Abstract
COVID-19 is a disease with a variable clinical course ranging from mild symptoms to critical illness, organ failure, and death. Prospective biomarkers may help to predict the severity of an individual’s clinical course and mortality risk. We analyzed asymmetric (ADMA) and symmetric dimethylarginine (SDMA) in blood samples from 31 patients hospitalized for COVID-19. We calculated associations of ADMA and SDMA with mortality and organ failure, and we developed a predictive algorithm based upon these biomarkers to predict mortality risk. Nine patients (29%) experienced in-hospital death. SDMA and ADMA serum concentrations were significantly higher at admission in COVID-19 patients who died than in survivors. Cut-offs of 0.90 µmol/L for SDMA (AUC, 0.904, p = 0.0005) and 0.66 µmol/L for ADMA (AUC, 0.874, p = 0.0013) were found in ROC analyses to best discriminate both subgroups of patients. Hazard ratio for in-hospital mortality was 12.2 (95% CI: 2.2–31.2) for SDMA and 6.3 (1.1–14.7) for ADMA above cut-off. Sequential analysis of both biomarkers allowed discriminating a high-risk group (87.5% mortality) from an intermediate-risk group (25% mortality) and a low-risk group (0% mortality). Elevated circulating concentrations of SDMA and ADMA may help to better identify COVID-19 patients with a high risk of in-hospital mortality.
Similar content being viewed by others
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 that has evolved into a pandemic since first observed in China in late 2019. Most SARS-CoV-2-infected patients remain asymptomatic or develop mild symptoms of respiratory infection and fever. However, about 19% of infected individuals develop a severe course of the disease requiring hospitalization1. The disease may aggravate in a few patients into life-threatening acute respiratory failure, requiring intensive care treatment and respiratory support including extracorporeal membrane oxygenation (ECMO). Ultimately, mortality is associated with multiple factors including comorbidities like diabetes or cardiovascular diseases2.
During the first surge of SARS-CoV-2 infection, the individual outcome of patients has remained rather unpredictable, and multiple efforts have been undertaken to identify co-morbidities, biomarkers, and clinical or imaging scores to better predict outcome of hospitalized COVID-19 patients3,4. A severity score consisting of age, oxygen saturation, mean arterial pressure, blood urea nitrogen, C-reactive protein, and the international normalized ratio was shown to improve prediction of COVID-19-related in-hospital mortality5. Other groups proposed redefining established risk factors of cardiac disease6 or organ damage markers like aspartate aminotransferase and alanine aminotransferase7 to better predict worse outcomes in COVID-19 patients. Another risk score for prediction of mortality of hospitalized COVID-19 patients comprised age, heart rate, oxygen saturation, lactate dehydrogenase activity, pro-calcitonin (PCT) and the presence or absence of chronic obstructive pulmonary disease or congestive heart failure8. Conditions that affect the course of the disease and outcome of COVID-19 patients include pre-existing morbidities like obesity or type 2 diabetes9, evolvement of thromboembolic vascular complications10, acute kidney failure11,12, and neurovascular damage4. The broad spectrum of individual disease courses and outcomes calls for continued efforts to identify biomarkers for risk prediction, as the second and, meanwhile, third waves of the pandemic are ongoing and numbers of infected individuals are rapidly rising. The identification of novel risk factors or biomarkers leading to early identification of patients at high risk of dying of the SARS-CoV-2 infection might help to better target these patients to early monitoring and intervention13.
Dysfunctional production of nitric oxide (NO) by endothelial cells may contribute to COVID-19-associated morbidities9,10,11. Endothelial cell infection by SARS-CoV-2 may lead to endothelial inflammation and dysfunction of its paracrine functions14. Angiotensin-converting enzyme 2 (ACE2), an important regulator of NO release15, is also the host cell receptor for SARS-CoV-216, thus linking SARS-CoV-2 infection and endothelial NO signaling. NO synthesis is tightly regulated17. Asymmetric (ADMA) and symmetric dimethylarginine (SDMA) are endogenous modulators of NO synthesis and intracellular L-arginine availability in the endothelium18; their circulating concentrations being dysregulated in hypoxia19. In addition, inhibition of NO synthesis by ADMA and SDMA may affect immune response and inflammatory reaction, as they also interfere with inducible NO synthase, an enzyme that is upregulated by inflammatory cytokines20.
Both metabolites have been shown to predict morbidity and mortality risk in populations with a wide range of risk: ADMA is associated with all-cause mortality in the general population21,22, in patients with end-stage renal disease23, and in patients with pre-existing cardiovascular disease24. SDMA is a predictor of mortality after acute ischemic stroke25 and in the general population. Both also help to identify high-risk patients within critically ill populations: ADMA and SDMA have been shown by our group to be predictors of mortality in patients with severe sepsis26. Nijveldt and co-workers proposed high circulating ADMA concentrations as risk factors of ICU mortality in critically ill patients27; moreover, ADMA is a marker of perioperative complications in patients undergoing major abdominal surgery28. These results were confirmed in a longitudinal study of ADMA metabolism in critically ill patients29.
We therefore retrospectively studied a cohort of consecutive COVID-19 patients hospitalized in a tertiary care medical center, of whom blood samples were stored under standardized conditions. We assessed whether ADMA and SDMA serum concentrations may help to better identify those at high risk of COVID-19-associated organ dysfunction and death.
Results
Baseline characteristics and clinical course of the patients
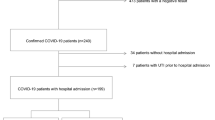
We included 31 patients (11 women, 20 men) with a mean age of 63.3 ± 17.8 years. 15 patients were primary admissions; 16 patients were referred from other hospitals (amongst them 8 with ongoing mechanical ventilation). With the exception of one, all patients had pre-existing co-morbidities. The mean duration from first symptoms to hospital admission was 6.8 ± 6.3 days. The mean duration of treatment in our medical center was 30.6 ± 27.0 days; need for oxygen insufflation varied from 0 to 97 days with a mean of 24.8 ± 29.1 days. 19 patients were treated on ICU for a mean of 34.7 ± 31.5 days. Detailed patient characteristics of this cohort are given in Table 1.
Nine patients (29%) died in-hospital. Causes of death were multi-organ failure in five patients (16%), respiratory failure in two (6.5%), and hemorrhagic complications in two patients with respiratory failure (6.5%). Patients who survived had a mean SOFA score at admission of 3.0 ± 3.5, whereas patients who died during in-patient treatment had a mean initial SOFA score of 7.0 ± 3.8 (p < 0.001). 14 patients had ARDS (seven of them died), five patients with ARDS were treated by ECMO (all of them died), 10 patients developed a high thromboembolic burden (six of them died), 14 patients had cardiac injury (nine of them died), 11 patients had liver injury (five of them died), eight patients developed acute kidney injury (three of them died), and ten patients developed circulatory insufficiency (three of them died). Patients who died in-hospital had significantly higher leukocyte count, higher CRP, and higher PCT concentrations at admission (Table 1). D-dimer concentrations and eGFR were not significantly different between survivors and non-survivors.
Serum concentrations of ADMA and SDMA
Mean ADMA serum concentration was significantly higher in patients referred to our hospital either with ongoing mechanical ventilation or without (0.84 ± 0.15 µmol/L and 0.66 ± 0.29 µmol/L, respectively; p = n.s.); it was lowest in primary admissions (0.58 ± 0.13 µmol/L; p = 0.010 for difference between groups in ANOVA). By contrast, there was no significant difference in mean SDMA serum concentration between patients referred to our ICU with ongoing mechanical ventilation (0.99 ± 0.40 µmol/L), patients referred without mechanical ventilation (0.79 ± 0.24 µmol/L; p = n.s. vs. patients with mechanical ventilation), and primary admissions (0.85 ± 0.46 µmol/L; p = 0.598 for difference between groups in ANOVA). ADMA concentration correlated significantly with CRP and leukocyte count, but not with PCT and eGFR (supplementary Fig. S1). SDMA concentration correlated inversely with eGFR, positively with CRP, but not with PCT nor with leukocyte count (supplementary Fig. S2).
The serum concentrations of ADMA and SDMA at hospital admission were significantly higher in patients who experienced in-hospital death versus those who survived (ADMA, 0.86 ± 0.07 µmol/L vs. 0.59 ± 0.03 µmol/L, p = 0.0004; SDMA, 1.15 ± 0.09 µmol/L vs. 0.78 ± 0.08 µmol/L, p = 0.017; Fig. 1). The ADMA serum concentration further increased significantly over time in patients who died, but not in survivors (Fig. 2a). The difference of SDMA levels between both groups remained significant during the course of hospitalization (Fig. 2b). The differences in ADMA and SDMA between survivors and non-survivors were stable when six patients with pre-existing chronic kidney disease were excluded (supplementary Fig. S3); the same was true for the comparison of eight patients admitted with ongoing mechanical ventilation versus those who were not (supplementary Fig. S4).
Time course of ADMA (a) and SDMA (b) serum concentrations of hospitalized COVID-19 patients during in-hospital treatment. N refers to the number of patients for whom blood samples were available for analysis at each time point. P values given in the legend refer to two-sided ANOVA for trend between groups; asterisk mark statistically significant differences between both group at a specific time point in Bonferroni’s multiple comparisons test. *p < 0.05, **p < 0.01 between groups.
Association of biomarkers with COVID-19-related outcome
ADMA and SDMA were differentially associated with organ injury evolving during COVID-19: Both ADMA and SDMA were significantly higher in 14 patients who developed cardiac injury (ADMA, 0.79 ± 0.06 vs. 0.57 ± 0.04 µmol/L, p = 0.048; SDMA, 1.02 ± 0.08 vs. 0.78 ± 0.11 µmol/L, p = 0.04). ADMA was also higher in 14 patients with ARDS (0.78 ± 0.07 vs. 0.58 ± 0.03 µmol/L, p = 0.01) and tended to be higher in 11 patients with liver injury, but this trend did not reach statistical significance. SDMA was significantly higher in patients with liver injury (1.07 ± 0.16 vs. 0.79 ± 0.07 µmol/L, p = 0.017) and showed a non-significant trend towards higher levels in ARDS. None of the two biomarkers was different in patients with (N = 8) or without acute kidney injury. The differences in the two biomarkers in these diseases are graphically shown in supplementary Fig. S5.
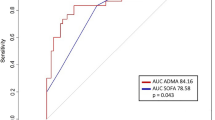
We performed ROC analyses with mortality as outcome for SDMA, ADMA, SOFA score, and other blood biomarkers that were significantly elevated at admission in non-survivors. The SOFA score showed an AUC of 0.819 (95% CI, 0.659–0.980; cut-off, 5.5 points; p = 0.007); CRP had an AUC of 0.843 (95% CI, 0.702–0.985; cut-off, 94.1; p = 0.003), and PCT had an AUC of 0.876 (95% CI, 0.745–1.000; cut-off, 0.32; p = 0.001). Leukocyte cell count had an AUC of 0.833 (95% CI, 0.687–0.980; cut-off, 10.7; p = 0.004). The ROC curves for these inflammatory parameters are given in supplementary Fig. S6.
ROC analysis further showed that an ADMA concentration of 0.66 µmol/L allowed to discriminate survivors from patients who experienced in-hospital death with 88.9% sensitivity and 81.1% specificity (AUC, 0.874 (95% CI, 0.743–1.000), p = 0.0013; Fig. 3a). The cut-off for SDMA was 0.90 µmol/L (sensitivity, 88.9%, specificity, 89.2%; AUC, 0.904 (95% CI, 0.793–1.000); p = 0.0005; Fig. 3b).
Prediction of COVID-19 mortality
The HR of in-hospital death for patients with a SOFA score ≥ or < 5.5 was 1.25 (95% CI, 0.29–5.66; p = n.s.). Addition of SDMA to the survival model significantly improved prediction of mortality; the HR was 6.31 for patients with high SOFA and high SDMA vs. those with both values low (95% CI, 1.07–37.16; p = 0.04). Likewise, neither CRP nor PCT when analyzed alone were significantly predictive of in-hospital death. For both inflammatory markers, addition of SDMA significantly improved predictive power (supplementary Fig. S7 and Table 2).
COVID-19 patients with ADMA serum concentration ≥ 0.66 µmol/L had a significantly higher probability of in-hospital death than those with ADMA < 0.66 µmol/L. The HR was 6.33 (95% CI, 1.06–14.69), p = 0.043 (Fig. 4a). Individuals with SDMA serum concentration ≥ 0.90 µmol/L had a HR for in-hospital death of 12.18 (95% CI, 2.16–31.23), p = 0.002 (Fig. 4b).
We performed multivariable-adjusted logistic regression analyses with SDMA and ADMA above and below the cut-off concentrations as categorical variables. Both markers were significantly associated with in-hospital mortality in models adjusted for age and sex and for age, sex, and eGFR (Table 3). In a fully adjusted model including inflammatory markers, SDMA remained highly significantly associated with survival, whilst this association lost significance for ADMA (Table 3).
We next tested whether SDMA and ADMA combined in a single variable improved predictive power. However, neither (SDMA + ADMA) nor (SDMAxADMA) showed significant improvement over SDMA used alone (supplementary Fig. S8). In addition, we tested three previously published COVID-19 mortality risk scores 5,7,8; none of these scores showed a significant prediction of mortality in our patient cohort (supplementary Fig. S9).
By contrast, we observed that sequential measurements of SDMA and ADMA significantly enhanced discrimination of mortality risk. Patients with high SDMA and high ADMA concentrations had a HR of in-hospital mortality of 9.30 (95% CI, 2.09–41.37), p = 0.0034, as compared to those with both biomarker levels low; individuals with only one biomarker level elevated had an intermediate risk (p = n.s. vs. both biomarkers low; Fig. 4c). Using a decision tree algorithm, we were able to discriminate high-risk patients (SDMA ≥ 0.90 µmol/L and ADMA ≥ 0.66 µmol/L) with an in-hospital mortality of 87.5%, intermediate-risk patients (either SDMA or ADMA elevated) with an in-hospital mortality of 25%, and low-risk patients (SDMA < 0.90 µmol/L and ADMA < 0.66 µmol/L), whose in-hospital mortality was 0% (Fig. 5). Sequential measurement of SDMA and ADMA therefore provided the best predictive power for in-hospital death when compared to traditional risk markers or their combination with either SDMA or ADMA.
Decision tree analysis to identify the risk of in-hospital mortality. Out of 31 patients of whom serum samples were available for day 1, nine died (30%). First decision step: Patients were identified as having elevated risk when SDMA levels were ≥ 0.90 μmol/L, and moderate risk when SDMA levels were < 0.90 µmol/L. Second decision step: Additional analysis of ADMA allowed identification of patients with high risk (SDMA ≥ 0.90 µmol/L and ADMA ≥ 0.66 µmol/L (mortality, 87.5%), intermediate risk (SDMA ≥ 0.90 µmol/L or ADMA ≥ 0.66 µmol/L (mortality, 25%), or low risk (SDMA < 0.90 µmol/L and ADMA < 0.66 µmol/L (mortality, 0%).
Discussion
The present study is the first to report two novel biomarkers beyond currently established clinical chemistry and blood hematology parameters to identify hospitalized COVID-19 patients at high risk of in-hospital mortality. These biomarkers, SDMA and ADMA, have a high sensitivity and specificity to predict mortality amongst hospitalized COVID-19 patients.
In the general population, pre-existing conditions like advanced age, obesity, type 2 diabetes, and hypertension are risk factors for a severe course of COVID-19 requiring hospitalization30,31. In our cohort of hospitalized COVID-19 patients, however, none of these conditions was significantly associated with in-hospital mortality or organ dysfunction. This is not astonishing, as 30 out of the 31 patients had pre-existing conditions and the mean age of our cohort was above 60 years. Although severe COVID-19 is an inflammation-driven disease, leukocyte cell count was the only traditional inflammatory marker that was weakly associated with mortality, whilst neither the SOFA score nor other commonly used laboratory parameters like CRP and PCT were able to significantly identify high-risk patients. In addition, three previously published risk scores that are based on a variety of different traditional diagnostic parameters5,7,8 failed to significantly predict mortality in our COVID-19 cohort.
Current data suggest that proper function of endothelial NO synthase (eNOS) may be an important mechanism of defense after infection with SARS-CoV-232. Endothelial cell tropism of the SARS-CoV-2 virus causes endothelial inflammation14, which possibly accelerates endothelial dysfunction and NO deficiency33. Endothelial dysfunction as marked by dysfunctional endothelium-dependent, NO-mediated vasodilation, and the ensuing high thrombosis risk contribute to COVID-19 morbidity and mortality34. ADMA is an endogenous, competitive inhibitor of NO synthesis an—like its congener molecule, SDMA—an inhibitor of cellular L-arginine uptake18. Both dimethylarginines are formed through the action of protein arginine methyltransferases (PRMTs), enzymes that are involved in innate immune responses and in the response to hypoxia in the lung19. PRMTs produce mainly SDMA in the central nervous system, but ADMA in many other organs including heart, circulatory system, and lungs (for review, cf.17). ADMA is enzymatically degraded by dimethylarginine dimethylaminohydrolases (DDAH), the activity of which is reduced by cysteine nitrosylation35, resulting in ADMA accumulation in a manner reversible by antioxidants. SDMA, by contrast, is inactivated by alanine-glyoxylate aminotransferase (AGXT2), an enzyme predominantly expressed in the kidneys and liver36. The underlying biochemistry may explain the associations of ADMA and SDMA with ensuing organ dysfunction typical for COVID-19 patients, i.e. acute kidney injury, cardiovascular thromboembolic events, neurological damage, and multi-organ failure. ADMA and SDMA, two biomarkers causing impaired NO production, may interfere with two essential pathophysiological steps in COVID-19-associated critical illness: vascular failure and immune response. Whilst the interaction of ADMA and SDMA with endothelial NO production has been extensively studied, there is much less information available on their interaction with inducible NO synthase20. Nonetheless, both mechanisms may contribute to their roles as predictive biomarkers in the present cohort. In line with this, we have previously reported that sequential measurement of SDMA and ADMA helps to predict the lethality of sepsis in ICU-treated patients26. Others have also reported ADMA to be associated with ICU death in a heterogeneous cohort of critically ill patients27.
Respiratory failure and global hypoxemia were major pathophysiological problems that led to hospital admission of the patients included in our present study. We have previously shown that ADMA continuously increases in humans exposed to chronic-intermittent hypoxia37, and that DDAH1-/- mice that have high circulating ADMA concentration are prone to develop pulmonary hypertension and right ventricular hypertrophy upon exposure to chronic hypoxia38. Based upon our data showing that inhibitors of NO synthesis are predictive biomarkers for COVID-19 survival with high discriminative power, and in line with published pilot studies that successfully administered inhaled NO to treat severe respiratory failure39,40, therapeutic approaches aimed at restoring physiological NO function may help to better treat severe COVID-19.
Combined analysis of ADMA and SDMA was previously shown by us to predict mortality risk in sepsis patients26. In that study, critically ill patients during ICU treatment were included, the cut-off values used were 1.34 µmol/L for SDMA and 0.97 µmol/L for ADMA. In the present cohort of COVID-19 patients, we found lower cut-off values for both biomarkers, which is in line with the unselected character of the present cohort, including hospitalized COVID-19 patients with a moderate to severe disease course and 19 out of a total of 31 patients being treated on ICU.
This is a retrospective cohort study and therefore has certain limitations. The study was carried out at a single center and involved a relatively small number of patients, which did not allow us to perform extensive subgroup analyses. The population comprised exclusively hospitalized patients with confirmed COVID-19 in a tertiary care hospital, with a relevant portion of patients that were referred as secondary admissions from other hospitals in the region, mostly but not exclusively because of ARDS. Although we used a validated liquid chromatography-tandem mass spectrometric (LC–MS/MS) method for biomarker analysis, ADMA and SDMA may be measured by ubiquitously available laboratory methods. We have previously validated an ADMA enzyme-linked immunosorbent assay (ELISA) and established reference ranges using this method41. However, in the absence of a widely used routine analytical method for ADMA and SDMA, reference ranges reported in the literature are broad and may vary according to the analytical method applied, and cut-off values reported in this study relate to the analytical method we used, LC–MS/MS. Nevertheless, our observations warrant follow-up studies with larger patient groups and a more formalized statistical approach to confirm the utility of SDMA and ADMA to independently predict COVID-19 outcome and severity.
In conclusion, we show here for the first time that ADMA and SDMA are biomarkers that allow us to prospectively identify COVID-19 patients with a high mortality risk beyond the diagnostic utility of the SOFA score and commonly used laboratory parameters. This may help to monitor such patients more closely, establish intensive care treatment earlier, and reduce the lethality of the COVID-19 pandemic.
Patients and methods
Study cohort and protocol
31 consecutive patients with confirmed SARS-CoV-2 infection were primarily admitted with symptomatic COVID-19 to the University Hospital Aachen (UKA) or referred from another hospital between March and May 2020. Patients were included in this study if the main cause for hospital admission was COVID-19 disease. Patients had to have a positive SARS-CoV-2 test result in respiratory samples that was performed in our hospital or externally before admission. Patients were only included if an initial blood sample for biomarker analysis was available within 24 h after admission. All patients gave their informed consent to have their blood samples included into the RWTH centralized Biomaterial Bank (RWTH cBMB) for further scientific study. The Ethics Committee of the Medical Faculty of RWTH Aachen had consented the Covid-19 Aachen study (COVAS) according to their vote EK080/20 and the regulations of the RWTH CBMB (vote EK206/09). All investigations were performed in accordance with the Declaration of Helsinki in its latest revision. No further selection criteria were applied.
All patients were treated according to best medical practice and individual clinical needs. Patients were either isolated under standard care or treated in our intensive care unit (ICU). The decision on treatment strategies was based on clinical judgment of the severity of the disease and the presence or absence of acute respiratory distress syndrome (ARDS). Severity of ARDS was classified according to the degree of hypoxia as defined by the “Berlin definition”42.
Comorbidities (such as hypertension, overweight or obesity, diabetes, pre-existing respiratory or cardiovascular diseases, smoking, chronic kidney disease, malignancies, chronic liver disease), and medications prescribed at the time of admission were recorded in hospital, or taken from existing medical records. Blood samples were drawn into EDTA vacutainers on day 1 after admission, after one week, two weeks, and six weeks. Samples were immediately centrifuged and stored at -80 °C until analysis. Sample storage and logistics were managed by the team of the RWTH cBMB.
Measurement of ADMA and SDMA by LC–MS/MS
Validated protocols for liquid chromatography-tandem mass spectrometry (LC–MS/MS) were used to quantify ADMA and SDMA in serum43. Briefly, 25 μl of serum were diluted in methanol to which stable isotope labelled internal standards had been added. Subsequently, the compounds were converted into their butyl ester derivatives and quantified by LC–MS/MS (Xevo TQ-S cronos, Waters GmbH, Eschborn, Germany). Compounds were separated on an Aquity UPLC BEH C18 column (2·1 × 50 mm, 1.7 µm, Waters GmbH). The coefficient of variation for the quality control samples was below 15% for both compounds.
Clinical and biochemical assessment of patient status
Patients were assessed for eligibility based on a positive RT-PCR assay for SARS-CoV-2 in a respiratory tract sample as previously described30,44. Vital parameters presented in this study were taken between four and 24 h following hospital admission or intubation, with the worst values being depicted. We defined multi organ failure (MOF) as a failure of at least four major organs (heart, lungs, liver, kidneys) because of complications of COVID 19. Severity of ARDS was defined using P/F-ratio or the Horowitz index. Acute kidney injury was defined according to the AKIN criteria45 and/or need for continuous veno-venous hemofiltration in patients with no pre-existing chronic renal failure. Cardiac injury was defined as troponin T levels > 52 ng/mL or a relative increase by ≥ twofold during in-hospital treatment. Liver injury was defined as an increase in serum total bilirubin by ≥ twofold and/or increases in serum ALT and/or AST activities by ≥ threefold. High thromboembolic burden was defined as a relative increase of D-dimer levels by ≥ twofold during in-hospital treatment. Circulatory insufficiency/shock was defined as the need for catecholamines at any time during in-hospital treatment. Febrile days were defined as the time from fever onset until the last documented value above 38.5 ℃. Patients with a body mass index (BMI) of 25 to < 30 kg/m2 were classified as overweight and those with BMI ≥ 30 kg/m2 as obese. Diabetes and prediabetes were defined by clinical history, medication and HbA1c values ≥ 6.5% or ≥ 5.7 to < 6.5%, respectively. Serum and whole blood samples were obtained routinely at the time of admission. Complete blood count, coagulation tests, inflammatory markers [circulating levels of C-reactive protein (CRP), pro-calcitonin (PCT)] and creatinine levels in blood were measured among other tests. Creatinine clearance was estimated using the CKD-EPI formula46.
Statistical analyses
All variables were tested for normal distribution using the Kolmogorov–Smirnov test. Data are presented as mean with standard deviation (SD). Differences between groups were tested for significance using the nonparametric Mann–Whitney U test for two groups or the Kruskal–Wallis analysis of variance for more than two groups. The Chi2 test was used for comparison of categorical variables between groups. Time courses of ADMA and SDMA concentrations were examined using repeated measures two-way ANOVA followed by Tukey’s multiple comparisons test. Spearman’s rank correlation was used to assess pairwise correlations. Survival analyses were performed using Kaplan–Meier curves comparing patients with ADMA and SDMA above or below the cut-off value determined in receiver-operated curve (ROC) analyses. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by multivariable-adjusted logistic regression analyses. As we identified two biomarkers, ADMA and SDMA, as predictors of COVID-19 mortality, we analyzed additional models using (SDMA + ADMA) or (SDMAxADMA) as variables, respectively. In addition, we performed a decision tree analysis to determine risk upon sequential analysis of SDMA and ADMA. Cut-offs to separate risk groups were based on values determined in ROC analysis for both biomarkers. All statistical analyses were performed using SPSS (version 25; IBM Corporation, Armonk, NY, USA) and GraphPad Prism (version 6.01, GraphPad Software, San Diego, CA, USA). For all tests, p < 0.05 was considered statistically significant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
28 August 2021
The original online version of this Article was revised: In the original version of this Article the author name Rainer Böger was incorrectly indexed. The original Article has been corrected.
11 November 2021
The original online version of this Article was revised: In the original version of this Article the Funding section was omitted. The correct Funding section now reads: “Open Access funding enabled and organized by Projekt DEAL”.
References
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA 323, 1239–1242. https://doi.org/10.1001/jama.2020.2648 (2020).
Phua, J. et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 8, 506–517. https://doi.org/10.1016/s2213-2600(20)30161-2 (2020).
Ponti, G., Maccaferri, M., Ruini, C., Tomasi, A. & Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin Lab Sci 57, 389–399. https://doi.org/10.1080/10408363.2020.1770685 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/s0140-6736(20)30566-3 (2020).
Altschul, D. J. et al. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci. Rep. 10, 16726. https://doi.org/10.1038/s41598-020-73962-9 (2020).
Qin, J. J. et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension 76, 1104–1112. https://doi.org/10.1161/hypertensionaha.120.15528 (2020).
Qin, C. et al. High aspartate aminotransferase to alanine aminotransferase ratio on admission as risk factor for poor prognosis in COVID-19 patients. Sci. Rep. 10, 16496. https://doi.org/10.1038/s41598-020-73575-2 (2020).
Zhao, Z. et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS ONE 15, e0236618. https://doi.org/10.1371/journal.pone.0236618 (2020).
Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8, 782–792. https://doi.org/10.1016/s2213-8587(20)30238-2 (2020).
Wichmann, D. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 173, 268–277. https://doi.org/10.7326/m20-2003 (2020).
Braun, F. et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396, 597–598. https://doi.org/10.1016/s0140-6736(20)31759-1 (2020).
Kolhe, N. V., Fluck, R. J., Selby, N. M. & Taal, M. W. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 17, e1003406. https://doi.org/10.1371/journal.pmed.1003406 (2020).
Harrison, S. L., Fazio-Eynullayeva, E., Lane, D. A., Underhill, P. & Lip, G. Y. H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 17, e1003321. https://doi.org/10.1371/journal.pmed.1003321 (2020).
Varga, Z. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418. https://doi.org/10.1016/s0140-6736(20)30937-5 (2020).
Rabelo, L. A. et al. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS ONE 11, e0150255. https://doi.org/10.1371/journal.pone.0150255 (2016).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271-280.e278. https://doi.org/10.1016/j.cell.2020.02.052 (2020).
Böger, R. & Hannemann, J. Dual role of the L-arginine-ADMA-NO pathway in systemic hypoxic vasodilation and pulmonary hypoxic vasoconstriction. Pulm. Circ. 10, 2045894020918850. https://doi.org/10.1177/2045894020918850 (2020).
Böger, R. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 59, 824–833. https://doi.org/10.1016/s0008-6363(03)00500-5 (2003).
Hannemann, J., Zummack, J., Hillig, J. & Böger, R. Metabolism of asymmetric dimethylarginine in hypoxia: from bench to bedside. Pulm. Circ. 10, 2045894020918846. https://doi.org/10.1177/2045894020918846 (2020).
Böger, R. Live and let die: asymmetric dimethylarginine and septic shock. Crit. Care 10, 169. https://doi.org/10.1186/xx5076 (2006).
Böger, R. et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 119, 1592–1600. https://doi.org/10.1161/circulationaha.108.838268 (2009).
Böger, R., Maas, R., Schulze, F. & Schwedhelm, E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—an update on patient populations with a wide range of cardiovascular risk. Pharmacol. Res. 60, 481–487. https://doi.org/10.1016/j.phrs.2009.07.001 (2009).
Zoccali, C. et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358, 2113–2117. https://doi.org/10.1016/s0140-6736(01)07217-8 (2001).
Schnabel, R. et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ. Res. 97, e53-59. https://doi.org/10.1161/01.Res.0000181286.44222.61 (2005).
Schulze, F. et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 208, 518–523. https://doi.org/10.1016/j.atherosclerosis.2009.06.039 (2010).
Winkler, M. S. et al. Symmetrical (SDMA) and asymmetrical dimethylarginine (ADMA) in sepsis: high plasma levels as combined risk markers for sepsis survival. Crit. Care 22, 216. https://doi.org/10.1186/s13054-018-2090-1 (2018).
Nijveldt, R. J. et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin. Nutr. 22, 23–30. https://doi.org/10.1054/clnu.2002.0613 (2003).
Appel, D. et al. Asymmetric dimethylarginine predicts perioperative cardiovascular complications in patients undergoing medium-to-high risk non-cardiac surgery. J. Int. Med. Res. 48, 300060520940450. https://doi.org/10.1177/0300060520940450 (2020).
Ghashut, R. A. et al. Assessment of asymmetrical dimethylarginine metabolism in patients with critical illness. Eur. J. Clin. Invest. 47, 279–288. https://doi.org/10.1111/eci.12710 (2017).
Dreher, M. et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl. Int. 117, 271–278. https://doi.org/10.3238/arztebl.2020.0271 (2020).
Ronderos Botero, D. M. et al. COVID-19 in the healthy patient population: demographic and clinical phenotypic characterization and predictors of in-hospital outcomes. Arterioscler. Thromb. Vasc. Biol. 40, 2764–2775. https://doi.org/10.1161/atvbaha.120.314845 (2020).
Ozdemir, B. & Yazici, A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths?. Med. Hypotheses 144, 109970. https://doi.org/10.1016/j.mehy.2020.109970 (2020).
Green, S. J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 22, 149–150. https://doi.org/10.1016/j.micinf.2020.05.006 (2020).
Nagashima, S. et al. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler. Thromb. Vasc. Biol. 40, 2404–2407. https://doi.org/10.1161/atvbaha.120.314860 (2020).
Leiper, J., Murray-Rust, J., McDonald, N. & Vallance, P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc. Natl. Acad. Sci. U. S. A. 99, 13527–13532. https://doi.org/10.1073/pnas.212269799 (2002).
Rodionov, R. N., Jarzebska, N., Weiss, N. & Lentz, S. R. AGXT2: a promiscuous aminotransferase. Trends Pharmacol. Sci. 35, 575–582. https://doi.org/10.1016/j.tips.2014.09.005 (2014).
Siques, P. et al. Asymmetric dimethylarginine at sea level is a predictive marker of hypoxic pulmonary arterial hypertension at high altitude. Front. Physiol. 10, 651. https://doi.org/10.3389/fphys.2019.00651 (2019).
Hannemann, J. et al. Upregulation of DDAH2 limits pulmonary hypertension and right ventricular hypertrophy during chronic hypoxia in Ddah1 knockout mice. Front. Physiol. 11, 597559. https://doi.org/10.3389/fphys.2020.597559 (2020).
Abou-Arab, O. et al. Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study. Crit. Care 24, 645. https://doi.org/10.1186/s13054-020-03371-x (2020).
Fang, W. et al. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 163, 153–162. https://doi.org/10.1016/j.freeradbiomed.2020.12.008 (2021).
Schulze, F. et al. Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur. J. Clin. Invest. 35, 622–626. https://doi.org/10.1111/j.1365-2362.2005.01561.x (2005).
Ranieri, V. M. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–2533. https://doi.org/10.1001/jama.2012.5669 (2012).
Schwedhelm, E. et al. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 851, 211–219. https://doi.org/10.1016/j.jchromb.2006.11.052 (2007).
Daher, A. et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir. Med. 174, 106197. https://doi.org/10.1016/j.rmed.2020.106197 (2020).
Mehta, R. L. et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31. https://doi.org/10.1186/cc5713 (2007).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Acknowledgements
We gratefully thank Mariola Kastner, Melanie Reckordt, Fiona Franke, and the team of the RWTH centralized Biomaterial Bank (RWTH cBMB) for their excellent technical assistance. This study was funded by institutional funds of the two contributing institutions and by the Joachim Herz-Stiftung, Hamburg, Germany. JH and RB received grants by the Bundesministerium für Bildung und Forschung (grant 01DN17046, DECIPHER) and the Georg & Jürgen Rickertsen Foundation, Hamburg, Germany (to JH). NM is funded by the Deutsche Forschungsgemeinschaft DFG; SFB TRR219 (M-03; M-05). The blood samples used were provided by the RWTH cBMB biobank of the Medical Faculty of the RWTH Aachen University and used for this study in compliance with the biobank regulations and the approvals EK206/09 and EK080/20 of the Ethics Committee of the Medical Faculty of the RWTH Aachen University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.H., N.M., and R.B. designed the study. J.H., P.B., E.S., B.H., J.U., D.M.W., E.D., and M.D. were involved in data acquisition. Data analysis and interpretation was performed by J.H., P.B., J.U., N.M., and R.B. J.H. drafted the manuscript, all other authors critically reviewed the manuscript; all authors approved the final version. R.B. and N.M. supervised the study. Funding for the study was obtained by J.H., N.M., and R.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hannemann, J., Balfanz, P., Schwedhelm, E. et al. Elevated serum SDMA and ADMA at hospital admission predict in-hospital mortality of COVID-19 patients. Sci Rep 11, 9895 (2021). https://doi.org/10.1038/s41598-021-89180-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89180-w
This article is cited by
-
SDMA attenuates renal tubulointerstitial fibrosis through inhibition of STAT4
Journal of Translational Medicine (2023)
-
The role of asymmetric dimethylarginine (ADMA) in COVID-19: association with respiratory failure and predictive role for outcome
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.