Abstract
Increasing evidence suggests a relationship between in vitro fertilization-embryo transfer (IVF-ET) and placenta accreta spectrum (PAS). Some studies have reported a lower rate of antenatal diagnosis of PAS after IVF-ET compared to PAS with spontaneous conception. This study aimed to review the diagnostic accuracy of PAS after IVF-ET and to explore the relationship between IVF-ET pregnancy and PAS. According to the PRISMA guidelines, a comprehensive systematic review of the literature was conducted through August 31, 2020 to determine the effects of IVF-ET on PAS. In addition, a meta-analysis was conducted to explore the relationship between IVF-ET pregnancy and PAS. Twelve original studies (2011–2020) met the inclusion criteria. Among these, 190,139 IVF-ET pregnancies and 248,534 spontaneous conceptions met the inclusion criteria. In the comparator analysis between PAS after IVF-ET and PAS with spontaneous conception (n = 2), the antenatal diagnosis of PAS after IVF-ET was significantly lower than that of PAS with spontaneous conception (22.2% versus 94.7%, P < 0.01; < 12.9% versus 46.9%, P < 0.01). The risk of PAS was significantly higher in women who conceived with IVF-ET than in those with spontaneous conception (odds ratio [OR]: 5.03, 95% confidence interval [CI]: 3.34–7.56, P < 0.01). In the sensitivity analysis accounting for the type of IVF-ET, frozen ET was associated with an increased risk of PAS (OR: 4.60, 95%CI: 3.42–6.18, P < 0.01) compared to fresh ET. Notably, frozen ET with hormone replacement cycle was significantly associated with the prevalence of PAS compared to frozen ET with normal ovulatory cycle (OR: 5.76, 95%CI 3.12–10.64, P < 0.01). IVF-ET is associated with PAS, and PAS after IVF-ET was associated with a lower rate of antenatal diagnosis. Therefore, clinicians can pay more attention to the presence of PAS during antenatal evaluation in women with IVF-ET, especially in frozen ET with hormone replacement cycle.
Similar content being viewed by others
Introduction
Placenta accreta spectrum (PAS) is associated with an increased maternal morbidity and mortality due to massive hemorrhage during cesarean delivery1,2,3. The mean blood loss during cesarean delivery for PAS is approximately 3000 mL, and the hysterectomy rate is around 40%4,5. The main risk factor of PAS is placenta previa, with an approximate odds ratio (OR) of 50–1006,7,8. Several risk factors have been linked to PAS, including a history of cesarean deliveries (OR: 5–9), uterine surgery (OR: 2–3), multiparity (OR: 3), and advanced maternal age (OR: 2.1)7,9. Recently, mounting evidence have suggested that in vitro fertilization-embryo transfer (IVF-ET) is associated with PAS, and the OR of PAS is roughly between 3 and 1410,11,12,13.
IVF-ET currently accounts for 1–4% of live births in the United States and Europe, and the rate has been rapidly increasing as experience with the procedure accumulates and success rates improve14. Similarly, the number of PAS after IVF-ET may be increasing15. Therefore, clinicians should pay attention to patients with PAS after IVF-ET; despite this, little is known about the characteristics of PAS after IVF-ET15. Moreover, to the best of our knowledge, no systematic reviews focusing on PAS after IVF-ET have been performed. A recent study has shown that the diagnostic accuracy of PAS by magnetic resonance imaging (MRI) is lower in PAS after IVF-ET than in PAS with spontaneous conception15. Therefore, there is a possibility that PAS after IVF-ET has different characteristics, compared to PAS with spontaneous conception.
Several studies have reported that preoperative assessment of PAS disorders and multidisciplinary surgical approaches are needed to decrease the surgical morbidity of patients with PAS1,16,17,18. Moreover, it has been widely known that the antenatal diagnosis of PAS helps to reduce hemorrhagic morbidity and improves the prognosis; this may be attributed to the comprehensive multidisciplinary care received by patients, which includes a planned cesarean hysterectomy19,20,21,22.
This study focused on assessing whether the diagnostic accuracy for PAS after IVF-ET is lower than that for PAS with spontaneous conception. Furthermore, a meta-analysis was conducted to explore the relationship between IVF-ET pregnancy and PAS.
Materials and methods
Approach for a systematic literature review
A systematic review was performed to review the diagnostic accuracy of PAS after IVF-ET comparing with PAS with spontaneous conception. This study also aimed to examine the effect of IVF-ET on the prevalence of PAS.
Eligibility criteria, information sources, search strategy
We conducted a systematic search of articles published through August 31, 2020 using PubMed, Scopus, and Cochrane Central Register of Controlled Trials as performed in our previous study2,23,24,25,26. We reviewed articles according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines27. Studies were identified by screening the titles, abstracts, and full texts of relevant articles, as previously described. All abstracts were screened by Sh.M. and Y.N.
The following terms were applied in PubMed, Scopus, and the Cochrane database to identify studies which examined the association between IVF-ET and PAS (Supplemental Table S1; MeSH terms were used in PubMed and Cochrane database search): fertilization in vitro [MeSH] OR Assisted Reproductive Techniques [MeSH Terms] OR Embryo Transfer [MeSH Terms] OR Intracytoplasmic Sperm Injection [MeSH Terms] OR "in vitro fertilization" OR “Cryopreserved” OR “Oocyte donation” OR "fresh cycle" OR "frozen cycle" were used to identify the studies regarding IVF-ET.
Studies investigating the effect of IVF-ET on PAS were then identified from this list using the following keywords: Placenta accreta [MeSH Terms] OR "Morbidly adherent placenta" OR "Morbid adherent placenta" OR "Placenta Accreta Spectrum" OR “placenta increta” OR “placenta percreta” OR "adherence of placenta" OR "adherence of the placenta" OR "adherent placenta."
Study selection
The inclusion criteria based on Patient/Population, Intervention, Comparator, Outcome, Study (PICOS) design are shown in Table 128. Studies were included if they met the following criteria: (1) the effect of IVF-ET on the risk of PAS or antenatal diagnostic accuracy for PAS or maternal outcome for PAS during cesarean delivery was examined; and (2) comparative study was performed between an experimental group and a control group (e.g., IVF-ET versus spontaneous conception, frozen ET versus fresh ET, etc.).
The exclusion criteria were as follows: (1) insufficient information about the outcome of interest; (2) studies lacking control arm; (3) article not written in the English language; and (4) conference abstracts, case reports, case series, reviews, systematic review, and meta-analysis.
Data extraction
Data were extracted by the author (Sh.M. and Y.N.) and the following variables were recorded: PAS type, year of study, first author’s name, study location, number of included cases, the definition of PAS, and outcomes of interest (the risk of PAS, maternal outcome, diagnostic accuracy for PAS).
Outcome measures analysis and assessment of risk of bias
The primary objective of the study was to review the diagnostic accuracy of PAS after IVF-ET. Two secondary objectives were also examined. First, the relationship between IVF-ET pregnancy and PAS was examined, comparing women who conceived with IVF-ET versus those with spontaneous conception. In the sensitivity analysis, the risk of PAS was examined according to the approach of IVF-ET (fresh ET versus spontaneous conception, frozen ET versus spontaneous conception, fresh ET versus frozen ET, frozen ET with hormone replacement cycle versus frozen ET with normal ovulatory cycle). Second, maternal outcome of PAS after IVF-ET was examined.
Risk of bias assessment was performed using the Risk Of Bias In Non-randomized Studies-of Interventions tool (ROBINS-I)29,30,31.
Meta-analysis plan
From the eligible study data, the risk of PAS estimates for the experimental and control groups was computed by using the 95% confidence intervals (CIs) of the reported values to estimate the odds ratios for the risk of PAS. Since most studies reported the odds ratio for PAS, the study which showed the risk ratio without showing crude data was excluded from the analysis. Heterogeneity across the studies was examined using I2 statistics, which measures the percentage of total variation across studies. The meta-analysis and the production of all graphics were performed using RevMan ver. 5.4.1 software (Cochrane Collaboration, Copenhagen, Denmark). For consistency, data from all outcomes (continuous and bivariate) were entered into RevMan ver. 5.4.1 in such a way that negative effect sizes or relative risks of < 1 favored active intervention.
Statistical analysis
Differences in baseline demographics between the two groups were assessed with Fisher exact test, or chi-square as appropriate. All statistical analyses were based on two-sided hypotheses, and a P-value of less than 0.05 was considered statistically significant. Statistical Package for Social Sciences (IBM SPSS, version 27.0, Armonk, NY) was used for the analysis.
Ethical committee exemption
The approval of Institutional Review Board exempted the use of publicly available data.
Results
Study selection
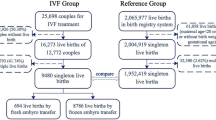
The study selection schema has been displayed in Fig. 1. A total of 214 studies were examined, and 12 studies, comprising of 190,139 IVF-ET pregnancies and 248,534 pregnancies with spontaneous conception met the inclusion criteria and were used for the descriptive analysis10,11,12,15,32,33,34,35,36,37,38,39.
Study characteristics
The meta-data of the evaluated studies are shown in Supplemental Table S2. One study was excluded due to the presence of overlapping cases13. The aims of the 12 studies (some studies were overlapping) were as follows: two studies examined the rate of antenatal diagnosis of PAS comparing between PAS after IVF-ET group and PAS with spontaneous conception group15,39, eight studies compared the effect of IVF-ET on PAS between IVF-ET group and spontaneous conception group10,11,12,32,34,36,37,38,39, three studies compared the prevalence of PAS between frozen ET and fresh ET group11,33,36, and one study compared the prevalence of PAS between frozen ET with hormone replacement cycle and frozen ET with normal ovulatory cycle35. The data on intracytoplasmic sperm injection were unavailable in most studies (n = 10).
No studies focused on the maternal outcomes of PAS after IVF-ET. Moreover, the severity (placenta accreta, increta, and percreta) of PAS was not compared between PAS after IVF-ET and PAS with spontaneous conception.
In the 12 included studies, the patients’ background was matched for maternal age in one study 34, and matched for maternal age and parity in the other32. Studies included in this review were published from 2011 to 2020. The majority of the studies were from Japan (58.3%)10,15,32,33,35,36,37, followed by United States (25.0%)11,38,39, China (8.3%)34, and Israel (8.3%)12.
Risk of bias of included studies
All studies were retrospective and of non-randomized comparative design10,11,12,15,32,33,34,35,36,37,38,39. No prospective studies were identified. The results of the risk of bias assessment for the comparative studies10,11,12,15,32,33,34,35,36,37,38,39 showed that there could be a moderate publication bias (moderate quality) in nine studies10,11,12,32,33,34,35,38,39 and severe publication bias (low quality) in three studies (Supplemental Table S3)15,36,37.
Definition of PAS
Among 12 studies, the definition of PAS was mentioned in six11,12,15,36,38,39. Of those, three studies defined PAS as a histopathological proven PAS15,38,39 and the remaining three defined PAS which was diagnosed by histopathological analysis or clinical diagnosis11,12,36.
Meta-analysis
Diagnostic accuracy on PAS after IVF-ET
Two studies compared the antenatal diagnostic accuracy of PAS after IVF-ET (Table 2)15,38, one study determined the diagnostic accuracy of PAS after IVF-ET with placenta previa in MRI (28 cases of PAS)15, and another one investigated the rate of antenatal diagnosis of PAS without placenta previa in ultrasonography (112 cases of PAS)39. The rate of antenatal diagnosis of MRI for placenta previa patients with PAS after IVF-ET was significantly lower 2/9 (22.2%) than that for PAS with spontaneous conception 18/19 (94.7%, P < 0.01)15. Similar to the MRI study, the rate of antenatal diagnosis of ultrasonography for PAS after IVF-ET was significantly lower than that for PAS with spontaneous conception (< 4/31 [< 12.9%] versus 38/81 [46.9%], P < 0.01)39.
These limited data suggested that the rate of antenatal diagnosis for PAS after IVF-ET may be lower than that of PAS with spontaneous conception both in women with and without placenta previa.
The risk of PAS between IVF-ET versus spontaneous conception
Nine studies (two of low and seven of moderate quality) examined the effect of IVF-ET on PAS (Table 3)10,11,12,32,34,36,37,38,39. In nine studies, there were 13,897 patients having IVF-ET pregnancies and 248,474 patients having pregnancies with spontaneous conception. Due to substantial heterogeneity, a random-effect analysis was performed. In the unadjusted pooled analysis (n = 9), IVF-ET was associated with an increased risk of PAS (OR: 5.03, 95%CI: 3.34–7.56, P < 0.01; heterogeneity: P < 0.01, I2 = 89%) (Fig. 2A). Egger’s test detected the presence of publication bias (P < 0.01). Of these nine studies, the effect of IVF-ET on placenta previa was examined in six. With regard to placenta previa, IVF-ET was associated with an increased risk of placenta previa (OR: 2.79, 95%CI: 2.11–3.69, P < 0.01; heterogeneity: P < 0.01, I2 = 71%) (Fig. 2B).
Results of the meta-analysis for the effect of IVF-ET on PAS. The pooled odds ratio for (A) PAS, (B) placenta previa, and (C) PAS (patient background matched) between IVF-ET patients versus spontaneous conception patients are shown. Since the numbers after the third decimal places for the lower or upper confidence interval were omitted in most studies, some calculated values of OR using Revman ver. 5.4.1™ may be different from the original values. Abbreviations: PAS, placenta accreta spectrum; OR, odds ratio; CI, confidence interval; and IVF-ET, in vitro fertilization-embryo transfer.
In the patient background-matched comparator analysis of the prevalence of PAS between IVF-ET versus spontaneous conception, a fixed-effect model was used as no heterogeneity was observed between the studies. In the adjusted pooled analysis (n = 2), IVF-ET was associated with an increased risk of PAS (OR: 2.41, 95%CI: 1.97–2.94, P < 0.01; heterogeneity: P = 0.74, I2 = 0%) (Fig. 2C). The risk of publication bias could not be calculated due to the small number of studies.
The risk of PAS: spontaneous conception versus fresh ET versus frozen ET
To determine the risk of IVF-ET on PAS according to the type of IVF-ET (spontaneous conception versus fresh ET, spontaneous conception versus frozen ET, fresh ET versus frozen ET), a sensitivity analysis was performed. Two studies were identified to examine the risk of fresh ET or frozen ET on PAS compared to that on spontaneous conception11,36. Among the two studies, there were 1378 fresh ET patients, 280 frozen ET patients, and 54,024 spontaneous conception patients. A fixed-effect analysis was performed due to the absence of heterogeneity. In the comparator analysis between fresh ET versus spontaneous conception (n = 2), PAS patients were more likely to be observed in the fresh ET group compared to that of the spontaneous conception group (OR: 3.04, 95%CI: 2.15–4.28, P < 0.01; heterogeneity: P = 0.85, I2 = 0%) (Fig. 3A). Similarly, frozen ET was associated with an increased risk of PAS compared to that of spontaneous conception (OR: 11.47, 95%CI: 7.61–17.31, P < 0.01; heterogeneity: P = 0.33, I2 = 0%) (Fig. 3B). The risk of publication bias could not be calculated due to the small number of studies.
The effect of fresh ET, frozen ET, FET with normal ovulatory cycle, and FET with hormone replacement cycle on PAS. The pooled odds ratio for PAS (A) fresh ET versus spontaneous conception, (B) frozen ET versus spontaneous conception, (C) fresh ET versus frozen ET, and (D) frozen ET with hormone replacement cycle versus frozen ET with normal ovulatory cycle are shown. Since the numbers after the third decimal places for the lower or upper confidence interval are omitted in most studies, some calculated values of OR using Revman ver. 5.4.1™ may be different from the original values. PAS placenta accreta spectrum, OR odds ratio, CI confidence interval, ET embryo transfer, FET frozen embryo transfer.
To determine whether fresh ET or frozen ET is a stronger risk factor of PAS, three studies were included in the analysis (one of low and two of moderate quality)11,33,36. Of those, there were 91,063 frozen ET patients and 51,833 fresh ET patients, respectively. Since no heterogeneity was observed, a fixed effect analysis was performed. In the pooled analysis (n = 3), frozen ET was associated with an increased risk of PAS (OR: 4.60, 95%CI: 3.42–6.18, P < 0.01; heterogeneity: P = 0.42, I2 = 0%) compared to that of fresh ET (Fig. 3C). The risk of publication bias could not be calculated due to the small number of studies. One study compared the rate of placenta previa between frozen ET versus fresh ET33. In this study, frozen ET was associated with a decreased rate of placenta previa compared to fresh ET (OR: 0.80, 95%CI: 0.70–0.89, P < 0.01).
The risk of PAS: frozen ET with hormone replacement cycle versus frozen ET with normal ovulatory cycle
One comparator study examined the prevalence of PAS between frozen ET with hormone replacement cycle versus frozen ET with normal ovulatory cycle groups (Table 4)35. This study did not include spontaneous conception patients and included only women with frozen ET. In this comparator analysis, frozen ET with hormone replacement cycle was significantly associated with the prevalence of PAS compared to that of frozen ET with normal ovulatory cycle (OR: 5.76, 95%CI 3.12–10.64, P < 0.01). The rate of placenta previa was similar between the two groups (OR: 0.80, 95%CI 0.59–1.08, P = 0.15).
Discussion
Main findings
The key findings of this study are the following: (i) the rate of antenatal diagnosis of PAS after IVF-ET may be lower than that of PAS with spontaneous conception, and (ii) IVF-ET was associated with an increased risk of PAS, especially in women who conceived with frozen ET with hormone replacement cycle.
Comparison with existing literature
Several studies have reported that IVF-ET is associated with an increased risk of PAS. The first study that showed the relationship between IVF-ET and PAS was published in 201112, but it did not determine the risk of PAS according to the type of IVF-ET12. Subsequently, Kaser et al. examined the risk of PAS in different groups (spontaneous conception [n = 53,376], fresh ET [n = 1,351], and frozen ET [n = 220])11. The authors found that women with an endometrial thickness of < 9 mm before ET had a greater risk of PAS than women with an endometrial thickness of ≥ 9 mm; thus, a thin endometrium may lead to PAS. In their analysis, the median endometrial thickness before ET was 8.4 mm in the frozen ET cycle and 10.6 mm in the fresh ET cycle. Therefore, frozen ET is considered a higher risk factor for PAS compared to that of fresh ET. Our systematic review found that Kaser et al.’s study is the only study that determined the association between endometrial thickness and PAS.
From the results of the meta-analysis, frozen ET with hormone replacement cycle appeared to be the most significant risk factor of PAS (Fig. 3A–D). Saito et al. compared the prevalence of PAS between women who conceived with frozen ET with hormone replacement cycle and those who conceived with frozen ET with normal ovulatory cycle35. They found that frozen ET with hormone replacement cycle is more likely to be complicated with PAS than frozen ET with normal ovulatory cycle. Although Saito et al.’s study did not examine the endometrial thickness, several studies have reported that endometrial thickness is lower in women who conceived with frozen ET with hormone replacement cycle than in those who conceived with frozen ET with normal ovulatory cycle or fresh ET40,41,42. Thus, our results are consistent with those of Kaser et al.’s study.
To discuss the relationship between IVF-ET and PAS, it is pertinent to mention that IVF-ET is associated with an increased rate of placenta previa, and placenta previa, in turn, is a high-risk factor for PAS (OR: 50–100)6,7,8. Therefore, it is possible that an increased risk of placenta previa contributes to an increased prevalence of PAS. A previous study that examined the relationship between endometrial thickness and placenta previa revealed that the risk of placenta previa is increased in women with an endometrial thickness of > 12 mm (adjusted OR: 3.74, 95%CI: 1.90–7.34) compared with women with an endometrial thickness of < 9 mm42. In contrast, Kaser et al.’s study has shown that an endometrial thickness of < 9 mm before ET had a greater risk of PAS11.
In our analysis, frozen ET was found to be associated with an increased risk of PAS compared to fresh ET, whereas the rate of placenta previa was not found to increase in the frozen ET group. Similarly, frozen ET with hormone replacement cycle is associated with an increased rate of PAS compared to frozen ET with normal ovulatory cycle, whereas the rate of placenta previa was similar between the two groups (Table 4). This study includes a comparator analysis to examine the association between IVF-ET and PAS. Since we did not focus on IVF-ET and placenta previa, our analysis missed a large study that compared the rate of placenta previa between women who conceived with fresh ET and frozen ET43.
A systematic review and meta-analysis that compared the risk of obstetrics complication showed no significant differences in the risk of placenta previa between the frozen ET and fresh ET groups (adjusted OR 0.70, 95%CI 0.46–1.08), which was consistent with our results43. These data support our findings suggesting that PAS can occur after IVF independently of the placental position.
We should note some limitations of this study in examining the effects of IVF-ET on the risk of PAS between women who conceived with IVF-ET and spontaneous conception. Advanced maternal age, increased parity, and prior cesarean delivery are the main risk factors for the development of placenta previa, and PAS in subsequent pregnancies has been reported in numerous large studies44,45,46,47,48. In general, women who require IVF to conceive are more often older and more likely to have a history of uterine surgery, including cesarean delivery or dilatation and curettage, compared with women who conceived with spontaneous conception39,49,50,51,52. We should note that these factors are the risk factors of placenta previa and PAS.
Since previous studies did not match the obstetric patient background or most studies did not perform a multivariate analysis with adjustments of various background factors in obstetrics patients, such as those mentioned above, our analysis cannot attribute IVF-ET as the risk factor of PAS while excluding the confounding factors.
We also should note that the quality of the diagnosis of PAS in this study may be low due to the following reasons: (i) all studies did not mention the severity or classification of PAS according to the previous literature45,53 and (ii) all studies did not report the detailed clinical findings at delivery and/or the histopathologic data. Therefore, there is a possibility that the present study has included women with less severe PAS (i.e., placenta adherent or creta) rather than women with the more severe type of PAS (i.e., placenta increta or percreta) 53. This is a strong limitation of this study, and further studies examining the effect of IVF-ET on the severity of PAS are warranted.
In summary, a lower endometrial thickness before ET may be associated with an increased risk of PAS; thus, confirmation of the endometrial thickness in women who have additional high-risk factors for PAS is recommended. Further studies investigating the relationship between endometrial thickness before ET and PAS are warranted.
The main cause of PAS, except for placenta previa, is damage to the endometrium-myometrial interface due to prior uterine surgeries, especially cesarean delivery45,54. These surgeries lead to abnormal decidualization in the area of the uterine scar, as well as myometrial invasion of the trophoblast and villi47. Previous studies have reported that an abnormally rich vascularity between the cesarean scar and placenta is observed in cases of PAS with uterine scars, even in early pregnancy. Unlike the typical cause of PAS with spontaneous conception, a thin endometrial thickness may be associated with PAS after IVF-ET11. It is speculated that abnormal vessels between the uterine scar and placenta are not newly developed in cases of PAS after IVF-ET without uterine scar; thus, the representative ultrasound or MRI findings are not observed due to the differences in the mechanisms of PAS. No studies have yet identified the specific findings of ultrasound or MRI in PAS after IVF-ET.
Although IVF-ET is associated with an increased risk of PAS, no study has yet compared the effects of IVF-ET on PAS in women with placenta previa. PAS complicated with placenta previa is one of the highest risk factors of massive postpartum hemorrhage1,45,47. Moreover, PAS after IVF-ET is associated with a lower rate of antenatal diagnosis. Therefore, if IVF-ET is associated with an increased risk of PAS in women with placenta previa, clinicians can be more aware of the presence of PAS in patients with placenta previa who have conceived with IVF-ET. On the other hand, since the technical ability for the antenatal diagnosis of PAS has been clearly shown in the literature55,56,57, we must appreciate the technical limitations of our diagnostic capabilities for PAS after IVF-ET.
Given the results of this study, it is important to anticipate that women who undergo IVF, especially frozen ET with hormone replacement cycle, are at increased risk for PAS which is difficult to identify antenatally, and some points as follows may be beneficial for the patients: (i) appropriate counseling prior to ET may be beneficial, as some women who have had successful pregnancies may not wish to assume this risk if known ahead of time, (ii) thorough and careful evaluations of the placenta by experienced sonologists and radiologists are key to identifying cases of PAS after IVF-ET, and (iii) preparations for the unidentified PAS after IVF-ET may help reduce hemorrhagic morbidity and improve prognosis, possibly because of multidisciplinary care, which includes cesarean hysterectomy, uterine artery embolization, transfusion preparation, and treatment by skilled physicians18,19,20,21,22.
Strengths and limitations
The strengths of the study are that this is the first systematic review to focus on PAS after IVF-ET. Our study revealed that IVF-ET is associated with the prevalence of PAS, and this risk appeared to be highest in women who conceived with frozen ET with hormone replacement cycle. However, this study has some limitations. First, there was an unmeasured bias due to the retrospective studies we included in our systematic review. Potential sources of confounding variables in the study include the following: the varying definition of PAS across studies, the unmatched patient background, the lack of specific reasons for infertility, and the severity of PAS was not examined.
Second, the quality of the diagnosis of PAS appears to be low in most studies. Notably, all studies did not mention the severity of PAS and did not report the detailed clinical findings at delivery and/or the histopathologic data of PAS. Therefore, many of the studies included in the present study may have simple PAS rather than the severe one. This is a notable limitation of this study; hence, it should be considered while interpreting the results and the study's discussion for the patients having IVF-ET. Third, none of the studies matched the obstetric patient background to examine the effects of IVF-ET on the prevalence of PAS; thus, this study cannot isolate IVF-ET as the risk factor of PAS while excluding the confounding factors. This is an important limitation of this study.
Fourth, publication bias is a concern because the negative relationship between IVF-ET and PAS might not have been reported in the original papers. Egger’s test detected severe publication bias in the comparator analysis of the risk of PAS between IVF-ET versus spontaneous conception. This is another important limitation of this study. Fifth, only two studies examined the diagnostic accuracy of PAS after IVF-ET. To clearly show the accuracy of our findings, a more robust study should be conducted. Since a randomized controlled study is difficult due to the rarity of PAS after IVF-ET, a prospective study seems appropriate.
Lastly, the sample size was limited, particularly for the examination of the antenatal diagnosis for PAS after IVF-ET. Moreover, no prospective studies compared the prevalence of PAS among different groups (spontaneous conception versus fresh ET versus frozen ET with normal ovulatory cycle versus frozen ET with hormone replacement cycle).
Conclusions
The antenatal diagnosis of PAS after IVF-ET was found to be significantly lower than that of PAS with spontaneous conception. Since IVF-ET is associated with an increased risk of PAS, we believe that clinicians can be more vigilant in ruling out “PAS after IVF-ET” in women with placenta previa who conceive with IVF-ET. To confirm the results of this study, further studies examining the diagnostic accuracy of PAS after IVF-ET are warranted. Future studies should also examine the risk of PAS among different types of IVF-ET to determine which type has the highest risk for PAS.
Data availability
All the studies used in this study are published in the literature.
References
Collins, S. L. et al. Evidence-based guidelines for the management of abnormally invasive placenta recommendations from the International Society for Abnormally Invasive Placenta. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2019.02.054 (2019).
Matsuzaki, S. et al. Conservative management of placenta percreta. Int. J. Gynaecol. Obstet. 140, 299–306. https://doi.org/10.1002/ijgo.12411 (2018).
Marcellin, L. et al. Placenta percreta is associated with more frequent severe maternal morbidity than placenta accreta. Am. J. Obstet. Gynecol. 219, 193 e191–193 e199, https://doi.org/10.1016/j.ajog.2018.04.049 (2018).
Collins, S. L. et al. Evidence-based guidelines for the management of abnormally invasive placenta: Recommendations from the International Society for Abnormally Invasive Placenta. Am. J. Obstet. Gynecol. 220, 511–526. https://doi.org/10.1016/j.ajog.2019.02.054 (2019).
Allen, L. et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. Int. J. Gynaecol. Obstet. 140, 281–290. https://doi.org/10.1002/ijgo.12409 (2018).
Silver, R. M. et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 107, 1226–1232. https://doi.org/10.1097/01.AOG.0000219750.79480.84 (2006).
Wu, S., Kocherginsky, M. & Hibbard, J. U. Abnormal placentation: Twenty-year analysis. Am. J. Obstet. Gynecol. 192, 1458–1461. https://doi.org/10.1016/j.ajog.2004.12.074 (2005).
Eshkoli, T., Weintraub, A. Y., Sergienko, R. & Sheiner, E. Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births. Am. J. Obstet. Gynecol. 208(219), e211-217. https://doi.org/10.1016/j.ajog.2012.12.037 (2013).
Baldwin, H. J. et al. Antecedents of abnormally invasive placenta in primiparous women: Risk associated with gynecologic procedures. Obstet. Gynecol. 131, 227–233. https://doi.org/10.1097/AOG.0000000000002434 (2018).
Nagata, C. et al. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: A nationwide birth cohort study of Japan environment and children’s study. BMC Pregnancy Childb. 19, 77. https://doi.org/10.1186/s12884-019-2213-y (2019).
Kaser, D. J. et al. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil. Steril. 103, 1176–1184 e1172, https://doi.org/10.1016/j.fertnstert.2015.01.021 (2015).
Esh-Broder, E., Ariel, I., Abas-Bashir, N., Bdolah, Y. & Celnikier, D. H. Placenta accreta is associated with IVF pregnancies: A retrospective chart review. BJOG 118, 1084–1089. https://doi.org/10.1111/j.1471-0528.2011.02976.x (2011).
Ishihara, O. et al. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil. Steril. 101, 128–133. https://doi.org/10.1016/j.fertnstert.2013.09.025 (2014).
CDC. Figures From the 2016 Assisted Reproductive Technology National Summary Report.
Nagase, Y. et al. In-vitro fertilisation-embryo-transfer complicates the antenatal diagnosis of placenta accreta spectrum using MRI: A retrospective analysis. Clin. Radiol. https://doi.org/10.1016/j.crad.2020.07.013 (2020).
Committee on Obstetric, P. Committee opinion no. 529: Placenta accreta. Obstet. Gynecol. 120, 207–211, https://doi.org/10.1097/AOG.0b013e318262e340 (2012).
Eller, A. G. et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet. Gynecol. 117, 331–337. https://doi.org/10.1097/aog.0b013e3182051db2 (2011).
Licon, E. et al. Implementation of multidisciplinary practice change to improve outcomes for women with placenta accreta spectrum. Eur. J. Obstet. Gynecol. Reprod. Biol. 246, 194–196. https://doi.org/10.1016/j.ejogrb.2020.01.010 (2020).
Tikkanen, M., Paavonen, J., Loukovaara, M. & Stefanovic, V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet. Gynecol. Scand. 90, 1140–1146. https://doi.org/10.1111/j.1600-0412.2011.01147.x (2011).
Warshak, C. R. et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet. Gynecol. 115, 65–69. https://doi.org/10.1097/AOG.0b013e3181c4f12a (2010).
Bouvier, A. et al. Planned caesarean in the interventional radiology cath lab to enable immediate uterine artery embolization for the conservative treatment of placenta accreta. Clin. Radiol. 67, 1089–1094. https://doi.org/10.1016/j.crad.2012.04.001 (2012).
Matsuzaki, S. et al. Horizontal cervix as a novel sign for predicting adhesions on the posterior extrauterine wall in cases of placenta previa. J. Clin. Med. 8, https://doi.org/10.3390/jcm8122141 (2019).
Matsuo, K. et al. Malignant peritoneal cytology and decreased survival of women with stage I endometrioid endometrial cancer. Eur. J. Cancer (Oxford, England : 1990) 133, 33–46, https://doi.org/10.1016/j.ejca.2020.03.031 (2020).
Matsuzaki, S. et al. Management of stage IIB cervical cancer: An overview of the current evidence. Curr. Oncol. Rep. 22, 28. https://doi.org/10.1007/s11912-020-0888-x (2020).
Matsuzaki, S. et al. Systematic review on the needle and suture types for uterine compression sutures: A literature review. BMC Surg 19, 196. https://doi.org/10.1186/s12893-019-0660-z (2019).
Matsuzaki, S. et al. Surgical and oncologic outcomes of hyperthermic intraperitoneal chemotherapy for uterine leiomyosarcoma: A systematic review of literature. Gynecol. Oncol. https://doi.org/10.1016/j.ygyno.2020.12.032 (2021).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339, b2535, https://doi.org/10.1136/bmj.b2535 (2009).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 62, e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006 (2009).
Sterne, J. A. et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. https://doi.org/10.1136/bmj.i4919 (2016).
Danna, S. M., Graham, E., Burns, R. J., Deschenes, S. S. & Schmitz, N. Association between depressive symptoms and cognitive function in persons with diabetes mellitus: A systematic review. PLoS ONE 11, e0160809. https://doi.org/10.1371/journal.pone.0160809 (2016).
ROBINS-I Detailed Guidance. https://www.riskofbias.info/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016. Accessed 20 Sep 2020 (2016).
Hayashi, M., Nakai, A., Satoh, S. & Matsuda, Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil. Steril. 98, 922–928. https://doi.org/10.1016/j.fertnstert.2012.05.049 (2012).
Takeshima, K. et al. Impact of single embryo transfer policy on perinatal outcomes in fresh and frozen cycles-analysis of the Japanese Assisted Reproduction Technology registry between 2007 and 2012. Fertil. Steril. 105, 337-346.e333. https://doi.org/10.1016/j.fertnstert.2015.10.002 (2016).
Zhu, L. et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Sci. Rep. 6, 35141. https://doi.org/10.1038/srep35141 (2016).
Saito, K. et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum. Reprod. (Oxford, England) 34, 1567–1575. https://doi.org/10.1093/humrep/dez079 (2019).
Sakai, Y. et al. Embryo transfer associated with hormone replacement therapy cycles using assisted reproductive technology increases placenta accreta spectrum. J. Obstet. Gynaecol. Res. 45, 2394–2399. https://doi.org/10.1111/jog.14111 (2019).
Tanaka, H. et al. Evaluation of maternal and neonatal outcomes of assisted reproduction technology: A retrospective cohort study. Medicina (Kaunas, Lithuania) 56, https://doi.org/10.3390/medicina56010032 (2020).
Salmanian, B. et al. In vitro fertilization as an independent risk factor for placenta accreta spectrum. Am. J. Obstet. Gynecol. 223(568), e561-568.e565. https://doi.org/10.1016/j.ajog.2020.04.026 (2020).
Modest, A. M., Toth, T. L., Johnson, K. M. & Shainker, S. A. Placenta accreta spectrum: In vitro fertilization and non-in vitro fertilization and placenta accreta spectrum in a Massachusetts cohort. Am. J. Perinatol. https://doi.org/10.1055/s-0040-1713887 (2020).
Liu, X., Shi, W. & Shi, J. Natural cycle frozen-thawed embryo transfer in young women with regular menstrual cycles increases the live-birth rates compared with hormone replacement treatment: A retrospective cohort study. Fertil. Steril. 113, 811–817. https://doi.org/10.1016/j.fertnstert.2019.11.023 (2020).
Pang, C. et al. A comparison of pregnancy rate between natural cycle and hormone replacement cycle in patients who underwent frozen embryo transfer using 2 consecutive hormone replacement regiments: A STROBE-compliant retrospective study. Medicine (Baltimore) 99, e22163, https://doi.org/10.1097/MD.0000000000022163 (2020).
Rombauts, L., Motteram, C., Berkowitz, E. & Fernando, S. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod. (Oxford, England) 29, 2787–2793. https://doi.org/10.1093/humrep/deu240 (2014).
Roque, M., Valle, M., Sampaio, M. & Geber, S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: A systematic review and meta-analysis. JBRA Assist. Reprod. 22, 253–260. https://doi.org/10.5935/1518-0557.20180049 (2018).
Silver, R. M. & Branch, D. W. Placenta accreta spectrum. N. Engl. J. Med. 378, 1529–1536. https://doi.org/10.1056/NEJMcp1709324 (2018).
Society of Gynecologic, O. et al. Placenta accreta spectrum. Am. J. Obstet. Gynecol. 219, B2–B16, https://doi.org/10.1016/j.ajog.2018.09.042 (2018).
Thurn, L. et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: Results from a large population-based pregnancy cohort study in the Nordic countries. BJOG 123, 1348–1355. https://doi.org/10.1111/1471-0528.13547 (2016).
Jauniaux, E., Collins, S. & Burton, G. J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 218, 75–87. https://doi.org/10.1016/j.ajog.2017.05.067 (2018).
Miller, D. A., Chollet, J. A. & Goodwin, T. M. Clinical risk factors for placenta previa-placenta accreta. Am. J. Obstet. Gynecol. 177, 210–214. https://doi.org/10.1016/s0002-9378(97)70463-0 (1997).
Ubaldi, F. M. et al. Advanced maternal age in IVF: Still a challenge? The present and the future of its treatment. Front. Endocrinol. 10, 94. https://doi.org/10.3389/fendo.2019.00094 (2019).
Goisis, A., Håberg, S. E., Hanevik, H. I., Magnus, M. C. & Kravdal, Ø. The demographics of assisted reproductive technology births in a Nordic country. Hum. Reprod. (Oxford, England) 35, 1441–1450. https://doi.org/10.1093/humrep/deaa055 (2020).
Chughtai, A. A. et al. Gestational age-specific perinatal mortality rates for assisted reproductive technology (ART) and other births. Hum. Reprod. (Oxford, England) 33, 320–327. https://doi.org/10.1093/humrep/dex340 (2018).
Vissers, J. et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous Caesarean section: A retrospective cohort study. Hum. Reprod. (Oxford, England) 35, 595–604. https://doi.org/10.1093/humrep/dez295 (2020).
Jauniaux, E. et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 146, 20–24. https://doi.org/10.1002/ijgo.12761 (2019).
Jauniaux, E., Bunce, C., Gronbeck, L. & Langhoff-Roos, J. Prevalence and main outcomes of placenta accreta spectrum: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 221, 208–218. https://doi.org/10.1016/j.ajog.2019.01.233 (2019).
Jauniaux, E., Collins, S. L., Jurkovic, D. & Burton, G. J. Accreta placentation: A systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am. J. Obstet. Gynecol. 215, 712–721. https://doi.org/10.1016/j.ajog.2016.07.044 (2016).
Comstock, C. H. & Bronsteen, R. A. The antenatal diagnosis of placenta accreta. Bjog 121, 171–181; discussion 181–172, https://doi.org/10.1111/1471-0528.12557 (2014).
Pilloni, E. et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet. Gynecol. 47, 302–307. https://doi.org/10.1002/uog.14893 (2016).
Acknowledgements
Editorial support, in the form of medical writing and collating author comments, was provided by Editage, Cactus Communications.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sh.M., Y.N., Ts.T., A.K., K.M., Sa.M.; Data curation: Sh.M., Y.N., L.M., Sa.M.; Formal analysis: Sh.M., Y.N., Y.U.; Investigation: all authors; Methodology: Sh.M., Y.N., L.M.; Project administration: Sh.M., Ta.T., M.E., T.K.; Resources: all; Software: Sh.M.; Supervision: Ta.T., M.E., T.K.; Validation: Sh.M., Y.N.; Visualization: Sh.M.; Writing—original draft: Sh.M., Y.N., Ts.T.; Writing—review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsuzaki, S., Nagase, Y., Takiuchi, T. et al. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: a systematic review and meta-analysis. Sci Rep 11, 9205 (2021). https://doi.org/10.1038/s41598-021-88551-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88551-7
This article is cited by
-
Assisted reproductive technology-associated risk factors for placenta accreta spectrum after vaginal delivery
Scientific Reports (2024)
-
Risk factors for placenta accreta spectrum disorders in women with any prior cesarean and a placenta previa or low lying: a prospective population-based study
Scientific Reports (2024)
-
A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.