90469-x (1993).
Hiramatsu, M. & Inoue, K. Nociceptin/orphanin FQ and nocistatin on learning and memory impairment induced by scopolamine in mice. Br. J. Pharmacol. 127, 655–660. https://doi.org/10.1038/sj.bjp.0702595 (1999).
Villard, V., Espallergues, J., Keller, E., Vamvakides, A. & Maurice, T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (sigma1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J. Psychopharmacol. 25, 1101–1117. https://doi.org/10.1177/0269881110379286 (2011).
Maurice, T., Roman, F. J., Su, T. P. & Privat, A. Beneficial effects of sigma agonists on the age-related learning impairment in the senescence-accelerated mouse (SAM). Brain Res. 733, 219–230. https://doi.org/10.1016/0006-8993(96)00565-3 (1996).
Maurice, T., Su, T. P. & Privat, A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 83, 413–428. https://doi.org/10.1016/s0306-4522(97)00405-3 (1998).
Ennaceur, A. & Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 31, 47–59. https://doi.org/10.1016/0166-4328(88)90157-x (1988).
Callahan, P. M., Hutchings, E. J., Kille, N. J., Chapman, J. M. & Terry, A. V. Jr. Positive allosteric modulator of alpha7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology 67, 201–212. https://doi.org/10.1016/j.neuropharm.2012.10.019 (2013).
Callahan, P. M., Terry, A. V. Jr. & Tehim, A. Effects of the nicotinic alpha7 receptor partial agonist GTS-21 on NMDA-glutamatergic receptor related deficits in sensorimotor gating and recognition memory in rats. Psychopharmacology 231, 3695–3706. https://doi.org/10.1007/s00213-014-3509-2 (2014).
Matta, S. G. et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319. https://doi.org/10.1007/s00213-006-0441-0 (2007).
Ennaceur, A. & Delacour, J. Effect of combined or separate administration of piracetam and choline on learning and memory in the rat. Psychopharmacology 92, 58–67. https://doi.org/10.1007/bf00215480 (1987).
Poddar, I. et al. Chronic oral treatment with risperidone impairs recognition memory and alters brain-derived neurotrophic factor and related signaling molecules in rats. Pharmacol. Biochem. Behav. 189, 172853. https://doi.org/10.1016/j.pbb.2020.172853 (2020).
Rushforth, S. L., Allison, C., Wonnacott, S. & Shoaib, M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci. Lett. 471, 114–118. https://doi.org/10.1016/j.neulet.2010.01.022 (2010).
Feduccia, A. A., Chatterjee, S. & Bartlett, S. E. Neuronal nicotinic acetylcholine receptors: Neuroplastic changes underlying alcohol and nicotine addictions. Front. Mol. Neurosci. 5, 83. https://doi.org/10.3389/fnmol.2012.00083 (2012).
dos Santos Coura, R. & Granon, S. Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology (Berl) 221, 1–18. doi:https://doi.org/10.1007/s00213-011-2596-6 (2012).
Chan, W. K., Wong, P. T. & Sheu, F. S. Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology 52, 1641–1649. https://doi.org/10.1016/j.neuropharm.2007.03.008 (2007).
Levin, E. D., Bradley, A., Addy, N. & Sigurani, N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience 109, 757–765. https://doi.org/10.1016/s0306-4522(01)00538-3 (2002).
Guha, R. On exploring structure-activity relationships. Methods Mol. Biol. (Clifton, N.J.) 993, 81–94. https://doi.org/10.1007/978-1-62703-342-8_6 (2013).
Talevi, A. et al. CNS drug development: Lost in translation?. Mini. Rev. Med. Chem. 12, 959–970. https://doi.org/10.2174/138955712802762356 (2012).
Dellu, F., Contarino, A., Simon, H., Koob, G. F. & Gold, L. H. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol. Learn. Mem. 73, 31–48. https://doi.org/10.1006/nlme.1999.3919 (2000).
van Goethem, N. P. et al. Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav. Brain Res. 232, 323–334. https://doi.org/10.1016/j.bbr.2012.03.023 (2012).
Petersen, D. R., Norris, K. J. & Thompson, J. A. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab. Dispos. Biol. Fate Chem. 12, 725–731 (1984).
Craig, E. L. et al. Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug Metab. Dispos. Biol. Fate Chem. 42, 1447–1455. https://doi.org/10.1124/dmd.114.058719 (2014).
Ghosheh, O., Dwoskin, L. P., Li, W. K. & Crooks, P. A. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-(14)C]nicotine. Drug Metab. Dispos. Biol. Fate Chem. 27, 1448–1455 (1999).
Mohler, E. G. et al. Cross-site strain comparison of pharmacological deficits in the touchscreen visual discrimination test. Psychopharmacology 232, 4033–4041. https://doi.org/10.1007/s00213-015-4012-0 (2015).
Hahn, B., Wells, A. K., Lenartowicz, A. & Yuille, M. B. Nicotine effects on associative learning in human non-smokers. Neuropsychopharmacology 43, 2190–2196. https://doi.org/10.1038/s41386-018-0183-9 (2018).
Warburton, D. M. Nicotine as a cognitive enhancer. Prog. Neuropsychopharmacol. Biol. Psychiatry 16, 181–191. https://doi.org/10.1016/0278-5846(92)90069-q (1992).
Mondadori, C. Nootropics: preclinical results in the light of clinical effects; comparison with tacrine. Crit. Rev. Neurobiol. 10, 357–370 (1996).
Acknowledgements
We thank Amylgen for their excellent work on the Y-maze project and PBTLI for their excellent work on the NOR project. Special thanks to Dr. Laura Ceolin and Dr. Francois Romain for countless valuable discussions regarding the behavioral study designs and analyses. We also thank Dr. Wenhao Xia, Dr. Karsta Luettich, Dr. Anatoly Mazurov, Dr. Omar Alijevic, Dr. Stefan Frentzel, and Dr. Damian McHugh for the insightful scientific discussions.
Author information
Authors and Affiliations
Contributions
P.C. and A.T. designed, conduced, and analyzed the NOR behavioral test. M.P. and J.H. critically reviewed the manuscript for important intellectual content. K.K. initiated, designed, and analyzed the experiments and wrote the manuscript. All authors approved the final version to be published and declare that all data were generated in-house and that no paper mill and other ways of manipulating research materials were used.
Corresponding authors
Ethics declarations
Competing interests
M.P., J.H., & K.K. are employed by Philip Morris International. P.C. and A.T. are employed by PBTLI and Medical College of Georgia, Augusta University. Philip Morris International employed PBTLI’s service for NOR experiment and Amylgen’s service for Y-maze experiment. This work was funded solely by Philip Morris International.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Callahan, P.M., Terry, A.V., Peitsch, M.C. et al. Differential effects of alkaloids on memory in rodents. Sci Rep 11, 9843 (2021). https://doi.org/10.1038/s41598-021-89245-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89245-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.