Abstract
We examined the associations of smoking status and urinary cotinine levels, an objective measure of smoking, with the development of new-onset HL. This cohort study was performed in 293,991 Korean adults free of HL who underwent a comprehensive screening examination and were followed for up to 8.8 years. HL was defined as a pure-tone average of thresholds at 0.5, 1.0, and 2.0 kHz ≥ 25 dB in both ears. During a median follow-up of 4.9 years, 2286 participants developed new-onset bilateral HL. Self-reported smoking status was associated with an increased risk of new-onset bilateral HL. Multivariable-adjusted HRs (95% CIs) for incident HL comparing former smokers and current smokers to never-smokers were 1.14 (1.004–1.30) and 1.40 (1.21–1.61), respectively. Number of cigarettes, pack-years, and urinary cotinine levels were consistently associated with incident HL. These associations were similarly observed when introducing changes in smoking status, urinary cotinine, and other confounders during follow-up as time-varying covariates. In this large cohort of young and middle-aged men and women, smoking status based on both self-report and urinary cotinine level were independently associated with an increased incidence of bilateral HL. Our findings indicate smoking is an independent risk factor for HL.
Similar content being viewed by others
Introduction
Hearing loss (HL) is an important issue in public health, having rising prevalence and a negative impact on social function and mental and physical health. The World Health Organization (WHO) approximates that around 466 million people had disabling HL in 2015, and over 900 million people will have disabling HL by 20501. Therefore, it is of great importance to develop preventive strategies by identifying modifiable risk factors and individuals at high risk for HL.
Cigarette smoking is a well-known risk factor for a wide range of diseases, but its association with HL has been inconsistent in previous studies. Some studies have suggested a positive association between smoking and HL2,3,4,5,6,7,8,9,10, while others did not11,12. However, previous studies have been limited by the ambiguous temporal relationship between smoking and HL due to the cross-sectional design2,3,5,6,7,8,9,10, insufficient sample size5,6,10,11,12, inclusion of mainly male or elderly participants3,4,6,8, and lack of adjustment for important confounders (i.e., noise exposure and health behaviors)3,6. Furthermore, most studies used subjective measures of smoking, mainly depending on self-report, and the misclassification of smoking status may potentially underestimate the true association of smoking exposure with HL. Since cotinine, a major metabolite of nicotine, is a reliable and objective biomarker that reflects smoking status, it can reduce misclassification bias in self-reporting methods13,14. Until now, however, no cohort study has shown the effects of both subjective and objective smoking measures on the development of HL.
Therefore, we investigated the prospective association between smoking status, cigarettes per day, pack-years and risk of developing HL in Korean young and middle-aged adults. In addition, by adding the level of urine cotinine to the smoking parameter, objective reliability could be additionally obtained, while considering time-dependent measures of change in smoking status and other confounders during follow-up.
Methods
Study population
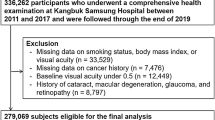
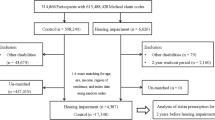
This cohort study was done in a subset of the population used in the Kangbuk Samsung Health Study, a cohort study of Korean adults who underwent a comprehensive annual or biennial health examination at the clinics of Kangbuk Samsung Hospital Total Healthcare Screening Center in Seoul and Suwon, South Korea15. The current analysis included all study participants who had a comprehensive health screening, including a pure-tone audiometry (PTA) between 2011 and 2017, and at least one follow-up PTA before December 31, 2019 (n = 336,262). After exclusion who met the exclusion criteria (Fig. 1), including HL with an average of PTA thresholds ≥ 25 dB at 0.5, 1.0 and 2.0 kHz in both ears (n = 3714), missing information on smoking, body mass index (BMI), or hearing tests (n = 33,118), or a history of cancer (n = 7,476), a total number of 293,991 participants were analyzed.
We followed the practice of the Declaration of Helsinki and this study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2020-03-021), which waived the requirement for written informed consent due to the use of anonymized data obtained as part of regular medical examinations.
Data collection
Physical measurements, hearing tests, and laboratory measurements were performed every 1–2 years. Demographic characteristics, diet, lifestyle factors, and medical history were also collected at each visit using standardized, self-administered questionnaires as previously described15,16. Questions regarding smoking, included lifetime and current smoking status, smoking duration, and number of cigarettes per day were included17. Participants who had smoked < 100 cigarettes during their lifetime were classified as never-smokers. Participants who had smoked > 100 cigarettes in their lifetime were further categorized as (1) current smokers who smoked currently or (2) former smokers who no longer smoked at the time of their screening examination. Pack-years were categorized as never (0), > 0–10, > 10–20, and ≥ 20 pack-years. Few female participants were identified as having ≥ 20 pack-years and were thus combined with the category of > 10–20 pack-years, resulting in a category of ≥ 10 pack-years in women. Average alcohol consumption was categorized into none, < 20 g of ethanol/day, and ≥ 20 g of ethanol/day18. Physical activity level was assessed using the validated Korean version of the International Physical Activity Questionnaire Short Form19 and was classified into three categories: inactive, minimally active, and health-enhancing physically active (HEPA)20. HEPA status was defined as (i) vigorous intensity activity on three or more days per week accumulating ≥ 1500 metabolic equivalent task (MET) min/week, or (ii) seven days of any combination of walking, moderate intensity, or vigorous intensity activities achieving at least 3000 MET min/week19. Cardiovascular disease (CVD) and cancer were defined as physician-diagnosed heart disease or stroke and physician-diagnosed malignancy of any type, respectively15.
Height, weight, and blood pressure (BP) were measured by trained nurses. Obesity was defined as BMI ≥ 25 kg/m2 according to Asia-specific criteria21. Hypertension was defined as a systolic BP ≥ 140 mmHg, a diastolic BP ≥ 90 mmHg, a self-reported history of hypertension, or current use of antihypertensive medications.
Fasting blood measurements included glucose, glycated hemoglobin (HbA1c), lipid profiles, insulin, and hsCRP. Insulin resistance was assessed using the following HOMA-IR equation: fasting blood insulin (uU/mL) × fasting blood glucose (mmol/L)/22.5. Diabetes mellitus (DM) was defined as a fasting serum glucose ≥ 126 mg/dL, HbA1c ≥ 6.5%, a history of physician-diagnosed diabetes, or current use of antidiabetic medications.
Urinary cotinine level was measured using the DRI Cotinine Assay (Microgenics Corp., Fremont, CA, USA) with a modular P800 chemistry analyzer (Roche Diagnostics, Tokyo, Japan). A urine cotinine cut-off point of 100 ng/mL has been used as a reference point in our hospital with analytical measurement range of 34 ng/mL, since nonsmoker urine levels have been reported not to exceed 100 ng/mL in several studies22,23. A urinary cotinine level of 50 ng/ml has also been widely used to distinguish tobacco use vs. no tobacco use24. Thus, in the present study, urinary cotinine levels were categorized into three groups: (1) < 50 ng/mL, (2) 50 ng/mL to 99 ng/mL, and (3) ≥ 100 ng/mL. Additionally, we performed analysis using categorization of urinary cotinine based on its distribution. Urinary cotinine levels above 50 ng/mL were divided into tertiles (1st tertile, 50–607 ng/mL; 2nd tertile, 608–1303 ng/mL; 3rd tertile, ≥ 1304 ng/mL).
Audiometric measurements
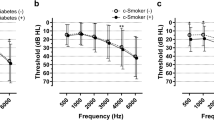
At baseline and follow-up visits, PTA was performed by trained audiometric technicians using a GSI 67 audiometer (Bedford, MA, USA) equipped with TDH-39 supra-aural earphones (Telephonics Co., Farmingdale, NY, USA) in a dedicated sound-treated booth25. Air conduction thresholds were measured in dB hearing level for both ears at 0.5, 1.0, and 2.0 kHz. HL was defined as an average of PTA thresholds ≥ 25 dB at 0.5, 1.0, and 2.0 kHz in bilateral ears.
The Korean Occupational Safety and Health Act 28 requires an annual hearing test at 3.0 and 4.0 kHz in addition to the regular frequencies (0.5, 1.0, and 2.0 kHz) for employees who are exposed to the equivalent sound pressure level of 85 dB(A) over an 8-h work day in the workplace26. Hearing tests at 3.0 and 4.0 kHz were performed only in a small proportion of the participants who met the above criteria; thus, these tests were not used to define HL, but as a proxy marker for occupational noise exposure26.
Statistical analysis
The baseline characteristics of the study participants were presented according to smoking status. The primary endpoint was the development of bilateral HL defined as a pure-tone average of thresholds at 0.5, 1.0, and 2.0 kHz ≥ 25 dB in bilateral ears. Each participant was followed from their baseline examination until either the development of bilateral HL or the last health exam conducted prior to December 31, 2019, whichever came first. The incidence rate was calculated as the number of incident cases divided by the number of person-years of follow-up. Since new-onset HL, if it did occur, would have occurred at an unknown time point between the visit at which HL was diagnosed by hearing tests and the prior visit, a parametric proportional hazards model was used to account for this type of interval censoring (stpm command in Stata)27.
The hazard ratio (HR) and 95% confidence interval (CI) were calculated for incident HL according to smoking status, cigarettes per day, pack-years smoked, and urinary cotinine levels. Models were initially adjusted for age and sex and then were further adjusted for BMI, alcohol intake (0 g/day, < 20 g/day, ≥ 20 g/day, or unknown), physical activity level (inactive, minimally active, HEPA, or unknown), education level (high school graduate or less, community college or university graduate, graduate school or higher, or unknown), total calorie intake (in quintiles or missing), history of diabetes (no vs. yes), history of hypertension (no vs. yes), history of CVD (no vs. yes), and occupational noise exposure (no vs. yes). To evaluate the effects of changes in smoking status and other covariates over time during follow-up, we conducted additional analyses introducing these variables as time-varying covariates in the models. The proportional hazards assumption was assessed by examining graphs of estimated log (−log) survival; ultimately, no violation of the assumption was found. To test for linear trends, we included the median value of each category (pack-years, cigarettes per day, and urinary cotinine) as a continuous variable in the models.
The sensitivity analyses were performed using different definitions of HL as follows: (1) pure-tone threshold at 0.5 kHz ≥ 25 dB in either ear, (2) pure-tone threshold at 1.0 kHz ≥ 25 dB in either ear, and (3) pure-tone threshold at 2.0 kHz ≥ 25 dB in either ear (separately). Additionally, subgroup analyses were performed by exposure to occupational noise (no vs. yes) and age (< 50 vs. ≥ 50 years).
All analyses were carried out using Stata version 16.0 (Stata Corp LP, College Station, TX, USA). All p-values less than 0.05 were considered to be statistically significant.
Results
The mean (standard deviation) age of all participants at baseline was 37.8 (8.0) years; 57.4 percent were male; and never, former, and current smokers were reported as 54.4%, 23.7%, and 21.8%, respectively (Table 1). Compared to never-smokers, current smokers were older, more male, and more likely to drink alcohol; to have unhealthy lipid profiles; to have a history of hypertension, diabetes, and CVD; and to have high levels of BMI, BP, glucose, liver enzymes, HOMA-IR, hsCRP, and total energy intake (Table 1). The median urinary cotinine level of current smokers was 818 ng/mL (interquartile range, 282–1445), whereas the median cotinine level of both never and former smokers was 34 ng/mL, which corresponded to the lower limit of the analytical measurement range.
Table 2 shows the relationship between subjective smoking measures and the incidence of bilateral HL. During follow-up of 1,406,455.9 person-years (median 4.9 years; interquartile range, 2.7–6.9 years; maximum 8.8 years), 2286 participants developed new-onset bilateral HL, which corresponds to an incidence rate (95% CI) of 1.6 (1.6–1.7) per 1000 person-years for the overall population, 1.3 (1.2–1.4) per 1000 person-years for women, and 1.8 (1.7–1.9) per 1000 person-years for men. Based on self-report, smoking status was positively associated with an increased risk of developing bilateral HL. Multivariable-adjusted HRs (95% CIs) for incident HL compared to never-smokers for former and current smokers were 1.14 (1.004–1.30) and 1.40 (1.21–1.61), respectively. Increasing baseline smoking-pack-years and smoking intensity, which is measured by the number of cigarettes smoked per day, had a dose–response relationship with the incidence of bilateral HL. For the amount of cigarettes per day, multivariable-adjusted HRs (95% CIs) for incident HL were 1.19 (0.95–1.48), 1.30 (1.13–1.48), and 1.29 (1.11–1.49) compared with < 10, 10–19, and ≥ 20 versus 0 cigarettes per day, respectively (P for trend < 0.001). For pack-years, multivariable-adjusted HRs (95% CIs) for incident HL were 1.00 (0.86–1.16), 1.10 (0.95–1.28), and 1.27 (1.10–1.46) compared with < 10, 10–19.9, and ≥ 20 versus 0 pack-years, respectively (P for trend = 0.001). A similar association was observed between pack-years and incident HL when introducing changes in smoking measures and other confounding factors during follow-up as covariates over time. None of the associations between subjective smoking measures and HL differed by sex (all P for interaction > 0.1), but these associations was not statistically significant among female participants (Supplementary Table S1).
Urinary cotinine levels were associated with an increased risk of developing HL (P for trend < 0.001) (Table 3). Cotinine levels of ≥ 100 ng/ml significantly related with incident HL in the multivariable-adjusted model with a corresponding HR (95% CI) of 1.30 (1.21–1.51). This pattern was observed in both men and women, but there was no significant interaction (P for interaction by sex = 0.891), and the association was not statistically significant among women. In a further time-dependent analysis, the association between cotinine levels and incident HL increased slightly than in the original analyses.
In the analysis using categories based on tertiles of urinary cotinine, multivariable-adjusted HRs (95% CI) for incident HL comparing 1st, 2nd and 3rd tertiles to low urinary cotinine of < 50 ng/ml were 1.10 (0.87–1.39), 1.35 (1.09–1.67) and 1.37 (1.12–1.69), respectively (Table 4). This pattern was similarly observed in both men and women. In the analysis using urinary cotinine as a continuous variable, multivariable-adjusted HR (95% CI) for incident HL was 1.013 (1.001–1.025) per 100 unit increase in cotinine level in overall subjects.
In a stratified subgroup analysis by occupational noise exposure (Supplementary Table S2), the association of smoking status, cigarettes per day, pack-years, and cotinine level with the risk of incident HL was consistently observed in participants without exposure to occupational noise. The average age of the HL group was 51.1 (standard deviation, 11.2) years. In the subgroup analysis by age (< 50 vs. ≥ 50 years), the association between self-reported smoking and incident HL was stronger in younger individuals aged < 50 years than in older individuals (p for interaction < 0.05) (Supplementary Table S2).
Sensitivity analyses were performed using different definitions of HL as follows: (1) pure-tone threshold at 0.5 kHz ≥ 25 dB in either ear, (2) pure-tone threshold at 1.0 kHz ≥ 25 dB in either ear28 and (3) pure-tone threshold at 2.0 kHz ≥ 25 dB in either ear (separately) (Supplementary Tables S3-S4). The incidence rate (95% CI) of HL at 0.5 kHz, 1.0 kHz, and 2.0 kHz were 4.9 (4.8–5.0) per 1000 person-years, 3.2 (3.1–3.3) per 1000 person-years, and 12.5 (12.3–12.8), respectively. In these sensitivity analyses, the associations of smoking status, cigarettes per day, pack-years, and cotinine level with incident HL were similar to in the original analyses, which HL defined as a pure-tone average of thresholds at 0.5, 1.0, and 2.0 kHz ≥ 25 dB in bilateral ears.
Discussion
In this longitudinal study of young and middle-aged adults in Korea, current smoking, cigarettes per day, and pack-years were significantly associated with an increased risk of developing HL. In addition, a significant association was found between HL and urine cotinine levels, which are objective smoking biomarker. The significance of the above associations were maintained even after adjusting for various confounders, including occupational noise exposure.
Previous longitudinal studies were limited by short follow-up periods4,12,29, small sample sizes6,11,12,29, audiometric measurements of single individual low frequency or self-reported hearing4,30,31, and inclusion of mainly male or elderly participants4,6,29,30. A previous meta-analysis including 4 cohort studies reported that the risk ratio (95% CI) for HL in current smokers was 1.97 (1.44, 2.70) and in former smokers was 1.49 (0.93, 2.39)32. In a recent cohort study of 1925 participants with a 15-year follow up, current smokers were more likely to cause HL than never-smokers (HR = 1.31, 95% CI = 1.003–1.71 in a model adjusted for age, sex, and education), but detailed information on smoking intensity, smoking duration, and other confounders was not available in this study33. In another cohort study of over 50,000 workers aged 20–64 years in Japan, the self-reported smoking status (current, former), smoking intensity (cigarettes smoked per day), and smoking pack-years were all significantly associated with an elevated risk of HL at an individual frequency (defined as > 30 dB at 1 kHz and > 40 dB at 4 kHz) after multivariable adjustment28. However, this study population was mainly composed of male workers (~ 85%), and information regarding important confounders including occupational noise exposure, alcohol drinking, and physical activity was only available in a subset of the study population28. In our study, we tried to reduce the measurement error by using the traditional standard definition of HL as an average of PTA thresholds ≥ 25 dB at 0.5, 1.0, and 2.0 kHz in bilateral ears34, rather than at one individual frequency in either ear. In addition, ≥ 25 dB was used as the cut-off threshold for HL, as opposed to the > 30 dB value used in the previous study28, enabling us to capture mild HL. Moreover, on the assumption that smoking causes HL through a systemic pathway35,36, we defined bilateral HL as the primary endpoint instead of unilateral HL, which may also be caused by a localized infection or accident, and found that current smoking status, intensity, and pack-years were still significantly associated with bilateral HL. When we repeated the analysis using the same definition as in the previous study for comparison28, the associations between smoking parameters and incident HL were consistently observed. We adopted urine cotinine as another measure of smoking to reduce the misclassification bias in the self-report method. and reaffirmed the robustness of our study findings on the associations between smoking and incident HL. Furthermore, all these associations were consistently observed in a time-dependent analysis that took into account changes over time in smoking parameters and other confoundings.
We found an association between smoking and low-frequency HL, defined as an average threshold of 0.5, 1.0, and 2.0 kHz. Previous studies have shown that smoking is associated with HL at both low and high frequencies9,37. Some studies have reported that the association of smoking with HL was stronger at high frequencies than at low frequencies8,35. In contrast, a Danish study reported that cardiovascular risk factors, including smoking, are related to low-frequency HL but not to high-frequency HL38. Studies using objective measures of auditory function, such as otoacoustic emission, also supported the link between smoking and hearing at various frequencies39,40. Smoking can cause negative alterations in the cochlea, leading to different effects on the base and apex of the cochlea via microvascular compromise and induced hypoxemia35,36. These pieces of evidence may describe that smoking can be associated with HL in the low-frequency range.
The associations between smoking and incidence of HL did not statistically differ by sex in our study, but the association was not statistically significant among women. The women’s smoking prevalence was very low, 7.4% being former smokers and 2.3% being current smokers, compared to the prevalence among men being 35.9% for former smokers and 36.3% for current smokers. Thus, the inconclusive association for women may be explained by the small number of smokers, which is insufficient to provide precise estimates. On the other hand, epidemiological studies have also reported that smoking-associated HL was more pronounced in men than in women31,41. A study of 85 healthy volunteers aged 25–45 years using otoacoustic emissions (OAEs), a test for activity in the outer hair cells of the cochlea, demonstrated a significant decline in OAEs among male smokers but not among the females, suggesting that men are more susceptible to smoking-induced adverse change in the organ of hearing39. These sex differences might be explained by the potential protective effect of female sex hormones in hearing, as previously proposed39,42. Future research is needed to interpret the mechanism between smoking and HL, while considering possible differences by sex.
The average age of the HL group was higher than the group without incident HL but the association between self-reported smoking and incident HL was stronger in younger individuals aged < 50 years than in older individuals. The reason for this difference is unclear. Participants aged ≥ 50 years may have more comorbidities than younger counterparts and relative contribution of smoking to incident HL might be lower in older age group. Due to the use of multiple comparisons, another explanation for the observed difference across age group can be attributed simply due to chance.
The strength of this study is that it is a large cohort, allows longitudinal analysis, and includes standardized laboratory data of a comprehensive health examination at all visits. And, unlike previous studies, urine cotinine provides less biased results compared to the self-reported smoking status. Our subjects were relatively healthy and young (average age, 37.8 years old), with a low occupational noise exposure rate (18.6%). Therefore, the findings are less likely to be biased by age-related HL and occupational noise exposure than previous studies conducted in older populations or those exposed to occupational noise.
There are some limitations to this study. First, hearing results at high frequencies above 2.0 kHz were not available. However, since HL was defined in the spectrum below 2.0 kHz, which is mainly used by human voices in real life, the association between smoking and hearing could be investigated with only low-frequency data. Second, leisure exposure, which is the main cause of noise-induced HL, was not measured at a comprehensive examination. However, since noise-induced HL is mainly related to high-frequency HL above 3.0 kHz, there was no significant interference even without leisure exposure data in our study. Third, information on ototoxic drugs (i.e., cisplatin and aminoglycoside antibiotics) was not available. However, by excluding subjects with a history of cancer, we tried to minimize the effects of ototoxic substances such as anticancer drugs. Fourth, even though we used cotinine level as an objective measure of smoking status, the biological half-life of cotinine is only 19 to 40 h in the body14. Therefore, measurement errors cannot be avoided in irregular smokers and persons who intentionally did not smoke temporarily17. Fifth, detailed analyses on secondhand smoking were not possible in our study even though secondhand smoking has been reported to be associated with HL and can affect urine cotinine levels43. While previous studies have suggested a cut-off for distinguishing between active and secondhand smokers of between 20 and 100 ng/mL23,44, the lower limit of detection of urinary cotinine was 34 ng/mL in our study. This limited our ability to further examine the dose–response relationship of urinary cotinine with incident HL at low levels of urinary cotinine that could correspond to secondhand smoking. Therefore, the effect of unmeasured secondhand smoke cannot be excluded. Additionally, we cannot ignore the possibility of bias related to residual confounding in regard to measured or unmeasured confounders in the associations observed in the present study. Lastly, our results were derived from young and healthy Korean adults, thus limiting the generalizability of our findings on different populations with various age and race/ethnicity.
In conclusion, smoking status in both subjective self-report and objective urinary cotinine levels were independently associated with increased risk of HL in a dose–response manner, after adjustment for confounders including occupational noise exposure. This association was evident even in individuals without occupational noise exposure. Our findings support the conclusion that smoking is an independent cause for HL even in young adults, underscoring the importance of smoking control on HL, in addition to the wide range of other diseases attributable to smoking.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- HR:
-
Hazard ratio
- HDL-C:
-
High-density lipoprotein cholesterol
- hsCRP:
-
High sensitivity C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Deafness and hearing loss, https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss
Cruickshanks, K. J. et al. Cigarette smoking and hearing loss: The epidemiology of hearing loss study. JAMA 279, 1715–1719. https://doi.org/10.1001/jama.279.21.1715 (1998).
Itoh, A. et al. Smoking and drinking habits as risk factors for hearing loss in the elderly: Epidemiological study of subjects undergoing routine health checks in Aichi, Japan. Public Health 115, 192–196. https://doi.org/10.1038/sj.ph.1900765 (2001).
Nakanishi, N., Okamoto, M., Nakamura, K., Suzuki, K. & Tatara, K. Cigarette smoking and risk for hearing impairment: A longitudinal study in Japanese male office workers. J. Occup. Environ. Med. 42, 1045–1049. https://doi.org/10.1097/00043764-200011000-00001 (2000).
Noorhassim, I. & Rampal, K. G. Multiplicative effect of smoking and age on hearing impairment. Am. J. Otolaryngol. 19, 240–243. https://doi.org/10.1016/s0196-0709(98)90125-9 (1998).
Rosenhall, U., Sixt, E., Sundh, V. & Svanborg, A. Correlations between presbyacusis and extrinsic noxious factors. Audiology 32, 234–243. https://doi.org/10.3109/00206099309072939 (1993).
Park, H. J., Yoo, M. H., Woo, S. Y., Kim, S. W. & Cho, Y. S. Prevalence of hearing loss and associated factors in subjects with normal otoscopy: A national cross-sectional study. Int. J. Audiol. 56, 951–957. https://doi.org/10.1080/14992027.2017.1373866 (2017).
Rigters, S. C. et al. Contributing determinants to hearing loss in elderly men and women: Results from the population-based Rotterdam Study. Audiol. Neurootol. 21(Suppl 1), 10–15. https://doi.org/10.1159/000448348 (2016).
Chang, J. et al. Effect of cigarette smoking and passive smoking on hearing impairment: Data from a population-based study. PLoS ONE 11, e0146608. https://doi.org/10.1371/journal.pone.0146608 (2016).
Lohi, V., Hannula, S., Ohtonen, P., Sorri, M. & Maki-Torkko, E. Hearing impairment among adults: The impact of cardiovascular diseases and cardiovascular risk factors. Int. J. Audiol. 54, 265–273. https://doi.org/10.3109/14992027.2014.974112 (2015).
Brant, L. J. et al. Risk factors related to age-associated hearing loss in the speech frequencies. J. Am. Acad. Audiol. 7, 152–160 (1996).
Karlsmose, B., Lauritzen, T., Engberg, M. & Parving, A. A five-year longitudinal study of hearing in a Danish rural population aged 31–50 years. Br. J. Audiol. 34, 47–55. https://doi.org/10.3109/03005364000000117 (2000).
Connor Gorber, S., Schofield-Hurwitz, S., Hardt, J., Levasseur, G. & Tremblay, M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 11, 12–24. https://doi.org/10.1093/ntr/ntn010 (2009).
Benowitz, N. L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 18, 188–204. https://doi.org/10.1093/oxfordjournals.epirev.a017925 (1996).
Chang, Y. et al. Metabolically healthy obesity and development of chronic kidney disease: A cohort study. Ann. Intern. Med. 164, 305–312. https://doi.org/10.7326/M15-1323 (2016).
Han, S. Y., Chang, Y., Shin, H., Choi, C. Y. & Ryu, S. Smoking, urinary cotinine levels and incidence of visual impairment. Sci. Rep. 11, 398. https://doi.org/10.1038/s41598-020-79865-z (2021).
Kim, K. et al. Smoking and urinary cotinine levels are predictors of increased risk for gastric intestinal metaplasia. Cancer Res. 79, 676–684. https://doi.org/10.1158/0008-5472.Can-18-2268 (2019).
Fernandez-Sola, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 12, 576–587. https://doi.org/10.1038/nrcardio.2015.91 (2015).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB (2003).
Ryu, S. et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J. Hepatol. 63, 1229–1237. https://doi.org/10.1016/j.jhep.2015.07.010 (2015).
Wen, C. P. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 12, 497–506. https://doi.org/10.1017/S1368980008002802 (2009).
Haufroid, V. & Lison, D. Urinary cotinine as a tobacco-smoke exposure index: A minireview. Int. Arch. Occup. Environ. Health 71, 162–168 (1998).
Zielińska-Danch, W., Wardas, W., Sobczak, A. & Szołtysek-Bołdys, I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers 12, 484–496. https://doi.org/10.1080/13547500701421341 (2007).
Verification, S. S. B. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 4, 149–159. https://doi.org/10.1080/14622200210123581 (2002).
Kim, M. B. et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int. J. Epidemiol. 46, 717–726. https://doi.org/10.1093/ije/dyw243 (2017).
Lee, W., Chang, Y., Shin, H. & Ryu, S. Hearing loss and risk of overall, injury-related, and cardiovascular mortality: The Kangbuk samsung health study. J. Clin. Med. https://doi.org/10.3390/jcm9051415 (2020).
Royston, P. & Parmar, M. K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 21, 2175–2197. https://doi.org/10.1002/sim.1203 (2002).
Hu, H. et al. Smoking, smoking cessation, and the risk of hearing loss: Japan epidemiology collaboration on occupational health study. Nic. Tob. Res. 21, 481–488. https://doi.org/10.1093/ntr/nty026 (2019).
Rigters, S. C., van der Schroeff, M. P., Papageorgiou, G., Baatenburg de Jong, R. J. & Goedegebure, A. Progression of hearing loss in the aging population: Repeated auditory measurements in the Rotterdam study. Audiol. Neuro-otol. 23, 290–297. https://doi.org/10.1159/000492203 (2018).
Shargorodsky, J., Curhan, S. G., Eavey, R. & Curhan, G. C. A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope 120, 1887–1891. https://doi.org/10.1002/lary.21039 (2010).
Burr, H., Lund, S. P., Sperling, B. B., Kristensen, T. S. & Poulsen, O. M. Smoking and height as risk factors for prevalence and 5-year incidence of hearing loss. A questionnaire-based follow-up study of employees in Denmark aged 18–59 years exposed and unexposed to noise. Int. J. Audiol. 44, 531–539. https://doi.org/10.1080/14992020500190045 (2005).
Nomura, K., Nakao, M. & Morimoto, T. Effect of smoking on hearing loss: Quality assessment and meta-analysis. Prev. Med. 40, 138–144. https://doi.org/10.1016/j.ypmed.2004.05.011 (2005).
Cruickshanks, K. J. et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J. Am. Geriatr. Soc. 63, 918–924. https://doi.org/10.1111/jgs.13401 (2015).
What the Numbers Mean: An Epidemiological Perspective on Hearing, https://www.nidcd.nih.gov/health/statistics/what-numbers-mean-epidemiological-perspective-hearing
Hossain, M. et al. Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier. Brain Res. 1287, 192–205. https://doi.org/10.1016/j.brainres.2009.06.033 (2009).
Pretorius, E., Oberholzer, H. M., van der Spuy, W. J. & Meiring, J. H. Smoking and coagulation: The sticky fibrin phenomenon. Ultrastruct. Pathol. 34, 236–239. https://doi.org/10.3109/01913121003743716 (2010).
Hong, J. W. et al. The prevalence and factors associated with hearing impairment in the Korean adults: The 2010–2012 Korea National Health and Nutrition Examination Survey (observational study). Medicine 94, e611. https://doi.org/10.1097/md.0000000000000611 (2015).
Frederiksen, T. W. et al. Atherogenic risk factors and hearing thresholds. Audiol. Neurootol. 19, 310–318. https://doi.org/10.1159/000365439 (2014).
Lisowska, G., Jochem, J., Gierlotka, A., Misiolek, M. & Scierski, W. Sex-related cochlear impairment in cigarette smokers. Med. Sci. Monit. 23, 377–397. https://doi.org/10.12659/msm.899589 (2017).
Prabhu, P., Varma, G., Dutta, K. K., Kumar, P. & Goyal, S. Influence of smoking on ultra-high-frequency auditory sensitivity. J. Int. Adv. Otol. 13, 110–112. https://doi.org/10.5152/iao.2017.3412 (2017).
Uchida, Y., Nakashimat, T., Ando, F., Niino, N. & Shimokata, H. Is there a relevant effect of noise and smoking on hearing? A population-based aging study. Int. J. Audiol. 44, 86–91. https://doi.org/10.1080/14992020500031256 (2005).
Nakamagoe, M., Tabuchi, K., Nishimura, B. & Hara, A. Effects of neuroactive steroids on cochlear hair cell death induced by gentamicin. Steroids 76, 1443–1450. https://doi.org/10.1016/j.steroids.2011.07.014 (2011).
Lin, Y. Y. et al. Secondhand smoke is associated with hearing threshold shifts in obese adults. Sci. Rep. 6, 33071. https://doi.org/10.1038/srep33071 (2016).
Florescu, A. et al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Ther. Drug Monit. 31, 14–30. https://doi.org/10.1097/FTD.0b013e3181957a3b (2009).
Acknowledgements
We thank our staff members at the Kangbuk Samsung Health Study for their hard work, dedication, and continuing support, and we thank study participants for their time, interest, and contribution to the Kangbuk Samsung Health Study.
Author information
Authors and Affiliations
Contributions
W.L., Y.C., and S.R. wrote the main manuscript text. Y.C. and S.R. performed statistical analysis. All authors participated in the hypothesis and design of the study, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, W., Chang, Y., Shin, H. et al. Self-reported and cotinine-verified smoking and increased risk of incident hearing loss. Sci Rep 11, 8103 (2021). https://doi.org/10.1038/s41598-021-87531-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87531-1
This article is cited by
-
Changes in Blood Pressure is Associated with Bone Loss in US Adults: A Cross-Sectional Study from NHANES 2005–2018
Calcified Tissue International (2024)
-
Hearing loss and depressive symptoms in older Chinese: whether social isolation plays a role
BMC Geriatrics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.