Abstract
Accurate methods for age determination are critical to the knowledge of wildlife populations' age structure and, therefore, to their successful management. The reliability of age estimation may have profound economic and ecological consequences on the management of the European wild rabbits, Oryctolagus cuniculus, in its native and introduced range, where it is a keystone species and a major pest, respectively. As in other mammal species, European rabbits' age is often estimated using the Gompertz relationship between age and lens' weight. The growth rate formula has been developed based on data collected from European rabbits introduced in Australia, where a single subspecies (O. cuniculus cuniculus, Occ) is present. However, this curve has never been validated in the species native range, the Iberian Peninsula, where two subspecies (Occ, and O. c. algirus, Oca) coexist naturally. In this study, we tested the relationship between age and lens' weight using 173 Occ and 112 Oca wild rabbits that were surveyed in two experimental facilities in Spain. Our findings show that, in the native range, the published growth curve formula fits well Occ but not Oca data. Therefore, we recommend using the formula reported in this study to estimate the age of Oca (Lens dry weight = 240 × 10(−64.9/(Age+32))). This study supports Oca rabbits' distinctiveness revealed by previous studies, which suggests that management interventions should be applied to protect this subspecies whose distribution range is very narrow and whose populations seem to be declining. More broadly, our findings point to the importance of testing the suitability of growth curves defined for other species with different genetic forms as occurs in the European wild rabbit case.
Similar content being viewed by others
Introduction
Accurate knowledge of the study system's biological and ecological parameters is essential for decision-making regarding wildlife management. One of these parameters is the population's age structure, which is useful for understanding its dynamics1. Age determination is, therefore, crucial to the effective management of wildlife species2. For example, reliable age techniques allow managers to establish the optimal wildlife harvesting or periods to control populations of pest species3,4.
A variety of methodologies has been used to determine the age of wild mammals. Traditionally, it has been estimated by using morphometric traits like body weight, total length, length of the trunk, length of the extremities, dental cementum layers, or dental wear5,6. The epiphysis or cranial suture fusion have been used too for this purpose7. However, many of these traits depend on age and on many other factors such as the environment, nutritional history or gender. For example, body mass relates well to the age only at the early stage of an individual's development8.
As regards mammals, there is currently a wide consensus that the dry weight of the eye lens9 (hereafter LDW) is not significantly affected by environmental variables such as those mentioned above10,11,12. Therefore, the LDW, first studied by Lord13 in cottontail rabbits (Sylvilagus floridanus), provides a good source of information to determine the age of a specimen. The constant proliferation of the lenses during an individual's lifetime, and the almost impossibility of cellular loss inside the lens, allow a good fit curve between age and dry-eye lens weight9,10,12,13. LDW method has been employed on different mammal species, including ungulates, rodents, or lagomorphs12,14. Among the latter, this method has been proved particularly useful in assessing the age of European wild rabbits (Oryctolagus cuniculus) in areas where the species was introduced15,16,17. In this species, the LDW growth was described as an asymptotic logistic growth curve:
where a is the maximum asymptotic value of LDW, b is the constant growth rate, A is the postnatal age, c is the time during prenatal life and, therefore, A + c corresponds to the elapsed growth time. Equation (1) is the logistic form of the linearized equation commonly used for describing asymptotic growth:
Previous works observed significant regional differences in LDW curves between populations of O. cuniculus from different areas of eastern and southwestern Australia15,16,17. All of them concluded that, although the use of joint curves was not inappropriate, defining alternatives at the regional level offered a more precise fit. However, more recently, Augusteyn11 rejected the idea of environmental influence and attributed the effect observed in previous studies to differences in the data's logistic analysis. He demonstrated that if the same value of c was used for all rabbits' populations analyzed until then in the literature, the other parameters in the logistic equation became almost identical11. Therefore, despite the large heterogeneity of rabbits' environmental conditions worldwide18 and the fact that the LDW method was originally calibrated from Australian specimens (where rabbits are heavier than in their native range19), a single equation should be valid for European wild rabbits regardless of their geographical origin. In other words, the curve described using Australian rabbits should work well also in the rabbit native range.
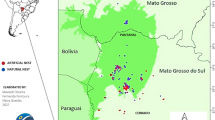
Besides, this independence of environmental variation proposed by Augusteyn11 suggests that the same curve should be valid for the two existing rabbit subspecies. Oryctolagus cuniculus cuniculus (hereafter Occ) and Oryctolagus cuniculus algirus (hereafter Oca) diverged approximately 1.8 million years ago20,21. Currently, they coexist naturally in the Iberian Peninsula (IP) where they hold differentiated natural distributions, with a narrow contact zone22 (Fig. 1). Domestic rabbits and nearly all European wild rabbits occurring out of the IP belong to Occ. A growing number of studies have revealed the critical genetic differences between both subspecies23,24. Furthermore, they also differ morphologically, being Oca lighter and with shorter ear and hind foot lengths19. Also, a recent study has suggested that both subspecies may be facing different trends in abundance in Spain after the outbreak of a new variant of rabbit haemorrhagic disease (RHD) virus, with Oca declining and Occ being stable or even increasing25.
Distribution areas of Oryctolagus cuniculus algirus and O. c. cuniculus in the Iberian Peninsula and their contact zone22. Putative glacial refugia of European rabbit lineages described by Branco et al.20 is shown. Black squares indicate the experimental facilities' locations where rabbits were kept in captivity at Ciudad Real (1) and Córdoba (2). Ellipses indicate the areas where initial stocks of wild rabbits for the facilities were captured.
In this study, we assessed if a unique curve (i.e. a single set of constants) describes the relationship between the LDW and the age in the European rabbit regardless of the origin of the animals (IP or Australia) or the subspecies (Occ or Oca) they belong to. Following the rationale of Augusteyn11, we did not expect differences in the relationship between the LDW and age between Australian and Iberian rabbits. Also, we hypothesized that the eye-lens growth curves should not vary between both rabbit subspecies.
Results
A total of 112 (38 from cages, 74 from enclosures) Oca and 173 (82 from cages, 91 from enclosures) Occ dead rabbits were dated, and their eye lenses weighed. Rabbits' age ranged from 17 to 1392 days (approx. 3.5 years), and distributed among all ages (< 3 months: 138 individuals ; 3–12 months: 76; > 12 months: 71). Model 4, which contemplates the different a and same b for the two subspecies, yielded the largest support (Table 1; R2 = 0.93). Model 2, which differs from the previous one in that it also includes a different b between subspecies, slightly exceeded the threshold of 2 AICc units and could be considered to be considerably less supported (Table 1). The rest of the models, which assigned the same a to the subspecies, were far less parsimonious, being their AICcWt = 0 (Table 1).
Our estimate of a from Occ in the IP (present study) was similar to that found in Australia10. Accordingly, the overlapping index computed by comparing the distribution of a in the Iberian and Australian Occ datasets was very high (η = 0.71; i.e. 71% overlap in their distribution), as can be visually appreciated in Fig. 2. Conversely, a of the Iberian Oca populations was clearly outside the lower range described for the Occ subspecies (aOca = 240; aOcc = 273; see Table 2). The overlapping index between a from the Oca and both Iberian or Australian Occ distributions indicates they are entirely different (η = 0 in both cases, see Fig. 2). Using the Australian age-LDW curve to estimate rabbits' age in IP would decrease very slightly the age estimated for Occ (Fig. 3). In contrast, such a bias would become very high if that curve was employed for determining the age of Iberian Oca rabbits, the error being huge for older animals (Fig. 3).
Estimated densities for the maximum asymptotic value of the growth curve of dry eye lens (MA) for Oryctolagus cuniculus algirus (Oca) and Oryctolagus cuniculus cuniculus (Occ) in the Iberian Peninsula (IP), and the latter subspecies in Australia (Aus). Dotted lines indicate the estimate of a for each group.
Age estimates for European rabbits calculated with the parameters obtained with the curve corresponding to each Iberian subspecies' dataset and the Australian or subspecies counterpart. The black line corresponds to the linear regression (y = 1x). Occ: Oryctolagus cuniculus cuniculus; Oca: Oryctolagus cuniculus algirus; Aus: Australia; IP: Iberian Peninsula.
Discussion
Our study reveals that the use of a single age-LDW curve can be inappropriate for age determination of all individuals within a particular species when different genetic forms exist. In the case of European rabbits, the same Australian age-LDW curve has been used previously to determine rabbit age both in its native range26 and in introduced areas like Australia10,13, Great Britain27, France28 or Argentina29. However, there is much evidence of the large genetic variability between rabbit subspecies30. For the first time, the published LDW-age has been fitted to data collected from the native range. By doing so, we demonstrated the existence of two well-differentiated curves depending on rabbit subspecies. The curve for Oca (LDW = 240 × 10(−64.9/(Age+32))) is different not only to that described in Australia but also to the curve estimated with Iberian Occ rabbits (LDW = 273 × 10(−64.9/(Age+32))) (Fig. 3). Our results show that estimating Oca rabbits' age through the curve used to date (i.e. Australian Occ) would lead to severe biases in age estimation. For example, the age of Iberian Oca rabbits of 180, 360 and 720 days old (~ 0.5, ~ 1 or ~ 2 y) would be underestimated 45, 124 and 360 days, respectively (Fig. 3). In contrast, such biases would be almost inappreciable in the case of Iberian Occ rabbits; only a few days (8, 19 or 40 days, respectively) in the example mentioned above.
This study demonstrated the existence of differences between the maximum asymptotic (MA) values of both rabbit subspecies in the IP. The MA value was near 15% smaller for Oca than for Iberian Occ rabbits (Table 2), approximately the same percentage of variation between their body weights (average weight Oca: 1040 g; Iberian Occ: 1240 g19). In contrast, our results showed that MA values of Iberian Occ rabbits did not differ substantially from those reported for Australian rabbits10, which are similar in body size (e.g. 1240 g19 vs 1300 g31 for Iberian Occ and Australian rabbits; respectively). Previous studies have proved that there is little advantage for individuals in increasing lens size over a given threshold, since this would require a reduction in its focal length, resulting in a loss of resolution at the retina11.
On this basis, Augusteyn10 asserted MA value should be the same for animals that belong to the same species, and therefore the estimation of animals of the same age should be accurate regardless of their body sizes. According to this statement, differences in MA between rabbit subspecies in the IP should not be related to their body size differences. Alternatively, such variation of the eye lenses between both subspecies could provide new evidence that Occ and Oca are already two well-differentiated species, as it has been previously suggested32. This would not contradict Augusteyn ideas and would indeed support recent studies that revealed reproductive isolation between Occ and Oca23,24,33, and by the growing number of studies showing genetic34,35,36, morphological19,37,38,39 and behavioral differences between both subspecies as well as by variations in their demographic trends after the outbreak of viral diseases25. Also, this study could stress the need of conducting additional studies to confirm the distinctiveness of O. c. algirus as this subspecies only occurs in the IP and few oceanic islands and its conservation status could be threatened25.
The European rabbit is likely one of the most managed vertebrate species in the world. In its native range many efforts are made to restore the declining rabbit populations40 either because rabbits are keystone species for predators or because of their role as game species41,42,43. Iberian rabbits are also managed in some farmland areas for population control and reduce of the damage the species causes to crops44. Furthermore, the European rabbit is one of the most devastating pests where it has been introduced and, as a consequence, it is the object of many control and eradication programs45,46. Developing accurate methods for rabbit aging is necessary to understand the species' dynamics (e.g. age-sex class composition) and condition (e.g. age-sex specific body mass), which may guide its conservation, and management as a game species or for population control.
Our proposed curves could be valuable for the long-term assessment of age structure of Iberian rabbit populations in the distribution area of both rabbit subspecies, which might be helpful to assess the sustainability of rabbit hunting as well as the success of rabbit restocking practices that are very often employed in the IP to feed endangered predators such as the Iberian lynx, Lynx pardinus40,42,47,48. Moreover, this study points to the convenience of assessing the reliability of age-LDW growth curves for other species with different genetic forms. Indeed, as it has been shown here, not doing so may lead to errors with negative implications for management and conservation. This could be the case for other ubiquitous mammalian species, such as the brown hare (Lepus europaeus), which has exceptional geographical and genetic heterogeneity and whose age is often estimated on the basis of a single age-LDW curve49,50.
Methods
European wild rabbit sampling
We collected data from a total of 112 Oca and 173 Occ European wild rabbits kept in captivity under controlled conditions in two experimental facilities in Spain: Dehesa de Galiana experimental facility (Instituto de Investigación en Recursos Cinegéticos, Ciudad Real), and the Research Center of Wild Lagomorphs facility (Instituto de Estudios Sociales Avanzados, Córdoba) (Fig. 1). The initial animal stocks for both facilities consisted of rabbits captured from wild populations in the Oca and Occ's distribution areas, southern and north-eastern Spain, respectively (Fig. 1). To ascertain the subspecies to which each captured rabbit belonged to, we collected samples of epithelial tissue from the ear and analyzed restriction fragment length polymorphisms (RFLPs) specific for four specific molecular markers located in different genomic regions51. The initial stocks were released in the facilities 31-01-2008 and 26-01-2015 respectively.
The experimental facilities consisted of six (Ciudad Real) and four (Córdoba) semi-captive populations enclosed, each one, in fenced plots of 2500 m2. At both facilities Occ rabbits occurred in half of the plots and Oca rabbits in the other half. As all the plots were fenced, both subspecies' rabbits were never in contact in the same plot. Each plot had several artificial warrens (nine in Ciudad Real, ten in Córdoba) surrounded with a wire net (approx. 1 m high), connected to three rabbit-traps. Such a system allows the capture of a large proportion of the rabbit population inside the warren52 (i.e. 50–60% in only one night). Rabbits were live-trapped in all warrens every month (28 days) from 2008 to 2011 in Ciudad Real and 2016–2018 in Córdoba.
Additionally, Córdoba facilities had 42 smaller outdoor cages (200 × 350 cm), which were specifically designed to improve the reproduction and monitoring of wild rabbits during the study period. These small enclosures had artificial cover tunnels, a wooden shelter (100 × 150 cm), and two wooden nest compartments filled with straw to improve insulation. On each cage, we introduced a pair of rabbits from the large enclosures. Both enclosures and cages had water suppliers and feeders; water and pelleted food were provided ad libitum. Overall conditions in both facilities (i.e. Ciudad Real and Córdoba) were very similar.
All animals (both in enclosures and cages) were marked with individually numbered ear tags and measured (sex, weight, tarsus, and ear length) when they were firstly captured in the facilities or when their birth was noticed in the cages. All cages were checked every 1–2 days to assess birth dates and survival of litters and breeders. As a consequence, we knew the exact age of the rabbits born in those cages. We estimated the age of those rabbits live-captured in the larger enclosures by using the linear equation (i.e. Eq. 3) described by Southern53 and revised by Dunnet54 with Occ in Australia and recently employed by Ferreira and Ferreira55 with Iberian Oca. This age calculation only works efficiently for juvenile animals because their weight range exhibits a perfectly linear relation with age55 (which does not occur for adults). For this reason, and to avoid potential biases associated with aging adult rabbits using this method, we only estimated the age of animals under 500 g at their first capture; this is almost half the weight considered for Oca adults19
where W is the body weight (g), wi is the weight at weaning age (g), r is the body growth rate (g/day), and A is the postnatal age (days). Postnatal age has been described as 21 days for both subspecies55. We used as body growth rate the same recommended by Dunnet54 (i.e. 9.77 g/day), which is almost the same as the one described for Oca (9.80 g/day; personal data). Similarly, we used as weight at weaning age the value recommended by Southern53 and Dunnet54 for Occ (200 g), which is also coincident with the one gathered for Oca (personal data).
Ethics declaration
Our sample collection was opportunistic as we did not sacrifice animals specifically for this study. We took eye lens samples from animals found dead by natural causes, including old animals and those killed by rabbit diseases (myxomatosis and RHD), or whose sacrifice was part of other research projects. Cervical dislocation was used for euthanasia of animals of less than 1 kg, while barbiturates were used for heavier animals. In particular, sodium pentobarbital by intracardiac injection was used after analgesia and anesthesia using xylazine and ketamine. Personnel trained consistently applied these methods humanely and effectively. All the proceedings agreed with the guidelines and regulations concerning animal welfare and experimentation set forth by Spanish legislation and were previously approved by the Animal Experimentation Ethical Committees of the Castilla-La Mancha University (Register Projects PAI06-0170, PEII09-0097-436, CGL2009-11665), and the Consejo Superior de Investigaciones Científicas (CGL2013-43197-R) for Ciudad Real and Córdoba facilities, respectively. The authors complied with ARRIVE guidelines56.
Eye lenses processing
Lenses were extracted from eyeballs with a scalpel. We always extracted the right eye lens of every individual, except when it was damaged; in those cases, we extracted the left eye. Lenses were immediately stored in 10% formaldehyde during a minimal period of two months to allow its protein's fixation. Fixed lenses were dried for 14 days at 85 °C as recommended by Wheeler and King17 and Augusteyn10 to stabilize their weight. We weighted lenses to the fourth decimal place (0.1 mg) with professionally calibrated Mettler AE260 scales.
Statistical analysis
All the statistical analyses were conducted in R version 4.0.257. We performed the comparative analysis of eye growth curves of Occ and Oca by fitting four different nonlinear regression models between the rabbit age and its corresponding LDW. These models were fit to estimate the parameters a (maximum asymptotic value; MA) and b (constant growth rate value; GR) in the Eqs. (1) and (2) (see above), by a nonlinear least square approach using function 'nls' from package 'stats'. Using this approach, we checked the potential similarity or difference of these parameters between rabbit subspecies.
We considered the AIC weight of each model (AICcWt) and the cumulative Akaike weight (Cum.Wt), which serve to describe the weight of evidence in support of each model. If two models differed less than two AICc units (ΔAICc < 2), they were both considered equally plausible58. To assess the predictive performance of the most supported model (the one with least AICc), we used a repeated (n = 100) ten-fold cross-validation. The training and testing subsets were randomly sampled 100 times (using a 90–10% split, respectively). The average R2 of all iterations was calculated.
As we did not have access to the original datasets employed for estimation of rabbit Australian age-LDW curve, we simulated the expected distribution of a (n = 1000) in Australian rabbit population and compared it to our a estimation of Occ and Oca Iberian populations. For that aim, we first estimated a from our Occ data in Iberia and then, we used the estimate of a and its standard error to simulate the expected distribution of the maximum asymptotic value (n = 1000) for Occ in the IP. We did the same with Oca population and then, given that the standard error of a for the combined Australian populations was not reported in Augusteyn10, we simulate the expected distribution of a (n = 1000) in Australia by using the coefficient value shown in Augusteyn10 and the standard error estimated in our study.
We employed the "overlapping" R package59 to quantify the differences of a between the Australian and Iberian datasets. The resulting overlapping index, η, is normalized between 0 and 1 (η = 0: distributions are entirely separated; η = 1: distributions are equal) and does not assume the normality of distributions nor any other distributional form60. We did not analyze the differences in b between populations because it varies according to the habitat quality61.
References
Stearns, S. C. The Evolution of Life Histories (Oxford University Press, 1992).
Caughley, G. & Sinclair, A. R. E. Wildlife Ecology and Management (Blackwell Science, 1994).
Servanty, S. et al. Influence of harvesting pressure on demographic tactics: Implications for wildlife management. J. Appl. Ecol. 48(4), 835–843 (2011).
Marboutin, E., Bray, Y., Péroux, R., Mauvy, B. & Lartiges, A. Population dynamics in European hare: Breeding parameters and sustainable harvest rates. J. Appl. Ecol. 40(3), 580–591 (2003).
Stoneberg, R. P. & Jonkel, C. L. Age determination of black bears by cementum layers. J. Wildlife Manage. 30(2), 411–414 (1966).
Roth, V. L. & Shoshani, J. Dental identification and age determination in Elephas maximus. J. Zool. 214, 567–588 (1988).
Dutta, S. & Sengupta, P. Men and mice: Relating their ages. Life Sci. 152, 244–248 (2016).
Dimmick, R. W. & Pelton, M. R. Criteria of sex and age. In Research and Management Techniques for Wildlife and Habitats 5th edn, (ed. Bookhout, T. A.) 169–214 (The Wildlife Society, Bethesda, MA, US, 1994).
Morris, P. A review of mammalian age determination methods. Mamm. Rev. 2, 69–103 (1972).
Augusteyn, R. C. On the relationship between rabbit age and lens dry weight: Improved determination of the age of rabbits in the wild. Mol. Vis. 13, 2030–2034 (2007).
Augusteyn, R. C. Growth of the lens: In vitro observations. Clin. Exp. Optom. 91(3), 226–239 (2008).
Augusteyn, R. C. Growth of the eye lens: I. Weight accumulation in multiple species. Mol. Vis. 20, 410–426 (2014).
Lord, D. R. The lens as an indicator of age in cottontail rabbits. J. Wildl. Manage. 23, 358–360 (1959).
Forsyth, D. M., Garel, M. & McLeod, S. R. Estimating age and age class of harvested hog deer from eye lens mass using frequentist and Bayesian methods. Wildlife biol. 22(4), 137–143 (2016).
Dudzinski, M. L. & Mykytowycz, R. The eye lens as an indicator of age in the wild rabbit in Australia. CSIRO Wildl. Res. 6, 156–159 (1961).
Myers, K. & Gilbert, N. Determination of age of wild rabbits in Australia. J. Wildl. Manage. 32, 841–849 (1968).
Wheeler, S. H. & King, D. R. The use of eye-lens weights for aging wild rabbits, Oryctolagus cuniculus (L.) in Australia. Aust. Wildl. Res. 7, 79–84 (1980).
Tablado, Z., Revilla, E. & Palomares, F. Breeding like rabbits: Global patterns of variability and determinants of European wild rabbit reproduction. Ecography 32, 310–320. https://doi.org/10.1111/j.1600-0587.2008.05532.x (2009).
Ferreira, C. et al. Biometrical analysis reveals major differences between the two subspecies of the European rabbit. Biol. J. Linn. Soc. 116, 106–116 (2015).
Branco, M., Monnerot, M., Ferrand, N. & Templeton, A. R. Postglacial dispersal of the European rabbit (Oryctolagus cuniculus) on the Iberian Peninsula reconstructed from nested clade and mismatch analyses of mitochondrial DNA genetic variation. Evolution 56, 792–803. https://doi.org/10.1111/j.0014-3820.2002.tb01390.x (2002).
Gómez, A. & Lunt, D. H. Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula. In Phylogeography in Southern European Refugia (eds Weiss, S. & Ferrand, N.) 155–188 (Springer, 2006).
Geraldes, A. et al. Reduced introgression of the Y chromosome between subspecies of the European rabbit (Oryctolagus cuniculus) in the Iberian Peninsula. Mol. Ecol. 17, 4489–4499 (2008).
Carneiro, M., Ferrand, N. & Nachman, M. W. Recombination and speciation: Loci near centromeres are more differentiated than loci near telomeres between subspecies of the European rabbit (Oryctolagus cuniculus). Genetics 181, 593–606 (2009).
Rafati, N. et al. A genomic map of clinal variation across the European rabbit hybrid zone. Mol. Ecol. 27, 1457–1478. https://doi.org/10.1111/mec.14494 (2018).
Vaquerizas, P. H. et al. The paradox of endangered European rabbits regarded as pests in the Iberian Peninsula: Subspecies differences in trends matter. Endang. Species Res. 43, 99–102 (2020).
Arques, J. & Peiró, V. Estructura de Sexos y Edades de una población de Conejos (Oryctolagus cuniculus) del sudeste de España. Mediterránea. Serie de Estudios Biológicos 18, 1–33 (2005).
Trout, R. C. & Smith, G. C. The reproductive productivity of the wild rabbit (Oryctolagus cuniculus) in southern England on sites with different soils. J. Zool. 237(3), 411–422 (1995).
Boussès, P., Arthur, C. & Chapuis, J. L. Rôle du facteur trophique sur la biologie des populations de lapins (Oryctolagus cuniculus L.) des Iles Kerguelen. Revue d’écologie 43, 329–343 (1988).
Bonino, N. & Donadio, E. Body parameters and sexual dimorphism in the European wild rabbit (Oryctolagus cuniculus) introduced in Argentina. Mastozool. Neotrop. 17(1), 123–127 (2010).
Carneiro, M. et al. Rabbit genome analysis reveals a polygenic basic for phenotypic change during domestication. Science 345(6200), 1074–1079 (2014).
Myers, K. The rabbit in Australia. In Dynamics of Numbers in Populations (eds den Boer, P. J. & Gradwell, G. R.) 478–506 (Proceedings of the NATO Advanced Study Institute Oosterbeek, 1970).
Delibes-Mateos, M., Villafuerte, R., Cooke, B. & Alves, P. C. Oryctolagus cuniculus (Linnaeus, 1758). In Lagomorphs: Pikas, Rabbits and Hares of the World (eds Smith, A. T. et al.) 99–104 (John Hopkins University Press, 2018).
Carneiro, M. et al. The genomic architecture of population divergence between subspecies of the European rabbit. PLoS. Genet. 10(8), e1003519. https://doi.org/10.1371/journal.pgen.1003519 (2014).
Bonino, N. & Soriguer, R. Genetic lineages of feral populations of the Oryctolagus cuniculus (Leporidae, Lagomorpha) in Argentina. Mammalia 72, 355–357 (2008).
Branco, M. & Ferrand, N. Biochemical and population genetics of the rabbit, Oryctolagus cuniculus, carbonic anhydrases I and II, from the Iberian Peninsula and France. Biochem. Genet. 41, 391–404. https://doi.org/10.1023/B:BIGI.0000007774.39262.8e (2003).
Geraldes, A., Ferrand, N. & Nachman, M. W. Contrasting patterns of introgression at X-linked loci across the hybrid zone between subspecies of the European rabbit (Oryctolagus cuniculus). Genetics 173, 919–933 (2006).
Lo Valvo, M., Scala, A. & Scalisi, M. Biometric characterization and taxonomic considerations of European rabbit Oryctolagus cuniculus (Linnaeus 1758) in Sicily (Italy). World Rabbit Sci. 22(3), 207–214. https://doi.org/10.4995/wrs.2014.1467 (2014).
Miller, G. S. Catalogue of the Mammals of Western Europe in the Collection of the British Museum (Trustees of the British Museum, 1912).
Sharples, C. M., Fa, J. E. & Bell, D. J. Geographical variation in size in the European rabbit Oryctolagus cuniculus (Lagomorpha: Leporidae) in western Europe and North Africa. Zool. J. Linn. Soc-Lond. 117, 141–158. https://doi.org/10.1111/j.1096-3642.1996.tb02153.x (1996).
Carro, F., Ortega, M. & Soriguer, R. C. Is restocking a useful tool for increasing rabbit densities?. Global Ecol. Conserv. 17, e00560. https://doi.org/10.1016/j.gecco.2019.e00560 (2019).
Angulo, E. & Villafuerte, R. Modelling hunting strategies for the conservation of wild rabbit populations. Biol. Conserv. 115, 291–301 (2003).
Delibes-Mateos, M., Delibes, M., Ferreras, P. & Villafuerte, R. Key role of European rabbits in the conservation of the Western Mediterranean Basin Hotspot. Conserv. Biol. 22, 1106–1117 (2008).
Garrido, J. L., Ferreres, J. & Gortázar, C. Las especies cinegéticas españolas en el siglo XXI. (eds. Garrido, J. L., Ferreres, J. & Gortázar, C.) (Independently published, Ciudad Real, Spain, 2019).
Ríos-Saldaña, C. et al. Control of the European rabbit in central Spain. Eur. J. Wildlife Res. 59, 573–580. https://doi.org/10.1007/s10344-013-0707-x (2013).
Lees, A. C. & Bell, D. J. A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Rev. 38, 304–320 (2008).
Cooke, B. D. Rabbits: Manageable environmental pests or participants in new Australian ecosystems?. Wildlife Res. 39, 279–289 (2013).
Calvete, C., Angulo, E. & Estrada, R. Conservation of European wild rabbit populations when hunting is age and sex selective. Biol. Conserv. 121(4), 623–634 (2005).
Delibes-Mateos, M., Ramírez, E., Ferreras, P. & Villafuerte, R. Translocations as a risk for the conservation of European wild rabbit Oryctolagus cuniculus lineages. Oryx 42(2), 259–264 (2008).
Andersen, J. & Jensen, B. Studies on the European hare. XXVIII. The weight of the eye lens in the European hares of known age. Acta Theriol. 17, 87–92 (1972).
Suchentrunck, F., Willing, R. & Hartl, G. B. On eye lens weights and other age criteria of the Brown hare (Lepus europaeus Pallas, 1778). Z. Säugetierkd. 56, 365–374 (1991).
Villafuerte, R. et al. Large-scale assessment of myxomatosis prevalence in European wild rabbits (Oryctolagus cuniculus) 60 years after first outbreak in Spain. Res. Vet. Sci. 114, 281–286 (2017).
Rouco, C., Villafuerte, R., Castro, F. & Ferreras, P. Effect of artificial warren size on a restocked European wild rabbit population. Anim. Conserv. 14, 117–123 (2011).
Southern, N. The ecology and population dynamics of the wild rabbit (Oryctolagus cuniculus). Ann. Appl. Biol. 27, 509–514 (1940).
Dunnet, G. M. Growth rate of young rabbits, Oryctolagus cuniculus (L.). CSIRO Wildl. Res. 1, 66–67 (1956).
Ferreira, A. & Ferreira, A. J. Post-weaning growth of endemic Iberian wild rabbit subspecies, Oryctolagus cuniculus algirus, kept in a semi-extensive enclosure: Implications for management and conservation. World Rabbit Sci. 22, 129–136. https://doi.org/10.4995/wrs.2014.1673 (2014).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (Vienna, Austria, 2020).
du Sert, N. P. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18(7), e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Burnham, K. P. & Anderson, D. R. Monte Carlo insights and extended examples. In Model Selection and Multimodel Inference, (eds. Burnham K. P. & Anderson D. R.) https://doi.org/10.1007/978-0-387-22456-5_5). (Springer, New York, NY, US, 2002).
Pastore, M. Overlapping: A R package for estimating overlapping in empirical distributions. J. Open Source Softw. 32, 1023 (2018).
Pastore, M. & Calcagnì, A. Measuring distribution similarities between samples: A distribution-free overlapping Index. Front. Psychol. 10, 1089 https://doi.org/10.3389/fpsyg.2019.01089 (2019).
Williams, C. & Moore, R. Phenotypic adaptation and natural selection in the wild rabbit, Oryctolagus cuniculus, Australia. J. Anim. Ecol. 58(2), 495–507. https://doi.org/10.2307/4844 (1989).
Acknowledgements
Special thanks go to all people who help during the fieldwork and to three anonymous reviewers for their constructive comments. PHV was supported by a scholarship (FPU17/04821) from Spanish Ministry of Science, Innovation and Universities. This study was partially funded by Projects PAI06-170, PEII09-0097-436, CGL2009-11665, CGL2013-43197-R.
Author information
Authors and Affiliations
Contributions
R.V., F.C. and M.D.M. conceived and designed the study. R.V., F.C., P.H.V. and M.D.M. carried out the methodology. S.S. performed the data analysis. P.H.V., R.V. and M.D.M. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaquerizas, P.H., Santoro, S., Delibes-Mateos, M. et al. Keeping an eye on the use of eye-lens weight as a universal indicator of age for European wild rabbits. Sci Rep 11, 8711 (2021). https://doi.org/10.1038/s41598-021-88087-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88087-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.