Abstract
Hungary has a single-payer health insurance system covering 10 million inhabitants. All medical reports of the in- and outpatient specialist services were collected in the NEUROHUN database. We used ICD-10 codes of Alzheimer’s disease (AD), vascular dementia (VaD), miscellaneous dementia group and mild cognitive impairment (MCI) for the inclusion of the patients. Incidence, prevalence and survival of different dementias and MCI were calculated and analyzed depending on the diagnoses given by neurological or psychiatric services or both. Between 2011 and 2016, the mean crude incidence of all dementias was 242/100,000/year, whereas the age standardized incidence was 287/100,000/year. Crude and age standardized mean prevalence rates were 570/100,000 and 649/100,000, respectively. There were significantly more VaD diagnoses than AD, the VaD:AD ratio was 2.54:1, being the highest in patients with psychiatric diagnoses only (4.85:1) and the lowest in patients with only neurological diagnoses (1.32:1). The median survival after the first diagnosis was 3.01 years regarding all dementia cases. Compared to international estimates, the prevalence of dementia and MCI is considerably lower in Hungary and the VaD:AD ratio is reversed.
Similar content being viewed by others
Introduction
Dementia, as a syndrome, is comprised of acquired cognitive and behavioral symptoms which are sufficiently severe enough to cause impairment in everyday and/or occupational activity of the patient, while the diagnosis of mild cognitive impairment (MCI) is used to describe symptoms that are measurable by cognitive testing but do not interfere with functional abilities1. The most common causes of dementia in older adults (> 65 years) are Alzheimer’s disease (AD), vascular (VaD) and Lewy body dementias2.
In aging societies, dementia is receiving increased attention due to its significant healthcare, societal and economic burden. Approximately 45–50 million people lived with dementia worldwide in 2015, and this number is expected to increase to 130 million by 2050. In addition to its adverse effects on patients' and caregivers’ quality of life and life expectancy, huge economic burden for the society is imposed by the increase in the prevalence of dementia: its cost was estimated to be $818 billion in 20153,4.
Analyzing and comparing epidemiological data from countries with different geographical and economic characteristics could be a useful opportunity to gain a better understanding of dementia as a multifactorial syndrome. Overall, population-based surveys should be conducted and regularly repeated in all countries to monitor changes in trends5.
Hungary is a country with 10 million inhabitants and the whole population is covered by a single-payer state health insurance system. Inpatient and outpatient care are documented in a unified system at a nationwide level enabling comprehensive data collection.
Our aim was to estimate the prevalence of MCI and dementia (with its subtypes) in Hungary using data from the health insurance database and to compare the Hungarian data with the international ones.
Methods
Our study was performed using the NEUROHUN 2004–2017 database, which was created from medical and medication prescription reports within the framework of the Hungarian National Brain Research Program6. Appearances in all in- and outpatient departments (except family medicine (FM)) in Hungary in the indicated period are documented in the database. In our study, data from 2011 to 2016 were analyzed. The original patient identifier codes were anonymized and encrypted identifiers were used.
All personal data protection regulations were followed. The study was approved by the Ethics Committee of Semmelweis University, Budapest, Hungary (Approval No: SE TUKEB 88/2015).
Selection of patients
First, all patients with a diagnosis of dementia and MCI were collected. Cases were categorized based on diagnoses according to the International Classification of Diseases (ICD-10). Although criticism is raised in the literature regarding the use of ICD-10 codes7, these are the exclusively used ones in the health insurance database. Only those ICD codes were selected which were given by medical specialty services; diagnostic and non-medical services (e.g. physiotherapy, psychology) were excluded. For the analysis, we used data of those patients only who had been assigned with dementia or MCI ICD codes at least twice and at least one of the ICD codes was given by neurological or psychiatric specialty services (Fig. 1).
Validation of the database
Validation of the clinical diagnosis criteria of dementias on a smaller subsample was performed. We checked patients who had records with the defined ICD codes in the local integrated hospital healthcare information technology system (MedSol, T-System, Hungary) of Semmelweis University, Budapest, in a selected period (October 2013) and compared them to the records in the NEUROHUN database. To match patients across the databases, we used the year of birth, postal code of the residence, the gender, the admission and the discharge date, together with the institutional code of the medical service provider (Department of Neurology, Semmelweis University, Budapest, Hungary).
First, we collected all records provided by our department in the selected period of time in the NEUROHUN database, then we checked whether these patients could be found in MedSol.
Second, we identified all patients at our department in MedSol who were treated with these ICD-10 codes either in the inpatient or the outpatient setting. Then, we checked these patients whether they did or did not appear in the NEUROHUN database.
Finally, for further clarification, we reviewed the medical records of these patients to ensure that the clinical findings support the diagnosis of dementia.
Statistical analysis
We calculated crude and age-standardized8 incidence and prevalence rates of dementias for each year for the period of 2011–2016. The newly diagnosed cases were analyzed by gender and age groups. For the analysis we used the date of first application of the diagnoses (without preceding diagnosis in the NEUROHUN database).
AD and VaD groups were analyzed separately. ICD-10 codes of G31.0 (corresponding to frontotemporal dementia with clinically heterogeneous diseases) and G31.8 (including diseases other than Lewy body dementias too) were included in the group of miscellaneous dementias (mD).
Population data were used from the database of the Hungarian Central Statistical Office based on the census in 20119.
Microsoft Excel 2016 (Microsoft Corporation, Redmond, Washington, USA) (descriptive statistics), TIBCO Statistica version 13 (TIBCO Software Inc, Palo Alto, California, USA) (one- and two-way ANOVAs) and GraphPad Prism 8 (GraphPad Software Inc., San Diego, California, USA) (Kaplan–Meier survival curves, log rank tests) were used for analyses.
Results
Validation of the database
In October 2013, 124 patients were registered in the MedSol system, of which 122 cases were found in the NEUROHUN database. The two missing patients from the NEUROHUN had a reporting error from the hospital and as a result, were not financed, so they did not appear in the NEUROHUN database. From the other direction, we were able to identify all 122 patients from NEUROHUN in MedSol.
Estimating the number of patients with dementia and MCI
During the examined six-year period, more than 1,956,000 (689,000 neurological, 1,087,000 psychiatric) appearances of 144,407 patients in the Hungarian health care system were associated with any type of dementia diagnosis and 467,063 (148,773 neurological and 318,290 psychiatric) appearances of 21,833 patients with MCI alone. At least one computed tomography (CT) scan of the head was performed in 70.3% (n = 101,559) of the patients, while only 12.0% (n = 17,339) had a magnetic resonance imaging (MRI) of the head. The proportion of patients without head CT or MRI was 26.2% (n = 37,838).
The number of new patients diagnosed with dementia during the observed period and the calculated incidence and prevalence results are summarized in Table 1.
A notable number of patients received multiple dementia diagnoses. Of the 33,584 AD patients, only 27% (n = 9165) were diagnosed with AD alone (with or without MCI diagnosis), 73% of the cases were associated with other subtypes of dementia. AD diagnosis was more often associated with mD (n = 17,451) than with VaD (n = 15,140).
Association of MCI
The association of MCI with different types of dementia is shown in Fig. 2. During the 6-year period, the number of patients without a diagnosis of dementia but receiving MCI was 21,833. Of the 9165 patients diagnosed with AD alone, 16.48% (n = 1510) had the diagnosis of MCI at some point during the course of the disease, but MCI was diagnosed only in 14.81% (n = 1357) preceding the diagnosis of AD. The same data for VaD were 8.05% (n = 2913) and 6.61% (n = 2392), for mD 10.05% (n = 4081) and 7.88% (n = 3198), while for all dementias 9.34% (n = 13,487) and 7.51% (n = 10,838), respectively.
Analysis of dementia subtypes
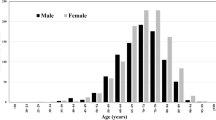
When examined by age groups, more men were diagnosed with dementia between the ages of 35 and 65, while the proportion of women under the age of 35 and over 65 was higher. In the total sample, more women were diagnosed with AD than men. With advancing age, the proportion of people diagnosed with AD is increased in both genders, peaking in the 80–84 age group, followed by a decrease in the incidence from the age of 85, with male predominance (Fig. 3).
The mean age is between 70 and 80 years for all forms of dementia, with a statistically significant difference by the types of dementia and gender except AD (men) vs. VaD (men) as well as AD (men) and VaD (men) vs mD (women) (two-way ANOVA, post hoc Tukey HSD p < 0.0001, for the matrix, see Supplementary information 1). In all dementia types, the mean age was lower in men. Case fatality was the highest in VaD and in all types the rate of death was higher in men. The median survival after the first diagnosis in all dementia cases was 3.01 years. There was a significant difference between the types: survival was the longest with AD and the shortest with VaD (Table 2 and Fig. 4).
Dementia diagnoses were given by psychiatric specialty alone in 45.1% (n = 65,118) of the cases, while in 20.6% (n = 29,720) and in 34.3% (n = 49,569) by neurological and both specialties, respectively. There was a significant difference in the mean age (one-way ANOVA, p < 0.00002) and median survival after the diagnosis (logrank test, p < 0.0001) between the three groups: the highest mean age and the shortest survival was observed in patients with diagnoses given by psychiatric specialties only. The survival was the longest in patients with neurological diagnosis.
The VaD:AD ratio was the highest in patients with psychiatric diagnoses only (4.85:1) and was the lowest in patients with only neurological diagnoses (1.32:1). Two-thirds of the patients without neuroimaging were in the group diagnosed by psychiatric specialty services alone (39.0% (n = 25,393) of the patients diagnosed by psychiatric specialty services only). The lowest rate of patients (13.1%, n = 6505) without neuroimaging was seen in patients diagnosed by both specialties (Table 3).
Discussion
AD and VaD are the two most common types of dementia. Based on clinical diagnostic criteria the ratio of VaD to AD is approximately 1:3 in Europe and in the United States, while in the developing countries it is 1:210,11,12. With advancing age, the incidence of degenerative and vascular diseases is increasing, together with the increase of vascular risk factors involved in the pathogenesis of AD; as a result of this, the frequency of dementias with mixed etiologies is increasing13,14.
According to the meta-analysis of Prince5, there are few epidemiological studies from the Central and Eastern European regions. However, data from the few available studies15,16 suggest that prevalence in these regions are similar to the Western European countries in terms of cognitive impairment and dementia. Based on these, the standardized prevalence among over 60 years of age is between 7.1 and 7.3% (meaning about 160–170,000 patients in Hungary). These numbers are similar to the estimation of Alzheimer Europe’s one (148,927 patients)17.
In previous Hungarian studies, the incidence of dementias was studied among residents of nursing homes. A study18 published in 1995 found the incidence of VaD to be more common compared to AD, while another study19 made no distinction in the etiology of dementia, but found that nearly half of the residents suffered from at least mild cognitive deficit.
In addition, in some of the studies20,21,22 small sample sizes (150020 and 40721 participants) were used from randomly selected FM practices to extrapolate the prevalence of dementia in patients over 55 years old at population level. As a result, prevalence data were overestimated, leading to the number of patients with any type of dementia in Hungary in 2008 between 530 and 917,000.
We did not find Hungarian data about clinical criteria based VaD:AD ratio, but according to a neuropathology analysis, AD-type pathology was seen in 49.2% of VaD cases; pure AD pathology was found in 26.3% and pure VaD in 17.3% of the examined patients, which was close to the international results23.
In our study, the ratio of the VaD and AD diagnoses was reversed, 2.54 times more VaD diagnoses were assigned than AD (this ratio includes mixed diagnoses also). When accounting pure VaD and AD diagnoses (only 36,204 and 9165 patients over the six years, respectively), VaD:AD ratio increases to 3.95:1. Our results showed that both dementia and MCI were significantly underdiagnosed and the categorization of patients into dementia subtypes is also different from international data (Table 4).
In Hungary, the type of dementia is defined by neurological or psychiatric specialist services after family physician referral, so FM records were not included in our analysis. However, dementias are underdiagnosed worldwide in FM practices, the national underdetection rate is about 52% in the United Kingdom24 and roughly 75% in middle or lower income countries25, thereby fewer patients can be admitted to the specialist care. In addition, it is possible that patients with MCI and in early stages of dementia were managed in the FM practices and they were referred in later stages for specialty services, as suggested by the shorter survival time, the low number of MCI diagnoses preceding dementia and the high mortality of patients with dementia in our study.
The limited availability of AD biomarkers (CSF beta-amyloid and phospho-tau and amyloid PET imaging)26 might partially explain the low rate of AD diagnoses. The high percentage of patients without neuroimaging could also be a factor for this.
The survival of patients without neuroimaging was shorter by 1.24 years compared to patients with neuroimaging (data not shown) and the ratio of patients without neuroimaging was higher among patients diagnosed in psychiatric services alone. The shorter survival and the less frequent use of neuroimaging among patients diagnosed with psychiatric services alone might also be explained by the more severe stage of dementia with prominent behavioral and psychological symptoms at the time of referral.
In addition, the VaD:AD ratio was the lowest in patients diagnosed by neurological specialty services alone. It could be hypothesized that neurologists might more often notice the focal signs of cerebral circulatory problems during physical examination and indicate head imaging, as well as in the absence of symptomatic vascular lesions on imaging studies they less frequently diagnose VaD. This might also be supported by our result that AD patients more frequently received unspecific dementia diagnosis (mD) than VaD patients. Moreover, compared to Western countries (e.g. USA, UK, Germany), the incidence of stroke is 1.3–2 times higher in Hungary27, which may partly explain the higher incidence of VaD. Accurate differentiation between types of dementias is important not only for the choice of the ideal treatment, but also because of the quality of life of patients and their caregivers, which could be significantly different28.
There is a significant difference in the course of different types of dementia: survival is worse in VaD followed by all dementias and AD29. Patients with dementia have a higher incidence and risk of death from stroke. In addition, the presence of cardiovascular risk factors is higher in VaD than in any other forms of dementia30. The shortest survival seen in VaD might be explained by the higher risk of stroke and other cardiovascular diseases, the late complications of cerebral infarctions and by the finding that demented patients receive poorer quality of care with worse outcomes after stroke31. These observations are supported by the findings of Broulikova et al.32 from Czech registers on hospitalized dementia patients.
In our study, the median survival of patients with all dementias (3.01 years) was shorter than the published ones (3.2–6.6 years)33, being the shortest in VaD (2.25 years). All types of dementia have higher case fatality in men, similarly to the literature29.
The reasons for the difference are unclear, however, as mentioned above, late detection of dementia and diagnosis at a more advanced stage may contribute, as indicated by the very low proportion of MCI codes that precede dementia. In addition, differences in baseline cognitive performance and rate of decline across European regions might also contribute to the observed differences in survival34. In addition, palliative and supportive care for patients with dementia and their caregivers is limited, although improving in Hungary. Optimal collaborative care is necessary to properly treat the wide range of cognitive, emotional, social or physical complications associated with dementia35. Development of extensive cooperation can effectively improve quality of life and the survival, as well as reduce social and economic burden36.
Limitations and strengths of the study
The NEUROHUN database allows us to estimate the number of patients with dementia and MCI at population level, with diagnoses confirmed by neurological and psychiatric providers, leading to more specific and reliable, but underestimated numbers of patients with dementia. Inclusion of data from FM practices could increase the number of identified patients, but without specifying the type of dementia. Dementia prevalence data are barely available from the Central and Eastern European regions and our results help to fill this gap and they are the first from a large sample size research from Hungary.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 (2004).
Elahi, F. M. & Miller, B. L. A clinicopathological approach to the diagnosis of dementia. Nat. Rev. Neurol. 13, 457–476 (2017).
Prince, M., Wimo, A.G.M., Ali, G.C., Wu, Y.T. & Prina, M. World Alzheimer Report 2015: The global impact of dementia: an analysis of prevalence, incidence, cost and trends. (Alzheimer’s Disease International, London). https://www.alzint.org/resource/world-alzheimer-report-2015/. Accessed 4 Jan 2021
GBD 2016 Dementia Collaborator. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88–106 (2019).
Prince, M. et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 9, 63-75.e2 (2013).
Oberfrank, F., Ajtay, A. & Bereczki, D. Demand for neurological services in Central Eastern Europe: A 10-year national survey in Hungary. Eur. J. Neurol. 25, 984–990 (2018).
Germaine-Smith, C. S. et al. Recommendations for optimal ICD codes to study neurologic conditions: A systematic review. Neurology 79, 1049–1055 (2012).
Pace, M. et al. Revision of the European Standard Population. Report of Eurostat's Task Force. Eurostat, European Union (2013). https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f. Accessed 4 Jan 2021.
Hungarian Central Statistical Office (HCSO). Hungary Population Census 2011. http://www.ksh.hu/nepszamlalas/?lang=en. Accessed 4 Jan 2021.
Lobo, A. et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic diseases in the Elderly Research Group. Neurology 54, S4–S9 (2000).
Goodman, R. A. et al. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement. 13, 28–37 (2017).
Kalaria, R. N. et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 7, 812–826 (2008).
Custodio, N. et al. Mixed dementia: A review of the evidence. Dement. Neuropsychol. 11, 364–370 (2017).
de la Torre, J. C. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke 33, 1152–1162 (2002).
Kiejna, A. et al. Epidemiological studies of cognitive impairment and dementia across Eastern and Middle European countries (epidemiology of dementia in Eastern and Middle European Countries). Int. J. Geriatr. Psychiatry. 26, 111–117 (2011).
Bacigalupo, I. et al. A systematic review and meta-analysis on the prevalence of dementia in Europe: Estimates from the highest-quality studies adopting the DSM IV diagnostic criteria. J. Alzheimers Dis. 66, 1471–1481 (2018).
Alzheimer Europe. The prevalence of dementia in Europe 2013. https://www.alzheimer-europe.org/Policy/Country-comparisons/2013-The-prevalence-of-dementia-in-Europe/Hungary. (Accessed 24 February 2014).
Leel-Őssy, L. Incidence of Alzheimer’s dementia in homes for the elderly. Arch. Gerontol. Geriatr. 21, 21–26 (1995).
Vincze, G. et al. Risk factors of cognitive decline in residential care in Hungary. Int. J. Geriatr. Psychiatry. 22, 1208–1216 (2007).
Linka, E., Kispál, G., Szabó, T. & Bartkó, G. Screening of dementia and one year follow-up of patients in a family medicine practice [A dementia szűrése és a betegek egyéves követése egy háziorvosi praxisban]. Ideggyogy Sz. 54, 156–160 (2001).
Leel-Őssy, L., Józsa, I., Szűcs, I. & Kindler, M. Screening for early detection of dementia (in family medicine practices and nursing homes) [Szűrővizsgálatok a dementia korai felderítésére (Családorvosi körzetekben és idősek otthonában)]. Medicus Universalis 38, 149–160 (2005).
Érsek, K. et al. Epidemiology of dementia in Hungary [A dementia epidemiológiája Magyarországon]. Ideggyogy. Sz. 63, 175–182 (2010).
Kovács, G. G., Kővári, V. & Nagy, Z. Incidence of dementia in the three-year neuropathological material of the National Institute of Psychiatry and Neurology [Dementiával járó kórképek gyakorisága az Országos Pszichiátriai és Neurológiai Intézet hároméves neuropatológiai anyagában]. Ideggyogy. Sz. 61, 24–32 (2008).
Connolly, A., Gaehl, E., Martin, H., Morris, J. & Purandare, N. Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment. Health. 15, 978–984 (2015).
Nakamura, A. E., Opaleye, D., Tani, G. & Ferri, C. P. Dementia underdiagnosis in Brazil. Lancet 385, 418–419 (2015).
Davda, N. & Corkill, R. Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J. Neurol. 267, 2475–2477 (2020).
GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 439–458 (2019).
Wu, Y. T. et al. Dementia subtype and living well: Results from the Improving the experience of Dementia and Enhancing Active Life (IDEAL) study. BMC Med. 16, 140 (2018).
Garcia-Ptacek, S. et al. Mortality risk after dementia diagnosis by dementia type and underlying factors: A cohort of 15,209 patients based on the Swedish Dementia Registry. J. Alzheimers Dis. 41, 467–477 (2014).
Subic, A. et al. Stroke as a cause of death in death certificates of patients with dementia: A cohort study from the Swedish Dementia Registry. Curr. Alzheimer Res. 15, 322–1330 (2018).
Callisaya, M. L., Purvis, T., Lawler, K., Brodtmann, A., Cadilhac, D. A. & Kilkenny, M. F. Dementia is associated with poorer quality of care and outcomes after stroke: An observational study. J. Gerontol. A. Biol. Sci Med. Sci. 76, 851–858 (2021).
Broulikova, H. M., Arltova, M., Kuklova, M., Formanek, T. & Cermakova, P. Hospitalizations and mortality of individuals with dementia: Evidence from Czech National Registers. J Alzheimers Dis. 75, 1017–1027 (2020).
Todd, S., Barr, S., Roberts, M. & Passmore, A. P. Survival in dementia and predictors of mortality: A review. Int. J. Geriatr. Psychiatry. 28, 1109–1124 (2013).
Formanek, T., Kagstrom, A., Winkler, P. & Cermakova, P. Differences in cognitive performance and cognitive decline across European regions: A population-based prospective cohort study. Eur. Psychiatry. 58, 80–86 (2019).
Grand, J. H., Caspar, S. & Macdonald, S. W. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 4, 125–147 (2011).
Galvin, J. E., Valois, L. & Zweig, Y. Collaborative transdisciplinary team approach for dementia care. Neurodegener. Dis. Manag. 4, 455–469 (2014).
Acknowledgements
This work was supported by the Hungarian Brain Research Program NAP2.0 (Nemzeti Agykutatási Program 2017-1.2.1-NKP-2017-00002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, data collection and analysis. The first draft of the manuscript was written by N.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balázs, N., Ajtay, A., Oberfrank, F. et al. Dementia epidemiology in Hungary based on data from neurological and psychiatric specialty services. Sci Rep 11, 10333 (2021). https://doi.org/10.1038/s41598-021-89179-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89179-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.