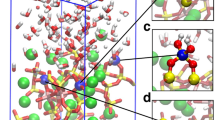
Abstract
Glass structures of multicomponent oxide systems (CaO–Al2O3–SiO2) are studied using a simulated pulsed laser with molecular dynamics. The short- and intermediate-range order structures revealed a direct correlation between the transformation of Al(IV) to Al(V), regions of increased density following laser processing, inherent reduction in the average T–O–T (T = Al, Si) angle, and associated elongation of the T–O bonding distance. Variable laser pulse energies were simulated across calcium aluminosilicate glasses with high silica content (50–80%) to identify densification trends attributed to composition and laser energy. High-intensity pulsed laser effects on fictive temperature and shockwave promotion are discussed in detail for their role in glass densification. Laser-induced structural changes are found to be highly dependent on pulse energy and glass chemistry.
Similar content being viewed by others
Introduction
The application of photo-modified materials in industry reflect the importance in understanding changes to the resolving microstructure. Early studies focused on micromachining surface structures, but quickly evolved into generating 3-dimensional refractive index-modified structures. Femtosecond pulsed laser exposure to materials has become an extensively studied topic1,2,3,4,5,6,7. Ultrafast pulsed lasers have undergone significant technological breakthroughs, enabling the development and innovation of materials in diverse fields8,9,10,11. The application of high-intensity laser pulses on transparent materials remains an active topic in large part due to the lack of understanding laser pulse—glass interaction.
Calcium aluminosilicate (CAS) glasses are well studied in materials research because of their superior mechanical- and physical-properties12,13,14,15. While prior works have investigated the structural dependence on composition and temperature16,17,18,19,20, studies of pressure induced structural changes primarily focus on peraluminous (R < 1)20 and percalcic (R > 1) glasses12,19,20,21,22. Limited work probing the silica-rich space along the tectosilicate join (R = CaO/Al2O3 = 1) has been reported13,14,15,23, and only a handful investigate pressure induced structural changes in the silica-rich CAS along the tectosilicate join23,24,25.
Fused silica has been the primary glass studied in femtosecond laser exposure due to its primitive structure; including experimental and simulated studies26,27,28. Its enhanced thermal–mechanical properties and wide optical transmissivity, extending from the infrared (IR) to ultraviolet (UV), make it an important material used in industry. The densification of fused silica has been documented under extensive experimental conditions such as: hydrostatic compression29, neutron irradiation30,31, femtosecond pulse laser irradiation32,33,34, and shock-wave propagation35,36. Experimental studies in which fused silica was exposed to femtosecond laser pulses, formation of filamentation, ablation, and waveguides have been reported. Chan et al.1 have shown a positive correlation between pulse energy (PE) and Si–O ring structures from 5- and sixfold to 3- and fourfold coordination. Computer simulations support the attribution of experimental densification in pure silicate glasses to the reduction of Si–O ring sizes35. Understanding the effects of glass modifiers added to the system under ambient conditions is pertinent to resolving thermal and pressurized effects.
The addition of Al and Ca into the pure SiO2 system creates sites with reduced bond strength, therefore more easily deformed in comparison to fused silica. Aluminum, like it’s silicon counterpart, is integrated into the SiOx structure with tetrahedral geometry, under four-fold coordination. However, it’s prone to form over-coordinated (Al(V)) species when exposed to elevated temperature and pressure, enhancing its mechanical properties. Five-coordinated Al, along the tectosilicate join, have been widely reported by Neuville19,37, Stebbins38 and others13,23,39, showing considerable deviation between Al(V) concentrations. Several MD and ab initio molecular dynamics (AIMD) simulated CAS glasses, along the tectosilicate join, report a range of five-coordinated aluminum from 1.4% to approximately 8% in CaAl2Si2O813,24,39. Using 27Al Nuclear Magnetic Resonance (NMR), Stebbins38 and Neuville37 reported a range of Al(V) for slow and fast quenched CaAl2Si2O8 from 7.5–9% and 6.5–7%, respectively. Based on these results, five-coordinated Al in CAS glasses thermally equilibrated to room temperature are highly dependent on potential energies as well as fictive temperature13,38. Increased Al-coordination occurs in conjunction with triclustered oxygen (TBO) to compensate for the presence of non-bridging oxygen (NBO). Due to the equal concentration of CaO and Al2O3, the system has no net charge. In theory, these glasses should be void of any NBO; however, Stebbins et al.38 have demonstrated, using 17O NMR, the presence of NBO in otherwise charge neutral CAS glasses, also revealing most NBO occur at SiO4 sites12. Agnello et al.13 provide a detailed analysis of the Al(V), in which all were Q5 (meaning no NBO bonds were present) and 2-out-of-5 were TBO, while the other three bonds are bridging oxygens (BO). For CAS systems along the tectosilicate join, the number of NBO decreases as silica content increases16.
Computer modeling of spatially confined laser-induced processes has largely focused on thermal evolution as a function of time, energy, and pressure. Recent studies have shown the effects of femtosecond pulse laser interaction with metallic surfaces through a two-temperature model (TTM)9,40. Applying this model to amorphous materials, like glass, is challenging due to lack of long-range order and covalent bonding. Instead, this study focuses on applying translational kinetic energy in a localized region and allowing the thermal energy to dissipate through the system. Due to experimental limitations, modeling provides a critical insight into highly localized structural gradients and chemistries as a function of glass composition and pulse energy. To the best of our knowledge, this is the first systematic effort of investigating simulated pulse laser-glass interaction with CAS glass and the resolving structural dependence on laser-energy and composition.
The motivation for this research stems from the lack of understanding localized glass structure after pulse laser exposure, and the effects of glass chemistry and pulse energy on the laser-modified region. In this work, we report the results of molecular dynamic (MD) simulations aimed at investigating the effects of composition and laser energy on short and intermediate range order, microstructure, and residual density profiles in silica-rich calcium aluminosilicate (CAS) glasses along the tectosilicate join (CaO/Al2O3 = 1). We will present the methodology used in simulating both glass formation as well as laser exposure and determine the mechanism responsible for laser induced densification.
Methods
Glass formation
Calcium aluminosilicate systems were modeled using Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS)41. We have chosen the force field developed by Pedone et al.42 based on adequate agreement with silicate structural features. The initial structures were created using the correct stoichiometric ratio and experimentally calculated mass densities, seen in Table 1, that produced roughly a 1 million atom sized system of the targeted composition. Each system had an approximate size of 240 × 240 × 240 Å3.
Long-range Coulombic interaction were represented by a particle–particle particle mesh (PPPM) solver at an accuracy of 10–4 and a timestep of 2 fs. The system was heated to a temperature of 4000 K, allowing the atoms to move for 50 ps. The randomized structure was quenched to 300 K over 0.5 ns, using the NVT ensemble. The simulated glass was finally relaxed to atmospheric pressure under a controlled pressure and temperature ensemble for 1 ns using a Berendsen thermostat and Berendsen barostat43. Quench rates of 3.7 and 0.74 K/ps were also tested to ensure little to no change in defect concentration. Radial distribution functions (g(r)) for initial configurations are shown in Fig. 1. The visual software OVITO44 was used to investigate microstructure and atom distribution of simulated glasses. To calculate structural characteristics, using Visual Molecular dynamics45 (VMD), cutoff distances were chosen based on the first minimum of the pair radial distribution function (PRDF): Si–O, Al–O, Ca–O cutoff distances of 2.15, 2.25 and 3.15 Å, respectively. Running coordination numbers for Al–Al, Si–Si and Al-Si were calculated by integrating the radial distribution functions, using a delta of 0.005.
Radial distribution functions for each starting glass composition (left), illustration of the simulated system with assigned shells ranging from 1 to 20 (Ovito, 2.6, https://www.ovito.org/) (right).
Laser exposure
Simulating laser exposure is applied through a heat flux, J, across a defined region which we will be refer to as the hotspot or focal region.
The first term in the equation for J is the per-atom kinetic and potential energy (ei), Si in the second term represents the calculated per-atom stress tensor, volume (V) and vi is a 3 × 3 matrix–vector multiplier. The laser source is applied at (x,y) = (116.5, 116.5) nm along the entire z-axis. Simulated laser heat is applied to this system in the form of a gaussian profile using the form of a normal distribution,
where \(\sigma\) is half pulse width divided by root two, A is number of atoms within the heated region multiplied by energy/atom (method for calculating this energy will be discussed later), x is step number multiplied by the timestep (2 fs), and μ is the pulse width (10 ps). During the laser exposure process, a Nose–Hoover global thermostat was applied outside of the heated region to simulate thermal dissipation from the laser pulse46. A total of 80 ps trajectory was conducted to ensure thermal equilibration. To maximize laser modified volume, the simulated focal point was elongated to a cylinder. As previously discussed, the structural modifications fluctuate across the length of the box, therefore we cylindrically chunk along the z-axis with a shell width of 5 nm, seen in Fig. 1. Although there are 48 chunks in the entire system, we will show perturbations are only observed in the first twenty shells. The remainder of this paper we will refer to specific shell numbers or a series of shells when referring to the modified system. For example, shell number 5 would be a shell starting from 25 nm and ending 30 nm from the center of the periodic box.
To calculate per atom energy, we used an in-house script that with inputs from experimental laser parameters (pulse width, peak intensity, repetition rate, and spot size) and material properties to model nonlinear laser absorption during experimentation. In this work, per atom energy represents the transfer of energy between the electronic and ionic systems while the experimental process (discussed later) involves phonon-electron coupling; both processes yielding similar results. A model similar to that of Couairon et al.47 was used, taking into account multiphoton and avalanche absorption, plasma defocusing, and Raman scattering. The amount of energy absorbed per atom in the center of the focal volume due to a Gaussian beam of pulse width of 10 ps and wavelength of 1064 nm focused by a lens of numerical aperture = 0.6 was modeled. Pulse energies of 4.5 uJ, 3.5 uJ, 2.9 uJ, 2.4 uJ, and 2.2 uJ were found to correspond to 100, 50, 25, 15 and 10 eV/atom respectively. Note that both the volume of the absorption region and the percentage of the beam absorbed vary with laser PE, resulting in a varying sensitivity to PE. The material parameters in the model were for fused silica which may cause some variance in the results; however, this range of energies was chosen to simulate common laser processing conditions. Energy per atom and spot size were optimized to reach target temperatures. A focal volume of less than 5% of the total volume is used to mitigate boundary effects.
Results and discussion
Residual density profile
Figure 2 shows a time-lapse of the simulated laser pulse interaction with CAS10.80100. Peak laser fluence occurs at the 10 ps timestep, after which thermal energy dissipates until it’s equilibrated to room temperature. High intensity pulse interaction induces void formation, commonly seen in experimental studies of silicate glasses6,7,48,49. Voids were observed in all compositions when the PE ≥ 15 eV/atom. While the pulse reaches significantly higher temperature than the glass, this is because not all energy is translated via heating the system (e.g. fluorescence or plasma-induced shockwave). Formation of voids is accompanied by compaction in the surrounding region.
Time-lapse of simulated kinetic energy(kg m2 s−2) during laser pulse interaction (PE = 100 eV/atom) with calcium aluminosilicate glass (Ovito, 2.6, https://www.ovito.org/).
The modified glass structure was first analyzed by mapping residual density profiles. Changes in density, and concurrent linear refractive index, align with Bhardwaj et al.50 results for aluminosilicate and alkali-aluminosilicate glass density after pulse laser exposure. Laser-induced refractive index gradients are the foundation for writing waveguides, and are reported to range 10–4 to 10–2 in fused silica2. These structural modifications, however, are highly dependent on glass composition and laser conditions.
Radial heatmaps, in Fig. 3, illustrate per shell density of the pristine and post-laser modified glasses. Simulated initial glass densities show good agreement with experimental values. Density within the focal volume reduces significantly due to void formation. This process occurs in experiments when tightly focused laser pulse energy is absorbed by electrons, producing an electron–ion plasma6. Electrons collisionally heat ions within the focal volume, and upon expansion significant pressures build and generate a pressure wave, or shockwave, resulting in artifacts such as voids, change in chemical species and density profiles. As anticipated, higher energies increase void size. Our results are in good agreement with previous studies investigating the effect of PE on void size in fused silica, borosilicate, and aluminosilicate glasses51. These studies demonstrate picosecond and femtosecond timescales produce similar trends in void size as a function of PE. The observed trend of increasing plasma formation, increasing void size, imposes a greater densification (likewise higher dielectric), strain, and subsequent pressure wave generation34.
Compositional dependence of localized residual density fields for each laser condition are shown in Table 2. Note the trend in single shell maximum density change—the addition of calcium and aluminum reduces the maximum change in density observed in a single shell. Considering the kinetic fragility index, which characterizes how rapidly the relaxation process occurs, we anticipate fragility to increase with larger initial densities52. As a result, glasses with high silica concentration (and lower initial density) are more difficult to relax53. Highly localized heating from the pulse-glass interaction produces the largest distribution in density profiles for these glasses. Similarly, we see a compositional dependence on the void diameter and change in density within the focal volume presented in Fig. 4 and Table 2. When x = 0.15, 0.20 and 0.25, focal volume density increases 4.2%, 10.8% and 13.5%, respectively, in comparison to CA10.80100. The addition of calcium creates NBO at either Si–O or Al-O sites, weakening the tetrahedral network. The reduction in network connectivity reduces the transition temperature (Tg) and viscosity, therefore increasing the observable viscous flow of the heated glass after removing the simulated pulse5. Lower viscosity provides more time for the glassy network to rearrange, confirmed based upon the direct correlation between density and fragility, causing the void to inherently collapse inward, reducing the diameter.
Figure 4 shows the change in density on a per shell basis, for each composition under all simulated laser-exposure conditions (Fig. 4a–d). Density change (Δρ) within the focal volume (diameter of 25 Å), seen in Fig. 4e, as a function of silica concentration and laser energy support the observation that modifier content and void size are inversely proportional. Focal volume Δρ, found in Table 2, varies 11% between CAS10.80 and CAS25.50 with PE = 100 µJ. Nearly every condition we find Δρ for shell 1 (25–30 Å from the origin) synonymous to the pristine glass, CAS25.50 shows slight deviates from this trend. Subsequent shells (representing 5 Å rings) show positive Δρ and maximum densified shell that is dependent on silica content and pulse energy. The maximum observed densification occurs in CAS10.80 (14%). Integrating the function, we calculate area under the curve and find total densification increases with silica concentration. As previously mentioned, glasses with higher silica content have lower fragility and therefore experience enhanced peak densification. The position of peak densification for all conditions support relaxation based on structural rigidity. Based on the overall Δρ between shells 6–11, densification trend becomes asymptotic as PE increases. Further extrapolation to higher energies would likely yield a plateau in densification. Experimental studies in laser exposure of soda-lime silicates (SLS)40,41 observe similar behavior in densification as a function of PE. Laser-induced densification gradually reduces at increased distance from the focal point; shell 13 (90 Å from the focal point) the density returns to its unmodified condition.
Based on simulated and experimental initial glass densities, we find the magnitude of laser-induced densification is dependent on the system’s free volume. Replacing Al and Ca for Si increases the structure’s packing density, therefore diminishing laser-exposure distortions to the glassy network. The position of peak densification occurring at greater distances from the focal point is attributed to a combination of enhanced fragility and reduced rigidity. Both factor into shockwave propagation during exposure and structural relaxation after the pulse is removed. Additionally, our results indicate a limit to PE effect on densification in area surrounding the focal point.
Coordination number distribution
Figure 5 shows the average coordination number distribution per shell of Al species in CAS10.80. The initial condition, shell zero, represents the total coordination within the focal volume (shells one through five). Pressure of the overall system at peak intensity of the simulated pulse was 6.1, 4.5, 2.9, 2.0 and 1.3 GPa for 100, 50, 25, 15 and 10 eV, respectively. Aluminum is predominately four coordinated in ambient conditions for all tectosilicate simulated systems20. The unmodified structure has predominantly four- and some five-fold Al species (CAS10.80: 94% and 5%, CAS15.70: 91% and 8%, CAS20.60: 89% and 9%, CAS25.50: 87% and 12%, Al(IV) and Al(V), respectively). Average aluminum coordination changes for CAS15.70, CAS20.60, and CAS25.50 can be found in the supplementary data.
The concentration of AlO3 in the unmodified system is ≤ 1% for all compositions and decreases with SiO2. These results are in agreement with Agnello13 and Tandia39, who modeled identical compositions. Hong et al.12 show that increasing pressure in percalcic CAS glasses reduces the fraction of AlO3 while increasing 4- and fivefold coordination. Based on shockwave propagation and direct relation to SiO2 concentration, it seems the increased Al(III) species within the focal volume is attributed to the tetrahedral substitution of Al3+ for Si4+.
The trend between PE and coordination is consistent across all compositions, while Al(V) concentrations increased with Al2O3/SiO2 ratio. Our results show that the presence of Al(V) in the glass structure are consistent with the observed increase in glass density (Fig. 3a). Previous studies53 demonstrated an increase in Al-coordination as a function of hardness and densification. Based on the residual density profiles, in Fig. 4, and the Al(V) coordination in Fig. 5, it is likely the increased coordination state plays a dominant role in the densification and crosslinking of the glass network. Amplified Al(V) species appear inversely proportional to the change in Al(IV). This change is largely attributed to the ability of Al to go from fourfold to fivefold under increased temperature and pressure35,54. The unmodified structure has predominantly four- and some five-fold Al species (CAS10.80: 94% and 5%, CAS15.70: 91% and 8%, CAS20.60: 89% and 9%, CAS25.50: 87% and 12%, Al(IV) and Al(V), respectively).
The fraction of AlO6 is less than 1% in all compositions in ambient conditions. Simulated PE = 100 eV increased Al(VI) concentration range from 1 to 4% for CAS10.80 and CAS25.50, respectively. Stebbins et al.21 show that annealing quenched glasses and lowering their fictive temperatures significantly reduces the population of high-coordinated Al species. Denser silica glasses are formed at fast quench rates and large fictive temperatures7. These findings are consistent with our results where the annealed starting structure has near zero Al(VI) concentration.
It is worth noting that the simulated laser-pulses induced a pressure-wave which increases the number density by approximately 12.4% in shells with the highest Al(V) and Al(VI) content. Although the increase in number density is not attributed the larger population of over-coordinated Al, we find that pulse energy and high-coordination are directly related, similar to Stebbins et al.21 use of high-resolution27Al and 17O NMR to show that the content of five-coordinated aluminum increases with fictive temperature.
Under ambient conditions the coordination number distribution of nearly all Si atoms is four-fold, forming SiO4 tetrahedra. The distributions of Si(IV) and Si(V), seen in Fig. 6, follow a similar trend as Al species where the density in regions surrounding voids increases due to the increased fictive temperature and pressure. As Stebbins38 and Lee54 highlight in aluminosilicates 5- and 6-coordinated Al form much more readily at high temperatures and pressures than Si(V). Previous studies12,23,54 show that the Si transition from four to five-fold coordination occurs at pressures ranging from 10 to 15 GPa, all the way up to 40 GPa; however, the maximum pressure predicted in the simulated laser pulse was 6.1 GPa. The densification process around silica tetrahedron primarily result from a reduction in the inter-tetrahedral angle (Si–O–Si), small or undetectable change of interatomic distances, and gradual increase of average atomic coordination29. Although our model generates pressures significantly lower than studies observing pressure induced over-coordinated Si (Si(V)), Stebbins et al.55 have shown its abundance also increases with fictive temperature. The simulated fraction of Si(V) in this study is small and may be unobservable using 29Si NMR—further experimental investigation should follow. The highest concentration of laser induced-five-coordinated silicon occurs in CAS10.80. Due to Si(V) dependence on silicon content, we anticipate lower concentrations in CAS15.70, CAS20.60 and CAS25.50. For this reason, our model shows silicon only exists as four-fold in those systems.
Interatomic bonding
Short range order
Simulated aluminum and silicon interatomic bonding distance distributions over the first eleven shells (55 Å)are shown in Figs. 7 and 8 (normalized y-axis) along with distribution statistics in Tables 3 and 4. To better understand the effect of pulse energy on localized structure, the topology of TO units (T = Al, Si) has been investigated via T–O and T–T radial distribution and O–T–O bond angle distributions; ambient conditions are plotted over each simulated laser condition, including the difference in distributions. Histogramed data and statistics for the other compositions can be found in the suplementary information. Distortions in the glass random network produce a distribution around the ideal bonding angle and interatomic bond distance. We show the effects pulse energy and composition have on the resolving distributions.
Histogram of (a) Al–O distance, (b) O–Al–O bond angle, and (c) Al–Al distance for 10CaO 10Al2O3 80SiO2 glass (shells 1–11) after simulating various pulse energy exposures (wide black line) and ambient structure (wide red line). The thin black line under each distribution shows the difference in distributions for visual assistance. Error within 1%.
Histogram of (a) Si–O distance, (b) O–Si–O bond angle, and (c) Si–Si distance for 10CaO 10Al2O3 80SiO2 glass (shells 1–11) after simulating various pulse energy exposures (wide black line) and ambient structure (wide red line). The thin black line under each distribution shows the difference in distributions to help illustrate the differences. Error is within 1%.
Four-coordinated Al and Si have a tetrahedral geometry and average bond angle of approximately 109.5°; tetrahedron geometry is comprised of four 109.5° angles, trigonal bipyramidal geometry has three 120°, two 90° and one 180° angle. The average O–Al–O bond angle in CAS10.80 is approximately 108.8° and average Al–O and Al–Al bonding distance of 1.76 and 3.09 Å, respectively. Average Al–O and Al–Al distances in the ambient system (CAS10.80) were 1.76 and 3.09 Å. These values are in agreement with previous simulation12,17,56,57,58,59 and experimental20,60,61 studies. Compositions CAS15.70, CAS20.60 and CAS25.50, under ambient conditions, have an average Al-O bond distance of 1.77 Å and Al-Al distance of 3.10, 3.11 and 3.12 Å, respectively. Minor elongation of the average bond length can be attributed to elevated population in high-coordinated polyhedra. Average O–Al–O bond angle is consistent across all compositions.
Bonding environment for Si under ambient conditions is extremely stable primarily due to its high bond energy62. The stability was previously eluded to in the discussion of nearly 100% tetrahedral geometry in high-silica content glasses under extreme conditions; similar findings have also been reported in experiments and simulations12,20,57,59,63. Average O–Si–O bond angle of 109.3° is constant in all simulated systems under ambient conditions. Likewise, the calculated Si–O and Si–Si bonding distance of 1.61 and 3.098 Å in agreement with experimental findings60,61,64.
Pulsed laser-glass interacion increases the systems disorder through broadening of the O–Al–O and O–Si–O anglular distributions. The standard deviation for both distributions increase with pulse energy (Tables 3 and 4). Although both angle distributions are mostly-symmetric under ambient conditions, laser exposure causes some shift and asymmetry of the peak(unmodified O–Al–O and O–Si–O are centered at 106.5° and 109°, respectively). The O–Al–O bond angle is more susceptible to distortions; PE = 10 and 15 eV show slight reduction around the average angle and increase around 90°, PE = 25, 50, and 100 eV show similar trend with 100 eV distirbution centered at 103° and average of 108.3°; the data reflects a positive skew. Bond angle distribution for O–Si–O shows no change in average bond angle; however, as pulse energy increases the distribution shifts to lower angles with PE = 100 eV centered at 107°. Increasing Al2O3/SiO2 ratio did not have a significant effect on O–Al–O peak position, where bond angle distribution (PE = 100 eV) in CAS15.70, CAS20.60 and CAS25.50 are centered at 102.5°, 102.5°, and 103°. However, it is important to note that enhancing the ratio increases the average O–Al–O distortions when compared to the ambient system.
Laser induced changes to interatomic bonding distance echo the bond angle data, where strucutral modifications are most prominent in Al species and the average Al-O bond length increases with succesively higher pulse energy. Because bond length and interbonding-angle are inversely proportional, the densification process reduces the average bond angle and increases bond length. At PE = 100 eV, Al–O bond length in CAS10.80 increased 0.04 Å, while the average bond distance in compositions CAS15.70, CAS20.60 and CAS25.50 show a slight reduction to 0.03 Å. Calculated increase in Al-O bonding distance is due to increased Al-coordiantion number12,65; these findings are consistent with empirical observations in ionic crystals66. It is also worth noting the averge Al-O bond length is dependent on temperature and pressure, based on the incremental trend towards longer bonds as pulse energy increases. Observed change in average Al-O bond length between PE = 100 and 10 eV decreases with Al2O3/SiO2 concentration (shown in Tables S1, S3 and S5). Calculated Si–O average bond length across all compositions increased by less than 0.01 Å. The aforementioned densification process around silicon species, which primarily occurs from a reduction in inter-tetrahedral angle and little to no detectable change in the interatomic distance, is supported by our study. Previous studies12,23 investigating compression of glass melts have found changes in the O–Al–O bond angle distribution are almost exclusively associated to Al(IV). Based on these results, we have determined that (1) Si species are far less susceptible to laser-induced structural modifications compared to Al, (2) distortion to the O–Al–O bond angle are dependent on temperature and pressure generated from pulse energies, and (3) increased Al2O3/SiO2 ratio amplifies laser modified structural distortions.
Intermediate range order
To better understand the effect of laser pulse interaction on intermediate range order of glass structure, we investigated the bond angle distribution between Si and Al species. Distribution statistics for Al–O–Al, Al–Al, Si–O–Si, Si–Si and Al–O–Si are provided in Tables 3 and 4 (statistics for CAS15.70, CAS20.60 and CAS25.50 are provided in the supplementary information, Tables S1–S6). Under ambient conditions, the average distance for Si–Si and Al–Al are approximately 3.10 Å59,65. Pulsed laser exposure increases the average distance for both; however, as previously noted in the interatomic bond angle analysis, Al species show a significantly higher capacity for distortion. An interesting observation from bond length calculations highlights a distinct elongation of Al–Al between ambient and laser-modified structure, but little to no change between PE = 10 and 100 eV. Similar trends are seen in Si–Si bonding, but the magnitude of change is smaller.
The average Al–O–Al, Si–O–Si and Al–O–Si bond angle in unmodified CAS10.80 are 116.1°, 148.4° and 136.8°, respectively. Based on the distributions presented in Fig. 9, the average bond angles all tend to decrease with pulse energies (resulting in concomitant increase in Al–Al and Si–Si distance). Contrary to aluminum avoidance principle, studies have shown the presence of Al–O–Al bonding in calcium aluminosilicate glasses19,67. In CAS10.80, average Al–O–Al angle is 116.1° under ambient conditions and 115.3° after exposure to PE = 100 eV. Increasing Al concentration results in a larger distinction between the pristine and laser-damaged (PE = 100 eV) average Al–O–Al bond angle (x = 0.15 117.9° and 116.3°, x = 0.2 119.3° and 117.2°, x = 0.25 119.8° and 117.6° for ambient and PE = 100 eV, respectively). Heightened standard deviation to Al–O–Al distribution reflects the transition of Al four- to five-fold coordination, considering laser effects on short-range order were significantly more pronounced in Al-species. Agnello et al.13 have shown deconvolution of Al–O–Al bond angle distribution, in high silica content glasses, contains two distributions, (1) Al connected by a BO (Al–BO–Al) and (2) Al bonded to a triclustered oxygen (Al–TBO–Al)13. Reduced intensity along the distribution tail at higher angles is attributed to decreased BO and an increase around 100° indicates the formation of TBO. As the initial glass system is charge neutral, triclustered oxygen and five-coordinated Al form to compensate for the formation of NBO.
Stability of Si–O and Si–Si distance (Table 4) after laser irradiation indicates the decrease in average Si–O–Si angle is likely unrelated. Reduction is attributed to the dissociation of high-coordinated ring structures (five- and six-fold) and increase in three- and four-membered rings. This phenomenon is reported in both simulated and experimental investigations of laser modified silicate glasses. Hehlen68 has shown the densification of vitreous silica at 1000 K under hydrostatic pressure decreases the Si–O-Si bond angle by roughly 5.7°, equating to nearly 20% densification, as a result of breaking six-membered rings and forming three- and four-fold rings. These studies are consistent with our findings in which increasing laser pulse energy reduces average Si–O–Si bond angle, likely through the dissociation of higher-membered rings and formation of three and four-membered rings.
Al–O–Si bonding follows a similar trend as Si–O–Si bonding when exposed to laser radiation. With increasing Al concentration, the unmodified distributions are centered at 136.8°, 136.6°, 136.5° and 136.4°, respectively. Pulse energy equal to 100 eV shifts the average Al–O–Si bond angle by 2.5°, 2.7°, 2.8° and 3.0° for CAS10.80, CAS15.70, CAS20.60 and CAS25.50, respectively. The distribution shows significant dependence on temperature and pressure. Based on the changes to Al–O–Al and Si–O–Si, we attribute the shift and population increase around 100° to triclustered oxygen formation. Forming five-coordinated Al and triclustered oxygen is necessary for charge compensation of NBO. Most NBO are associated with SiO4 units12. The Ca2+ cation tends to be close to negatively charged species like [AlO4]−, [AlO5]− and [NBO]−, therefore they are most likely to be found connecting [AlOx]− units or along –Si–NBO units.
Figure 10 illustrates the coordination number between Al–Al, Si–Si and Al–Si; calculated by integrating the pair radial distribution function (PRDF). The shoulders correspond to PRDF first minimum for cutoff distance; intensity represents the average coordination number of the pair. In the case of Si–Si under ambient conditions, each SiOx unit connects to approximately 3 other SiOx units. Laser exposure causes a slight decrease in shoulder height, indicating a reduction in silica clusters and forming of NBO. As previously discussed, this is to be expected with increased Al(V) and TBO concentrations, in order to maintain charge neutrality. Conversely, AlOx clustering increases as a function of pulse energy. As previously mentioned, Al(IV) species transitioning to Al(V) is accompanied by an increase in TBO (predominately bonded to AlOX units). Under ambient conditions Al–Si has an average coordination of 1. Similar to Al–Al, due to a slight increase in coordination as a function of pulse energy we show the pairing is susceptible to laser affects. As a result, the coordination change indicates mixing between AlOX and SiOX units increases with pulse energy. Based on the fact that NBO are most often found with SiO4 units, we can expect the modifier ion (Ca2+) to bridge two negatively charged species.
Conclusion
Ultrafast pulse-laser exposure of CAS glasses was modeled using MD simulations. We have compiled a comprehensive study on the effects of pulse energy and glass composition (along the tectosilicate join) on the resolving structure. After analyzing the data, we find (1) thermally induced shockwave generation caused permanent perturbations to the average number density per shell, but does not alter localized chemistry, (2) glasses with the highest silica content incur the largest void size and densification in a single shell (up to 14%), (3) formation of fivefold Al increases with pulse energy, and (4) distortion in SiOx units primarily occur to intermediate-range order(Si–O–Si, Si–Si), with minimal modification in the short-range order(O–Si–O, Si–O).
Systems with increased Al2O3 concentration have a smaller void diameter, this is attributed to longer relaxation times. Observations of the structure’s short-range order, including coordination number distribution and first nearest-neighbor bonding between Al–O and Si–O, showed the ability of Al-species to switch from four- to five-fold coordination, increasing the average Al–O bond length and reducing the O–Al–O bond angle. Shells with enriched Al(V) content showed the most significant change in density. Based on statistical trends relating to Al structure, we have determined that Al coordination and the subsequent bonding environment are dependent pulse energy. Our results are consistent with the literature, confirming that structural changes of Al species have significantly higher temperature and pressure dependences compared to Si. Formation of triclustered oxygen and reduction in silicon ring size were both observed as a result of laser modification to intermediate-range order. Based on a decreased intensity in Si–Si running coordination number shoulder and increase to Al-Al and Al-Si coordination number, we postulate that the laser effects reduce clusters of SiOX units via enhanced mixing of AlOX species and creation of NBO to charge balance TBO and Al(V).
In conclusion, we claim the laser-damaged densification process is two-fold; (1) increase in the AlV concentration and (2) distortion to the silica inter-tetrahedral bonding environment (i.e. Si–O bonding distance and Si–O–Si angular bonds). Densification in glasses with higher Al2O3/SiO2 ratio densify less; smaller increase in AlV occurs in comparison to Si-rich compositions. Understanding the complexities of laser modified glass structure, in multicomponent systems such as CAS, requires strong fundamental comprehension of both short- and medium-range order. This work serves as an important first step in creating a material map of laser-glass interaction studies. Future work should investigate structural evolution after exposure to multiple pulses and defect stability post annealing. By developing a comprehensive model, we can begin to lay the groundwork for improved structural optimization via laser-exposure, and even bridge towards laser-induced nucleation.
Change history
20 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-03906-4
References
Chan, J. W., Huser, T., Risbud, S. & Krol, D. M. Structural changes in fused silica after exposure to focused femtosecond laser pulses. Opt. Lett. 26, 1726–1728. https://doi.org/10.1364/OL.26.001726 (2001).
Krol, D. Femtosecond laser modification of glass. J. Non-Cryst. Solids 354, 416–424 (2008).
Locker, S., Clark, J. A. & Sundaram, S. K. Structural modifications of soda-lime silicate glasses using femtosecond pulse-laser irradiation. Int. J. Appl. Glass Sci. https://doi.org/10.1111/ijag.15823.
Locker, S. & Sundaram, S. Ultrafast modification of oxide glass surface hardness. Appl. Phys. B 125, 225 (2019).
Tan, D., Sharafudeen, K. N., Yue, Y. & Qiu, J. Femtosecond laser induced phenomena in transparent solid materials: fundamentals and applications. Prog. Mater Sci. 76, 154–228 (2016).
Schaffer, C., Glezer, E., Nishimura, N. & Mazur, E. Ultrafast laser-induced microexplosions: explosive dynamics and submicrometer structures. Vol. 3269 PW (SPIE, 1998).
Glezer, E. N. & Mazur, E. Ultrafast-laser driven micro-explosions in transparent materials. Appl. Phys. Lett. 71, 882–884 (1997).
Gottmann, J., Hermans, M., Repiev, N. & Ortmann, J. Selective laser-induced etching of 3D precision quartz glass components for microfluidic applications—up-scaling of complexity and speed. Micromachines 8, 110. https://doi.org/10.3390/mi8040110 (2017).
Rouleau, C. M. et al. Nanoparticle generation and transport resulting from femtosecond laser ablation of ultrathin metal films: Time-resolved measurements and molecular dynamics simulations. Appl. Phys. Lett. 104, 193106–193106. https://doi.org/10.1063/1.4876601 (2014).
Wang, Z. et al. High-throughput microchannel fabrication in fused silica by temporally shaped femtosecond laser Bessel-beam-assisted chemical etching. Opt. Lett. 43, 98–101. https://doi.org/10.1364/OL.43.000098 (2018).
Szustakiewicz, K. et al. Femtosecond laser-induced modification of PLLA/hydroxyapatite composite. Polym. Degrad. Stab. 149, 152–161. https://doi.org/10.1016/j.polymdegradstab.2018.01.015 (2018).
Hong, N. V., Ha, N. T. T., Hung, P. K. & Iitaka, T. Pressure-induced structural change of CaO–Al2O3–SiO2 melt: insight from molecular dynamics simulation. Mater. Chem. Phys. 236, 121839. https://doi.org/10.1016/j.matchemphys.2019.121839 (2019).
Agnello, G. et al. Bulk structures of silica-rich calcium aluminosilicate (CAS) glasses along the molar CaO/Al2O3 = 1 join via molecular dynamics (MD) simulation. J. Non-Cryst. Solids 519, 119450. https://doi.org/10.1016/j.jnoncrysol.2019.05.026 (2019).
Lamberson, L. Influence of atomic structure on plastic deformation in tectosilicate calcium-aluminosilicate, magnesium-aluminosilicate, and calcium galliumsilicate glasses PhD thesis, Cornell University, (2016).
Cormier, L., Ghaleb, D., Neuville, D. R., Delaye, J.-M. & Calas, G. Chemical dependence of network topology of calcium aluminosilicate glasses: a computer simulation study. J. Non-Cryst. Solids 332, 255–270. https://doi.org/10.1016/j.jnoncrysol.2003.09.012 (2003).
Bouhadja, M., Jakse, N. & Pasturel, A. Structural and dynamic properties of calcium aluminosilicate melts: a molecular dynamics study. J. Chem. Phys. 138, 224510. https://doi.org/10.1063/1.4809523 (2013).
Zheng, K., Zhang, Z., Yang, F. & Sridhar, S. Molecular dynamics study of the structural properties of calcium aluminosilicate slags with varying Al2O3/SiO2 ratios. ISIJ Int. 52, 342–349. https://doi.org/10.2355/isijinternational.52.342 (2012).
Thompson, L. M. & Stebbins, J. F. Non-bridging oxygen and high-coordinated aluminum in metaluminous and peraluminous calcium and potassium aluminosilicate glasses: high-resolution 17O and 27Al MAS NMR results. Am. Miner. 96, 841–853. https://doi.org/10.2138/am.2011.3680 (2011).
Neuville, D. R., Cormier, L. & Massiot, D. Al coordination and speciation in calcium aluminosilicate glasses: effects of composition determined by 27Al MQ-MAS NMR and Raman spectroscopy. Chem. Geol. 229, 173–185. https://doi.org/10.1016/j.chemgeo.2006.01.019 (2006).
Hennet, L. et al. Neutron diffraction of calcium aluminosilicate glasses and melts. J. Non-Cryst. Solids 451, 89–93. https://doi.org/10.1016/j.jnoncrysol.2016.05.018 (2016).
Allwardt, J. R. et al. Aluminum coordination and the densification of high-pressure aluminosilicate glasses. Am. Miner. 90, 1218–1222. https://doi.org/10.2138/am.2005.1836 (2005).
Allwardt, J. et al. Effect of structural transitions on properties of high-pressure silicate melts: Al-27 NMR, glass densities, and melt viscosities. Am. Miner. 92, 1093–1104. https://doi.org/10.2138/am.2007.2530 (2007).
Wiles, N. T., Goyal, S. & Baker, S. P. Geometric configuration of five-coordinated Al and Si in tectosilicate calcium aluminosilicate glasses and its effect on plastic flow. J. Non-Cryst. Solids 543, 120129. https://doi.org/10.1016/j.jnoncrysol.2020.120129 (2020).
Drewitt, J. W. E. et al. Development of chemical and topological structure in aluminosilicate liquids and glasses at high pressure. J. Phys. Condens. Matter 27, 105103. https://doi.org/10.1088/0953-8984/27/10/105103 (2015).
Ghosh, D. B. & Karki, B. B. First-principles molecular dynamics simulations of anorthite (CaAl2Si2O8) glass at high pressure. Phys. Chem. Miner. 45, 575–587. https://doi.org/10.1007/s00269-018-0943-4 (2018).
Cheng, C., Wu, A. Q. & Xu, X. Molecular dynamics simulation of ultrafast laser ablation of fused silica. J. Phys. Conf. Ser. 59, 100–104. https://doi.org/10.1088/1742-6596/59/1/022 (2007).
Wang, Y., Xu, X. & Zheng, L. Molecular dynamics simulation of ultrafast laser ablation of fused silica film. Appl. Phys. A 92, 849–852. https://doi.org/10.1007/s00339-008-4579-y (2008).
Petrovic, J. S., Mezentsev, V., Schmitz, H. & Bennion, I. Model of the femtosecond laser inscription by a single pulse. Opt. Quant. Electron. 39, 939–946. https://doi.org/10.1007/s11082-007-9158-5 (2007).
Rouxel, T., Ji, H., Hammouda, T. & Moréac, A. Poisson’s ratio and the densification of glass under high pressure. Phys. Rev. Lett. 100, 225501. https://doi.org/10.1103/PhysRevLett.100.225501 (2008).
Chan, S. L., Gladden, L. F. & Elliott, S. R. In The Physics and Technology of Amorphous SiO2 (ed Roderick A. B. Devine) 83–89 (Springer US, 1988).
Wright, A. C. et al. A neutron diffraction and MAS-NMR study of the structure of fast neutron irradiated vitreous silica. J. Non-Cryst. Solids 150, 69–75. https://doi.org/10.1016/0022-3093(92)90097-4 (1992).
Shcheblanov, N. S. & Povarnitsyn, M. E. Bond-breaking mechanism of vitreous silica densification by IR femtosecond laser pulses. EPL (Europhys. Lett.) 114, 26004. https://doi.org/10.1209/0295-5075/114/26004 (2016).
Shcheblanov, N. S., Povarnitsyn, M. E., Mishchik, K. N. & Tanguy, A. Raman spectroscopy of femtosecond multipulse irradiation of vitreous silica: experiment and simulation. Phys. Rev. B 97, 054106. https://doi.org/10.1103/PhysRevB.97.054106 (2018).
Sakakura, M., Terazima, M., Shimotsuma, Y., Miura, K. & Hirao, K. Thermal and shock induced modification inside a silica glass by focused femtosecond laser pulse. J. Appl. Phys. 109, 023503. https://doi.org/10.1063/1.3533431 (2011).
Kubota, A., Caturla, M. J., Stölken, J. S. & Feit, M. D. Densification of fused silica due to shock waves and its implications for 351 nm laser induced damage. Opt. Express 8, 611–616. https://doi.org/10.1364/OE.8.000611 (2001).
Jeanloz, R. et al. Achieving high-density states through shock-wave loading of precompressed samples. Proc. Natl. Acad. Sci. 104, 9172–9177. https://doi.org/10.1073/pnas.0608170104 (2007).
Neuville, D. R., Cormier, L. & Massiot, D. Al environment in tectosilicate and peraluminous glasses: A 27Al MQ-MAS NMR, Raman, and XANES investigation. Geochim. Cosmochim. Acta 68, 5071–5079. https://doi.org/10.1016/j.gca.2004.05.048 (2004).
Stebbins, J. F., Dubinsky, E. V., Kanehashi, K. & Kelsey, K. E. Temperature effects on non-bridging oxygen and aluminum coordination number in calcium aluminosilicate glasses and melts. Geochim. Cosmochim. Acta 72, 910–925. https://doi.org/10.1016/j.gca.2007.11.018 (2008).
Tandia, A., Timofeev, N. T., Mauro, J. C. & Vargheese, K. D. Defect-mediated self-diffusion in calcium aluminosilicate glasses: a molecular modeling study. J. Non-Cryst. Solids 357, 1780–1786. https://doi.org/10.1016/j.jnoncrysol.2010.12.078 (2011).
Karim, E. T. et al. Atomistic simulation study of short pulse laser interactions with a metal target under conditions of spatial confinement by a transparent overlayer. J. Appl. Phys. 115, 183501. https://doi.org/10.1063/1.4872245 (2014).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19. https://doi.org/10.1006/jcph.1995.1039 (1995).
Pedone, A., Malavasi, G., Menziani, M. C., Cormack, A. N. & Segre, U. A New self-consistent empirical interatomic potential model for oxides, silicates, and silica-based glasses. J. Phys. Chem. B 110, 11780–11795. https://doi.org/10.1021/jp0611018 (2006).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. v., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690. https://doi.org/10.1063/1.448118 (1984).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the open visualization tool. Modell. Simul. Mater. Sci. Eng. 18, 015012. https://doi.org/10.1088/0965-0393/18/1/015012 (2009).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38. https://doi.org/10.1016/0263-7855(96)00018-5 (1996).
Evans, D. J. & Holian, B. L. The nose-hoover thermostat. J. Chem. Phys. 83, 4069–4074. https://doi.org/10.1063/1.449071 (1985).
Couairon, A., Sudrie, L., Franco, M., Prade, B. & Mysyrowicz, A. Filamentation and damage in fused silica induced by tightly focused femtosecond laser pulses. Phys. Rev. B 71, 125435. https://doi.org/10.1103/PhysRevB.71.125435 (2005).
Watanabe, W., Li, Y. & Itoh, K. [INVITED] Ultrafast laser micro-processing of transparent material. Opt. Laser Technol. 78, 52–61. https://doi.org/10.1016/j.optlastec.2015.09.023 (2016).
Bressel, L. et al. Femtosecond laser induced density changes in GeO2 and SiO2 glasses: fictive temperature effect [Invited]. Opt. Mater. Express 1, 605–613. https://doi.org/10.1364/OME.1.000605 (2011).
Bhardwaj, V. R. et al. Femtosecond laser-induced refractive index modification in multicomponent glasses. J. Appl. Phys. 97, 083102. https://doi.org/10.1063/1.1876578 (2005).
Mauclair, C. et al. Single-pulse ultrafast laser imprinting of axial dot arrays in bulk glasses. Opt. Lett. 36, 325–327. https://doi.org/10.1364/OL.36.000325 (2011).
Wang, L., Guan, P. & Wang, W. H. The correlation between fragility, density, and atomic interaction in glass-forming liquids. J. Chem. Phys. 145, 034505. https://doi.org/10.1063/1.4958628 (2016).
Bouhadja, M., Jakse, N. & Pasturel, A. Striking role of non-bridging oxygen on glass transition temperature of calcium aluminosilicate glass-formers. J. Chem. Phys. 140, 234507. https://doi.org/10.1063/1.4882283 (2014).
Lee, S. K. Structure of silicate glasses and melts at high pressure: quantum chemical calculations and solid-state NMR. J. Phys. Chem. B 108, 5889–5900. https://doi.org/10.1021/jp037575d (2004).
Stebbins, J. F. & McMillan, P. Compositional and temperature effects on five-coordinated silicon in ambient pressure silicate glasses. J. Non-Cryst. Solids 160, 116–125. https://doi.org/10.1016/0022-3093(93)90292-6 (1993).
Ganster, P., Benoit, M., Delaye, J. M. & Kob, W. Structural and vibrational properties of a calcium aluminosilicate glass: classical force-fields vs. first-principles. Mol. Simul. 33, 1093–1103. https://doi.org/10.1080/08927020701541006 (2007).
Ganster, P., Benoit, M., Kob, W. & Delaye, J.-M. Structural properties of a calcium aluminosilicate glass from molecular-dynamics simulations: a finite size effects study. J. Chem. Phys. 120, 10172–10181. https://doi.org/10.1063/1.1724815 (2004).
Gutiérrez, G. & Johansson, B. Molecular dynamics study of structural properties of amorphous Al2O3. Phys. Rev. B 65, 104202. https://doi.org/10.1103/PhysRevB.65.104202 (2002).
Okhotnikov, K., Stevensson, B. & Edén, M. New interatomic potential parameters for molecular dynamics simulations of rare-earth (RE = La, Y, Lu, Sc) aluminosilicate glass structures: exploration of RE3+ field-strength effects. Phys. Chem. Chem. Phys. 15, 15041–15055. https://doi.org/10.1039/C3CP51726H (2013).
Petkov, V., Billinge, S. J. L., Shastri, S. D. & Himmel, B. Polyhedral units and network connectivity in calcium aluminosilicate glasses from high-energy X-ray diffraction. Phys. Rev. Lett. 85, 3436–3439. https://doi.org/10.1103/PhysRevLett.85.3436 (2000).
Petkov, V., Gerber, T. & Himmel, B. Atomic ordering in CaX/2AlXSi1-XO2 glasses (x=0,0.34,0.5,0.68) by energy-dispersive x-ray diffraction. Phys. Rev. B 58, 11982–11989 (1998).
Luo, Y. R. Comprehensive Handbook of Chemical Bond Energies. (CRC Press, 2007).
Navrotsky, A., Geisinger, K. L., McMillan, P. & Gibbs, G. V. The tetrahedral framework in glasses and melts—inferences from molecular orbital calculations and implications for structure, thermodynamics, and physical properties. Phys. Chem. Miner. 11, 284–298. https://doi.org/10.1007/BF00307406 (1985).
Himmel, B., Weigelt, J., Gerber, T. & Nofz, M. Structure of calcium aluminosilicate glasses: wide-angle X-ray scattering and computer simulation. J. Non-Cryst. Solids 136, 27–36. https://doi.org/10.1016/0022-3093(91)90115-M (1991).
Ruiz, J. M., McAdon, H., M. & Garcés, J. Aluminum complexes as models for broensted acid sites in zeolites: structure and energetics of [Al(OH)4]−, [Al(H2O)6]3+, and intermediate monomeric species [Al(OH)x(H2O)n-x·mH2O]3−x obtained by hydrolysis. J. Phys. Chem. B 101, 1733–1744 (1997).
The nature of the chemical bond and the structure of molecules and crystals. Nature 148, 677–677. https://doi.org/10.1038/148677c0 (1941).
Stebbins, J. F., Lee, S. K. & Oglesby, J. V. Al-O-Al oxygen sites in crystalline aluminates and aluminosilicate glasses; high-resolution oxygen-17 NMR results. Am. Miner. 84, 983–986. https://doi.org/10.2138/am-1999-5-635 (1999).
Hehlen, B. Inter-tetrahedra bond angle of permanently densified silicas extracted from their Raman spectra. J. Phys. Condens. Matter 22, 025401. https://doi.org/10.1088/0953-8984/22/2/025401 (2009).
Author information
Authors and Affiliations
Contributions
S.L. carried out the project and wrote the manuscript. S.G. and M.M. assisted in data collection and interpretation. SK.S motivated the research. C.U. provided technical analysis and supervised the work. All authors review the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, the values in Table 3 were a duplication of the values in Table 4. In addition, the legend of Table 4, “Distribution statistics for Al–O, O–Al–O, and Al–Al under ambient and laser modified conditions in CAS10.80.” now reads: “Distribution statistics for Si-O, O-Si-O, and Si-Si under ambient and laser modified conditions in CAS10.80.”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Locker, S., Goyal, S., McKenzie, M.E. et al. Laser-induced structural modification in calcium aluminosilicate glasses using molecular dynamic simulations. Sci Rep 11, 9519 (2021). https://doi.org/10.1038/s41598-021-88686-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88686-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.