Abstract
The contribution of endogenous insulin secretion to glycemic variability (GV) may differ between patients with impaired insulin secretion and those with preserved secretion. Our objective was to determine the linearity of the relationship between fasting C-peptide (CPR) as a marker of endogenous insulin secretion and GV in type 2 diabetes (T2DM), regardless of the type of antidiabetic treatment. We conducted a prospective observational study using continuous glucose monitoring obtained from 284 Japanese outpatients with T2DM with various HbA1c values and antidiabetic treatment. We constructed a prediction curve of base-line CPR versus coefficient of variation (CV) and identified the clinical factors associated with CV using multiple regression analysis. Fasting CPR showed a significant negative log-linear relationship with CV (P < 0.0001), and the latter being strikingly high in the low-CPR group. The multiple regression analysis showed that low CPR was an independent predictor of high CV (P < 0.0001). The significant correlations were sustained in both patients with/without insulin treatment. The contribution of endogenous insulin secretion to GV depends on the extent of insulin secretion impairment. Fasting CPR may represent a useful indicator of GV instability in T2DM.
Similar content being viewed by others
Introduction
The primary objective of diabetes treatment is to help patients live without the anxiety associated with the risk of hypoglycemia and diabetes-related complications1. Continuous glucose monitoring (CGM) is able to track the changes in glucose concentration throughout the day and permits the assessment of glycemic variability (GV)2. CGM-based studies have shown that GV instability is not only associated with higher risks of hypo- and hyperglycemia3, but also of the onset and progression of diabetes-related microvascular4,5,6, and macrovascular complications7, 8. Therefore, antidiabetic treatment strategies should be aimed at reducing GV. To achieve this target, it is necessary to clarify the patient factors that influence GV. In type 1 diabetes, low endogenous insulin secretion is associated with GV instability9. In addition, we have previously shown that impaired endogenous insulin secretion affects GV stability in type 2 diabetes10. However, other previous studies of type 2 diabetes have shown the inconsistent results regarding the relationship between endogenous insulin secretion and GV, including a lack of relationship between fasting C-peptide (CPR) concentration and GV in type 2 diabetes11,12,13. These previous studies were conducted in patients with preserved insulin secretion. To explain such heterogeneity in the correlation, comprehensive data regarding GV in type 2 diabetes using CGM, including in patients with severe insulin secretion disorders, were to be interpreted. Such investigations may demonstrate that the contribution of endogenous insulin secretion to GV differs between patients with impaired insulin secretion and those with preserved secretion. Therefore, we hypothesized that the relationship between endogenous insulin secretion and GV is not linear, but instead log-linear.
In the present study, we used CGM to investigate whether endogenous insulin secretion, evaluated using fasting CPR, has a log-linear relationship with GV in type 2 diabetes, including those with severely impaired endogenous insulin secretion10. Furthermore, we explored whether fasting CPR concentration could be a predictor of GV instability, regardless of the type of antidiabetic treatment used in those patients.
Methods
Study population and design
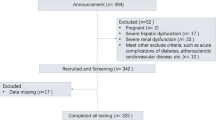
We conducted a prospective observational study. Japanese outpatients with type 2 diabetes were recruited between April 2018 and September 2019 at four medical institutions, as described previously10. Briefly, patients of ≥ 20 years of age were eligible if they consented to undergo ambulatory CGM, regardless of their HbA1c level, sex, duration of diabetes, or the presence of complications of diabetes. The exclusion criteria were as follows: (1) type 1 diabetes meeting the diagnostic criteria14, (2) hospitalization within the preceding 3 months, (3) diabetic ketosis/coma, (4) serious infection, (5) recent or planned surgery, (6) trauma within the preceding 6 months, (7) current glucocorticoid therapy, (8) difficulty with dietary intake, or (9) pregnancy or lactation. CGM data, fasting blood samples, and clinical information (age, sex, anthropometric measurements, duration of diabetes in years, treatment regimen, and medical history) were obtained from each participant.
The study was registered with the University Hospital Medical Information Network Center (registration number UMIN 000029993). The study protocol was approved by the Institutional Review Board of Hokkaido University Hospital Clinical Research and Medical Innovation Center (017-0147), and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Biochemical analyses and data collection
Ambulatory CGM (using a FreeStyle Libre Pro sensor; Abbott Diabetes Care, Alameda, CA, USA) was performed for 14 consecutive days. The CGM data remained blinded for patients and physicians because the CGM system used was a professional version that permitted blinded CGM. Thus, patients were not able to access any real time information on their GV that might have influenced their lifestyle behaviors, including their dietary choices, during the study. After the monitoring period, we excluded data collected during the first and last days of wearing the device, considering possible inaccuracies relating to its attachment and removal15. We analyzed the CGM data for the remaining period using GlyCulator2 software16. Because an international consensus statement recommends the coefficient of variation (CV) as the primary measure of GV17, we analyzed the CV as an index of GV using the following formula: 100 × [SD of glucose]/[mean glucose]. The standard deviation (SD) glucose concentration, the mean amplitude of glycemic excursions (MAGE)18, and the mean glucose concentration were also analyzed as secondary indices of GV. The low blood glucose index (LBGI) and high blood glucose index (HBGI), which increase with the frequency and extent of hypoglycemia and hyperglycemia, respectively, were also analyzed19. Furthermore, we calculated three key CGM-related indices: the percentage of readings and time per day within the target glucose range (TIR: 3.9–10.0 mmol/L), time below target glucose range (TBR: < 3.9 mmol/L), and time above the target glucose range (TAR: > 10.0 mmol/L), as recommended by the international consensus statement20.
Blood samples were collected after an overnight fast. Fasting plasma glucose (FPG), CPR, HbA1c, and estimated glomerular filtration rate (eGFR) were measured using standard techniques. The body weight and height of the patients were measured using a calibrated scale. Body mass index (BMI) was calculated as body weight (in kg) divided by height (in m2). Other data, including age, sex, the diabetes medications being used, and medical history were collected using a questionnaire that was administered by the attending physicians.
Data analysis
To define the relationship between endogenous insulin secretion and GV instability, we constructed a scatter plot of fasting CPR versus CV. The prediction formula for CPR versus CV was estimated after both CPR and CV were logarithmically transformed. The clinical factors related to GV, including CPR, were determined after CPR was logarithmically transformed. Spearman rank-order correlation was used to determine the relationships between continuous variables, such as age. Bivariate analyses of CGM-based metrics of GV and categorical variables, such as sex, were performed using the Mann–Whitney test. The results are shown as the median (interquartile range). Multiple regression analysis was performed using variables identified in previously published research11 and those that were significant (P < 0.05) in the univariate analysis. We have demonstrated that these items had linear relationships with CGM-based metrics of GV (Supplementary Figs. S1, S2).
To further evaluate the contribution of CPR to CV, the patients were allocated to three subgroups according to whether they had low (CPR < 1 ng/mL), moderate (1 ng/mL ≤ CPR < 2 ng/mL), or high (CPR ≥ 2 ng/mL) CPR concentrations, based on our prediction curve for CPR versus CV. Then, the biochemical and anthropometric characteristics of the three subgroups were compared using one-way analysis of variance, the chi-square test, or the Kruskal–Wallis test, as appropriate. Because the international consensus statement defines stable GV using a CV < 36% and unstable GV using a CV ≥ 36%17, the number of patients with CV ≥ 36% was compared among the three subgroups using the CV distribution and the chi-square test. Next, to determine whether insulin and antidiabetic treatment use modified the association of interest, the patients were allocated to two subgroups according to whether they received insulin and antidiabetic treatments or not. Additionally, to investigate whether the use of a basal-only or basal-bolus insulin regimen modified the association of interest, we allocated the patients on insulin to two subgroups, according to their insulin regimen, and a similar stratified analysis was carried out.
All the statistical tests performed were two-sided, and P < 0.05 was considered to represent statistical significance. Statistical analyses were performed using JMP Pro 14.0.0 (SAS Inc., Cary, NC, USA).
Results
Patients characteristics
Of a total of 311 patients enrolled in the study, 27 were excluded because they met one or more of the exclusion criteria described previously10. The remaining 284 patients (123 women) were included in the subsequent analyses. Their characteristics are shown in Table 1. The overall median age of the patients was 68 years, their median BMI 25.0 kg/m2, and their median duration of diabetes 14 years. The median CPR concentration was 1.7 ng/mL and the median CV was recorded as 27.8.
A prediction curve of baseline C-peptide versus glucose variability
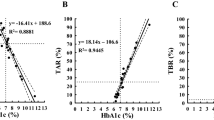
We first constructed a scatter plot of CPR versus CV to visualize the potential relationship between these two parameters. For a better understanding of the results, Fig. 1 shows the log-linear relationship between CPR and CV. The prediction curve, constructed using this scatter plot, showed a marked increase in CV with low CPR and relatively low CV with high CPR. CV had significant negative correlation with CPR (ρ = − 0.39, P < 0.0001). The prediction formula was estimated to be the following Eq. (1).
CV also showed significant negative correlation with BMI, FPG, and eGFR (Table 2). In addition, CV had significant positive correlation with the duration of diabetes. The CV in patients on insulin was significantly greater than that of non-insulin patients. The CV in patients treated with an alpha-glucosidase inhibitor (α-GI) or dipeptidyl peptidase-4 (DPP-4) inhibitor was significantly smaller than in patients not treated with these drugs. No relationships were identified between CV and other factors, such as HbA1c, age, and other antidiabetic treatments. We assessed the multicollinearity in the variables shown to be associated with CV using Spearman’s correlation analysis (Supplementary Table S1). There was a relatively strong correlation between CPR and BMI, with a correlation coefficient of 0.51. The absolute values of the correlation coefficient for other variables were lower. The variance inflation factor (VIF) for these parameters was less than 2.0 (Supplementary Table S2). Thus, there were no indications of multicollinearity between these variables. Low CPR was an independent predictive marker for high CV, according to multiple regression analysis (regression coefficient (β): − 0.285, 95% confidence interval [CI] − 0.234 to − 0.092, P < 0.0001). Insulin use, non-use of an α-GI or DPP-4 inhibitor, and low eGFR were also predictors of high CV (Table 3). We also performed the calculation after correcting CPR for the concomitant plasma glucose level using CPR index, calculated using the formula: 100 × fasting CPR (ng/mL)/FPG (mg/dL)21. The findings were similar to those obtained using the fasting CPR (data not shown).
Relationship between baseline C-peptide and hypo- or hyperglycemia
A log-linear relationship between CPR and LBGI was observed. LBGI showed a significant negative correlation with CPR (ρ = − 0.25, P < 0.0001) as well as the relationship between CPR and CV. As shown in Supplementary Table S3, LBGI also exhibited a significant negative correlation with BMI, FPG, HbA1c, and eGFR. The LBGI in patients treated with insulin was significantly greater than that in patients not treated with insulin. No relationships were identified between the LBGI and other factors. According to multiple regression analysis, low CPR was an independent predictive marker for a high LBGI (β: − 0.163, 95% CI − 0.327 to − 0.043, P = 0.0107). Insulin use, low FPG, low HbA1c, and low eGFR were also predictors of a high LBGI (Supplementary Table S4). In contrast, a HBGI was not significantly correlated with CPR (ρ = − 0.11, P = 0.0742).
The contribution of C-peptide to coefficient of variation
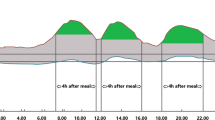
The 284 patients were allocated to three subgroups: low CPR (CPR < 1 ng/mL, n = 62), moderate CPR (1 ng/mL ≤ CPR < 2 ng/mL, n = 113), and high CPR (CPR ≥ 2 ng/mL, n = 109). The biochemical and anthropometric characteristics of each group are shown in Table 4. Age, BMI, the duration of diabetes, the type of antidiabetic treatment, and FPG were associated with the CPR concentration. The indices of GV (CV, SD, and MAGE), and the key CGM indices (LBGI, HBGI, TBR, TIR, and TAR), were also associated with the CPR concentration. The mean glucose profiles of the patients in each of the three CPR groups are shown in Fig. 2. In summary, the GV appeared to be more unstable in the low CPR group than in the moderate and high CPR groups. Supplementary Figure S3 shows the CV distribution among the three subgroups. The number of patients with CV ≥ 36%, defined as unstable GV, was significantly higher in the low CPR subgroup than in the medium and high CPR subgroups (Table 4).
Mean glucose profile of patients in the three C-peptide subgroups: (A) low C-peptide (CPR < 1 ng/mL, n = 62), (B) moderate C-peptide (1 ng/mL ≤ CPR < 2 ng/mL, n = 113) and (C) high C-peptide (CPR ≥ 2 ng/mL, n = 109). Smoothed curves represent the 10th (fine dotted line), 25th (dotted line), median (50th, solid line), 75th (broken line), and 90th (thick dotted line) frequency percentiles. Unstable glucose variability appears to be present in the low C-peptide subgroup.
Relationship between glucose variability and insulin use
Subsequently, the patients were allocated to two subgroups according to whether they were insulin user (n = 120) or non-user (n = 164). We then constructed a scatter plot and a prediction curve of CPR versus CV for each of the two subgroups. As shown in Fig. 3, the relationship between CPR and CV was log-linear, regardless of insulin use. The prediction curve showed a marked increase in CV when CPR was low. CV had significant negative correlation with CPR, but the correlation was enhanced for patients who were being treated with insulin (patients being treated with insulin: ρ = − 0.47, P < 0.0001; patients not being treated with insulin: ρ = − 0.19, P = 0.0162) (Fig. 3A). In addition, we allocated the patients who were taking insulin to groups according to whether they were on a basal-only regimen (n = 65) or a basal-bolus regimen (n = 55), and found that CV was negatively correlated with CPR significantly, regardless of the type of insulin regimen (patients on a basal-only regimen: ρ = − 0.42, P = 0.0006; patients on a basal-bolus regimen: ρ = − 0.50, P = 0.0001) (Fig. 3B). There were no relationships between CPR and the frequency of insulin injection or the total daily dose of insulin (data not shown). These results suggest that neither insulin use, nor the insulin regimen, modified the association between CPR and CV.
Scatter plot and prediction curve of fasting C-peptide versus coefficient of variation after stratification according to insulin use and insulin regimen. (A) Shows data for patients treated with insulin (n = 120, Scatter plot; closed circle, prediction curve; solid line) and those not treated with insulin (n = 164, cross, broken line). (B) Shows data for patients on a basal-only regimen (n = 65, closed circle, solid line) and patients on a basal-bolus regimen (n = 55, cross, broken line).
Relationship between glucose variability and the use of an alpha glucosidase inhibitor or dipeptidyl peptidase-4 inhibitor
Next, we examined the relationship between GV and the use of an α-GI or DPP-4 inhibitor, as the use of these treatments may impact GV in our study (Table 3). The patients were allocated to two subgroups according to whether they received α-GI therapy (n = 43) or not (n = 241). We then constructed a scatter plot and prediction curve of CPR versus CV for each subgroup. As shown in Fig. 4A, CV exhibited a significant negative correlation with CPR (patients being treated with an α-GI: ρ = − 0.33, P = 0.0313; patients not being treated with an α-GI: ρ = − 0.43, P < 0.0001). In addition, we allocated all patients to two groups according to whether they received a DPP-4 inhibitor (n = 194) or not (n = 90) and found that CV was significantly negatively correlated with CPR, regardless of the use of a DPP-4 inhibitor (patients being treated with a DPP-4 inhibitor: ρ = − 0.35, P < 0.0001; patients not being treated with a DPP-4 inhibitor: ρ = − 0.49, P < 0.0001) (Fig. 4B).
Scatter plot and prediction curve of fasting C-peptide versus coefficient of variation for (A) patients treated with an alpha-glucosidase inhibitor (n = 43, Scatter plot; closed circle, prediction curve; solid line) and those treated without an alpha-glucosidase inhibitor (n = 241, cross, broken line). (B) Shows data for patients treated with a dipeptidyl peptidase-4 inhibitor (n = 194, closed circle, solid line) and those treated without a dipeptidyl peptidase-4 inhibitor (n = 90, cross, broken line).
Discussion
This study verified that there was a log-linear relationship between the fasting CPR concentration and CV, both in insulin patients and non-insulin patients. These findings were consistent with the hypothesis that the contribution of endogenous insulin secretion to GV differs between patients with impaired and preserved endogenous insulin secretion. Our results also suggested that the fasting CPR concentration could be used as a predictor of GV instability, regardless of the antidiabetic treatment.
In our study, multiple regression analysis showed that fasting CPR is an independent predictor of GV instability. However, previous studies have generated inconsistent findings. A retrospective study of 53 patients with type 2 diabetes that was conducted using CGM did not show a correlation between fasting CPR and CV22. In addition, a cross-sectional study of 59 patients who were not on insulin therapy showed that fasting endogenous insulin secretion did not contribute to MAGE, but insulin secretion after glucose loading did13. However, in these studies, the relationship between endogenous insulin secretion and GV was assessed in patients who had preserved endogenous insulin secretion with mean CPR was > 2.7 ng/mL. In contrast, we assessed this relationship in a sample that included patients with relatively severe endogenous insulin secretion disorders, whose median was CPR 1.7 ng/mL. Similar to the present study, a previous cross-sectional study enrolled 208 Japanese patients with relatively severely impaired endogenous insulin secretion12, but there was no correlation between fasting CPR and GV. However, this cross-sectional study enrolled both hospitalized and ambulatory patients. In contrast, we enrolled only real-world outpatients. Taken together, the difference in the patient backgrounds might have contributed to the inconsistent findings regarding GV. The results of these studies suggest that endogenous insulin secretion is an independent predictor of unstable GV in outpatients with type 2 diabetes, including in those with severely impaired endogenous insulin secretion. The contribution of GV to hypoglycemia was reported in a CGM-based study23. We have also previously shown that GV, including hypoglycemia, was related to fasting CPR index, which is calculated by fasting serum CPR level and fasting blood glucose level10. Consistently, low CPR independently affected the increase in the LBGI in this study.
Our results are physiologically plausible because differences in the counter-regulatory response to hypoglycemia might explain GV instability24, 25. However, in patients with preserved endogenous insulin secretion, GV may be affected by factors other than endogenous insulin secretion. Moreover, our results suggest that the effects of drugs on GV should be considered in the context of whether the patient has impaired or preserved endogenous insulin secretion.
We also examined whether antidiabetic treatment could impact GV. Patients treated with insulin are known to have a higher risk of insulin-induced hypoglycemia26. Previous studies of hospitalized patients with type 2 diabetes due to hyperglycemia showed that GV was correlated with fasting CPR concentrations in patients on insulin, but there was no correlation in non-insulin patients11, 27. In the present study, we enrolled outpatients regardless of their daily glycemic control status, and the results suggested that insulin use did not affect the log-linear relationship between fasting CPR and CV. The previous CGM-based study comparing GV between different insulin regimens in patients with type 2 diabetes showed that the highest GV and prevalence of hypoglycemia were observed in patients on a basal-bolus regimen28. However, interestingly, our results showed that the type of insulin regimen did not affect the relationship between fasting CPR and GV (Fig. 3B). These conflicting results indicate that the instability of GV was inevitable in patients with severely impaired endogenous insulin secretion, regardless of the insulin regimen. For these patients, assessing hypoglycemia unawareness using CGM should be promoted in daily clinical examination. Moreover, antidiabetic treatment strategies should be aimed at the preservation of pancreatic beta cells to maintain the stability of GV throughout the patient’s life.
As previously reported in a cross-sectional study with patients taking an α-GI12 and a randomized trial with sitagliptin29, the use of an α-GI or DPP-4 inhibitor caused a greater reduction in daily glucose fluctuation. However, our current study showed that the use of an α-GI or DPP-4 inhibitor did not affect the log-linear relationship between fasting CPR and CV. These results suggest that the instability of GV could not be avoided in patients with severely impaired endogenous insulin secretion, regardless of the antidiabetic treatment.
To the best of our knowledge, the present study is the first to assess the relationships between CPR and key CGM indices, such as CV, TIR, TAR, TBR, which are recommended by the international consensus, in type 2 diabetes, including in patients with impaired endogenous insulin secretion, and regardless of glycemic control status. We have shown that GV instability appears to occur when the CPR concentration is low. CPR is easy to measure in a fasting blood sample, and we have shown that it could help identify patients at high risk of unstable GV. Furthermore, the results suggest that it is useful to know the CPR concentration of patients with type 2 diabetes to achieve a reduction in GV in general clinical practice. Intensive therapy to preserve CPR would be expected to reduce GV, thereby preventing the onset and progression of diabetic complications.
The present study had several limitations. First, the study population included only Japanese patients whose duration of diabetes was relatively long, and the proportion of patients on insulin was high. Considering that the insulin secretory capacity of Japanese patients with type 2 diabetes is less than that of Western patients21, it is not certain if our findings are applicable to other populations. However, the heterogeneity of the patients in the present study was representative of the characteristics in Japanese patients with diabetes30, because we included patients with various glycemic control statuses. These findings may be relevant to Asians and other ethnicities. Second, the present study was carried out in slightly obese Japanese patients with type 2 diabetes. Because the mean baseline BMI of Japanese patients is lower than that of Western patients, the present findings may not be directly applicable to patients of all ethnicities. Third, the findings of the present study need to be validated in a prospective study of a randomly selected sample. Fourth, the reliability of the CGM measurements, particularly in the hypoglycemic range, may have been a limitation. However, we aimed to minimize inaccuracies by excluding data from the first and last days of CGM data collection. Fifth, the existence of unassessed confounders, including lifestyle factors, compliance with medication, and the fact that the choice of treatment was made by individual physicians may represent a limitation of the study. Therefore, further randomized trials are needed to determine whether these factors might have affected the results.
In conclusion, we found a log-linear relationship between fasting CPR and GV. This relationship suggested that the contribution of endogenous insulin secretion to GV depends on the extent of insulin secretion impairment. Fasting CPR could therefore represent a useful indicator of GV instability, especially in patients with type 2 diabetes and impaired endogenous insulin secretion.
Data availability
The analyzed datasets are available from the corresponding author on reasonable request.
References
U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: A progressive disease. UK Prospective Diabetes Study Group. Diabetes 44, 1249–1258 (1995).
Kovatchev, B. P. Metrics for glycaemic control—From HbA1c to continuous glucose monitoring. Nat. Rev. Endocrinol. 13, 425–436 (2017).
Rodbard, D. Hypo- and hyperglycemia in relation to the mean, standard deviation, coefficient of variation, and nature of the glucose distribution. Diabetes Technol. Ther. 14, 868–876 (2012).
Gabbay, M. A. L. et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol. Metab. Syndr. 12, 22 (2020).
Lu, J. et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41, 2370–2376 (2018).
Jun, J. E. et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc. Diabetol. 14, 70 (2015).
Tang, X. et al. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin. Chim. Acta 461, 146–150 (2016).
Gohbara, M. et al. Glycemic variability on continuous glucose monitoring system correlates with non-culprit vessel coronary plaque vulnerability in patients with first-episode acute coronary syndrome—Optical coherence tomography study. Circ. J. 80, 202–210 (2016).
Gibb, F. W., McKnight, J. A., Clarke, C. & Strachan, M. W. J. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia 63, 906–914 (2020).
Miya, A. et al. Impaired insulin secretion predicting unstable glycemic variability and time below range in type 2 diabetes patients regardless of glycated hemoglobin or diabetes treatment. J. Diabetes Investig. https://doi.org/10.1111/jdi.13426 (2020).
Jin, S. M. et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: An analysis of 480 subjects. Diabetes Res. Clin. Pract. 104, 266–272 (2014).
Ohara, M. et al. Relationship between glucose variability evaluated by continuous glucose monitoring and clinical factors, including glucagon-stimulated insulin secretion in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 158, 107904. https://doi.org/10.1016/j.diabres.2019.107904 (2019).
Kohnert, K. D. et al. Glycemic variability correlates strongly with postprandial beta-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care 32, 1058–1062 (2009).
Araki, E. et al. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 11, 165–223 (2020).
Bailey, T., Bode, B. W., Christiansen, M. P., Klaff, L. J. & Alva, S. the performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 17, 787–794 (2015).
Pagacz, K., Stawiski, K., Szadkowska, A., Mlynarski, W. & Fendler, W. GlyCulator2: An update on a web application for calculation of glycemic variability indices. Acta Diabetol. 55, 877–880 (2018).
Danne, T. et al. International consensus on use of continuous glucose monitoring. Diabetes Care 40, 1631–1640 (2017).
Service, F. J. et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 19, 644–655 (1970).
Fabris, C., Patek, S. D. & Breton, M. D. Are risk indices derived from CGM interchangeable with SMBG-based indices?. J. Diabetes Sci Technol. 10, 50–59 (2015).
Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 42, 1593–1603 (2019).
Funakoshi, S. et al. Utility of indices using C-peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J. Diabetes Investig. 2, 297–303 (2011).
Crăciun, C. I. A. C. et al. The C-peptide correlations and effect on the glycemic variability parameters in patients with type 2 diabetes. Hum. Vet. Med. 10, 111–116 (2018).
Rodbard, D. Glucose time in range, time above range, and time below range depend on mean or median glucose or HbA1c, glucose coefficient of variation, and shape of the glucose distribution. Diabetes Technol. Ther. 22, 492–500 (2020).
Lachin, J. M., McGee, P. & Palmer, J. P. Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes 63, 739–748 (2014).
Fukuda, M. et al. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes 37, 81–88 (1988).
Ben-Ami, H., Nagachandran, P., Mendelson, A. & Edoute, Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch. Intern. Med. 159, 281–284 (1999).
Christensen, M. B., Gæde, P., Hommel, E., Gotfredsen, A. & Nørgaard, K. Glycaemic variability and hypoglycaemia are associated with C-peptide levels in insulin-treated type 2 diabetes. Diabetes Metab. 46, 61–65 (2020).
Bajaj, H. S. et al. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (variation study). Diabetes Care 40, 194–200 (2017).
Suzuki, R. et al. Effect of short-term treatment with sitagliptin or glibenclamide on daily glucose fluctuation in drug-naïve Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 20, 2274–2281 (2018).
Japan Diabetes Clinical Data Management Study Group; JDDM (2020). http://jddm.jp/data/index-2019/. Accessed 29 Sept 2020
Acknowledgements
The authors thank Mark Cleasby, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. We would like to express special gratitude to all the patients and staff who participated in this study.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and were involved in all stages of manuscript development. A.M. and A.N. analyzed the data and wrote the manuscript. A.N. was a guarantor of this study. T.H. researched the data. Y.M.I. contributed to the statistical analysis. H.N., H.K., K.C. and H.M. contributed to the discussion. S.N., H.M. and T.A. critically revised the manuscript and have primary responsibility for the final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miya, A., Nakamura, A., Handa, T. et al. Log-linear relationship between endogenous insulin secretion and glycemic variability in patients with type 2 diabetes on continuous glucose monitoring. Sci Rep 11, 9057 (2021). https://doi.org/10.1038/s41598-021-88749-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88749-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.